Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2190
Peer-review started: April 5, 2020
First decision: April 22, 2020
Revised: April 27, 2020
Accepted: May 19, 2020
Article in press: May 19, 2020
Published online: June 6, 2020
Processing time: 63 Days and 22.4 Hours
Claudin 7 is often abnormally expressed in cancers and promotes the progression of some malignancies. However, the role of claudin 7 in stage II colorectal cancer (CRC) has not been studied.
To assess the expression and prognostic value of claudin 7 in stage II CRC.
We retrospectively studied 231 stage II CRC patients who underwent radical surgery at our hospital from 2013 to 2014. The protein expression level of claudin 7 was assessed and its relationship with clinicopathological features and prognosis was statistically analyzed. The independent prognostic factors were identified by Cox proportional hazards models. A prognostic grading system was constructed to stratify the survival of CRC patients.
The expression of claudin 7 was significantly reduced in cancer tissues compared with normal tissues (P < 0.001), and its low expression was closely related to recurrence of the disease (P = 0.017). Multivariate analysis confirmed that claudin 7 low expression (claudin 7-low) (P = 0.028) and perineural invasion positivity (PNI+) (P = 0.026) were independent predictors of poor disease-free survival (DFS). A prognostic grading system based on the status of claudin 7 and PNI classified the patients into three prognostic grades: grade A (claudin 7-high and PNI-), grade B (claudin 7-low and PNI-, claudin 7-high and PNI+), and grade C (claudin 7-low and PNI+). The DFS was significantly different among the three grades (grade B vs grade A, P = 0.032; grade C vs grade A, P < 0.001; grade C vs grade B, P = 0.040).
Claudin 7 can be used as a new prognostic marker to predict the DFS of patients with stage II CRC. The prognostic grading system with the addition of claudin 7 can further improve prognosis stratification of patients.
Core tip: The clinical significance of claudin 7 in stage II colorectal cancer has not been studied. This is believed to be the first study to assess the expression and prognosis of claudin 7 in stage II colorectal cancer. We found that claudin 7 and perineural invasion were independent prognostic factors associated with disease-free survival. A prognostic grading system was constructed based on the status of claudin 7 and perineural invasion, which can better stratify disease-free survival.
- Citation: Quan JC, Peng J, Guan X, Liu Z, Jiang Z, Chen HP, Zhuang M, Wang S, Sun P, Wang HY, Zou SM, Wang XS. Evaluation of clinical significance of claudin 7 and construction of prognostic grading system for stage II colorectal cancer. World J Clin Cases 2020; 8(11): 2190-2200
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2190.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2190
Colorectal cancer (CRC) is a common malignancy, and its incidence and mortality rank fourth and second, respectively[1]. Furthermore, the incidence and mortality of CRC is still on the rise in many countries[2], which seriously threatens human health; thus, it is crucial to identify the factors influencing prognosis. At present, the International Union Against Cancer tumor node metastasis (TNM) staging system is widely used to predict prognosis and guide treatment. For patients with the same stage, they should have similar outcomes according to the TNM staging system. However, there are often different outcomes among these patients in clinical practice, including stage II CRC patients.
Although the overall prognosis of stage II patients is relatively good, some patients will develop local recurrence or distant metastasis within 5 years after radical surgery, which is the leading reason for poor prognosis and cancer-associated death. At present, although some conventional clinicopathologic factors have been used to distinguish these high risk patients, use of only these conventional factors can not better classify such patients. Therefore, the identification of risk factors and precise classification of these patients are still challenging. In addition to conventional clinicopathological factors, more and more attention has been paid to the study of molecular markers, as the application of molecular markers may be helpful in further stratifying prognosis.
Claudins, the main constituents of intercellular tight junctions, play a crucial role in maintaining the paracellular barrier[3]. However, the deregulation of claudins can lead to the development of many diseases[4,5]. Currently, the claudin family is known to contain 27 members[6], and as one of the important claudin family members, claudin 7 has received a remarkable amount of attention. It has been reported that claudin 7 is abnormally expressed in many types of tumors[7-9], and its deregulation promotes the invasion and metastasis of cancer[10,11]. In addition, the aberrant expression of claudin 7 was also found to be related to prognosis of breast carcinoma[12], lung cancer[13], nasopharyngeal carcinoma[14,15], oral and oropharyngeal squamous cell cancer[16], hepatocellular cancer[17], ovarian cancer[18], laryngeal carcinoma[19], gastric carcinoma[20], and clear cell renal cell carcinoma[21]. However, only limited studies have focused on CRC, and, as far as we know, there have been no studies on the clinical significance of claudin 7 in stage II CRC to date. Therefore, it is necessary to clarify the potential value of claudin 7 in stage II CRC.
In our study, the purpose was to assess the protein expression of claudin 7 in stage II CRC and its relationship with clinicopathological features and prognosis. In addition, we also comprehensively analyzed the prognostic factors in stage II CRC and developed a prognostic grading system to accurately classify the survival difference among patients.
This retrospective study included 231 stage II CRC patients who underwent radical surgery at the Cancer Hospital, Chinese Academy of Medical Sciences from 2013 to 2014. All patients had histopathology-confirmed stage II CRC. The patients’ clinicopathological data, including age, gender, tumor location (colon or rectum), preoperative carcinoembryonic antigen (CEA) level (negative or positive), T stage (T3 or T4), tumor differentiation (well/moderate or poor), perineural invasion (PNI, negative or positive), lymphovascular invasion (negative or positive) and postoperative adjuvant chemotherapy, were obtained. This study was approved by the Institutional Review Board of the Cancer Hospital, Chinese Academy of Medical Sciences.
Paraffin-embedded cancer tissue blocks were acquired from all the patients. An additional 72 paraffin-embedded adjacent normal tissue blocks were also prepared. After assessing the hematoxylin and eosin-stained slides, the donor paraffin blocks were punched (1.0 mm diameter of the core), and the tissue cores were placed into recipient paraffin blocks to construct the tissue microarray (TMA) blocks. Following construction of the TMA blocks, 5 µm-thick tissue sections were cut for subsequent immunohistochemical study.
The protein expression level of claudin 7 was investigated by immuno-histochemistry staining of the above TMAs. The immunohistochemical procedures were as follows: TMA sections were deparaffinized and rehydrated in xylene and gradient ethanol, respectively. After deparaffination and rehydration, the sections were heated following immersion in ethylenediaminetetraacetic acid buffer and using a microwave oven for antigen retrieval, and then washed with distilled water. Afterwards, the sections were incubated in 3% hydrogen peroxide for 30 min, and then washed in phosphate buffered saline (referred to as PBS) before blocking of nonspecific binding. Rabbit polyclonal antibody against claudin 7 (ab27487, 1:200; Abcam, Cambridge, United Kingdom) was used as the primary antibody and the sections were incubated overnight at 4 °C. After washing with PBS, the sections were incubated with the corresponding secondary antibody for 30 min at 37 °C, and then washed with PBS again. Subsequently, diaminobenzidine and hematoxylin were used for developing the color and counterstaining, respectively.
The immunoreactivity of claudin 7 was evaluated by two independent investigators, based on the staining intensity and percentage of positively stained cells. The immunostaining intensity was divided into four categories: 3, strong intensity; 2, moderate; 1, weak; 0, negative. The percent of positive cells was classified as 0, less than 5%; 1, 6%-25%; 2, 26%-50%; 3, 51%-75%; 4, > 75%. The results of staining intensity and staining percentage were multiplied to obtain the final comprehensive score (range: 0-12), and 0 score was defined as negative expression, scores of 1-5 as weak expression, scores of 6-8 as moderate expression, scores of 9-12 as strong expression. According to the comprehensive score, the immunoreactivity of claudin 7 was further classified as high expression (scores of 6-12) and low expression (scores < 6) for subsequent statistical analysis.
Clinicopathological variables are expressed in the form of median with corresponding range (continuous variables) and number with percent (categorical variables). The χ2 test was used to evaluate the relationship between claudin 7 expression and clinicopathological features. The Mann-Whitney U test was applied to examine the difference between groups. The prognostic factors were identified by Cox proportional hazards models. Disease-free survival (DFS) rates were assessed by the Kaplan-Meier method, and the differences were compared with the log-rank test. In further analysis, we performed a stratified analysis of prognosis using a combination of independent prognostic factors, and divided patients into different prognostic grades. The DFS among different grades was compared by Cox analysis, and the corresponding hazard ratio (HR) and 95% confidence interval (CI) were estimated. All statistical analyses were performed using SPSS version 20.0 (IBM Corp., Armonk, NY, United States), and the difference was considered statistically significant when the P value was less than 0.05.
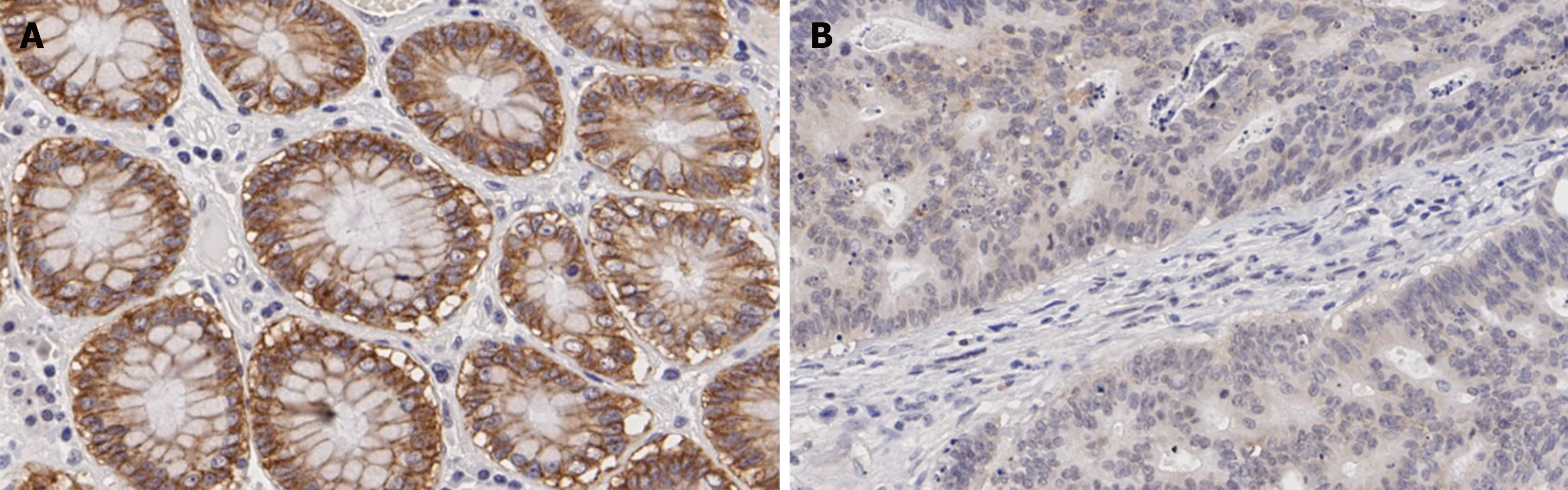
The claudin 7 expression status in normal tissues and cancer tissues was evaluated, respectively. The immunohistochemical results showed that 87.50% of normal tissue samples had strong expression and the remaining 12.50% had moderate expression, while the proportion of strong expression, moderate expression and weak/negative expression in cancer tissue samples was 23.81%, 50.65% and 25.54%, respectively. The representative images are shown in Figure 1. Compared with normal tissues, the claudin 7 protein expression in cancer tissues was statistically reduced (P < 0.001; Figure 2). Table 1 shows the detailed patient characteristics. Table 2 presents the analysis of relationships between claudin 7 expression and clinicopathological features. The results revealed that claudin 7 expression was only related to disease recurrence, and the incidence of disease recurrence was higher in the claudin 7 low expression group than in the high expression group (P = 0.017). However, no correlations were found regarding age, gender, CEA level, tumor location, T stage, tumor differentiation, PNI, and lymphovascular invasion.
| Variable | No. of patients |
| Age in yr | |
| Median (range) | 60 (30-79) |
| ≤ 60 | 124 (53.7) |
| > 60 | 107 (46.3) |
| Gender | |
| Male | 140 (60.6) |
| Female | 91 (39.4) |
| CEA level | |
| Negative | 119 (51.5) |
| Positive | 54 (23.4) |
| Unknown | 58 (25.1) |
| Tumor location | |
| Colon | 130 (56.3) |
| Rectum | 101 (43.7) |
| Tumor differentiation | |
| Well/moderate | 209 (90.5) |
| Poor | 22 (9.5) |
| T stage | |
| T3 | 189 (81.8) |
| T4 | 42 (18.2) |
| Lymphovascular invasion | |
| Negative | 200 (86.6) |
| Positive | 31 (13.4) |
| Perineural invasion | |
| Negative | 191 (82.7) |
| Positive | 40 (17.3) |
| Postoperative chemotherapy | |
| No | 147 (63.6) |
| Yes | 84 (36.4) |
| Recurrence | |
| No | 204 (88.3) |
| Yes | 27 (11.7) |
| Variable | No. of patients | Claudin 7 expression | P value | |
| High | Low | |||
| Age in yr | ||||
| ≤ 60 | 124 | 97 (78.2) | 27 (21.8) | 0.158 |
| > 60 | 107 | 75 (70.1) | 32 (29.9) | |
| Gender | ||||
| Male | 140 | 99 (70.7) | 41 (29.3) | 0.106 |
| Female | 91 | 73 (80.2) | 18 (19.8) | |
| CEA level | ||||
| Negative | 119 | 91 (76.5) | 28 (23.5) | 0.708 |
| Positive | 54 | 40 (74.1) | 14 (25.9) | |
| Unknown | 58 | 41 (70.7) | 17 (29.3) | |
| Tumor location | ||||
| Colon | 130 | 96 (73.8) | 34 (26.2) | 0.809 |
| Rectum | 101 | 76 (75.2) | 25 (24.8) | |
| Tumor differentiation | ||||
| Well/moderate | 209 | 159 (76.1) | 50 (23.9) | 0.082 |
| Poor | 22 | 13 (59.1) | 9 (40.9) | |
| T stage | ||||
| T3 | 189 | 143 (75.7) | 46 (24.3) | 0.374 |
| T4 | 42 | 29 (69.0) | 13 (31.0) | |
| Lymphovascular invasion | ||||
| Negative | 200 | 150 (75.0) | 50 (25.0) | 0.632 |
| Positive | 31 | 22 (71.0) | 9 (29.0) | |
| Perineural invasion | ||||
| Negative | 191 | 144 (75.4) | 47 (24.6) | 0.477 |
| Positive | 40 | 28 (70.0) | 12 (30.0) | |
| Recurrence | ||||
| No | 204 | 157 (77.0) | 47 (23.0) | 0.017 |
| Yes | 27 | 15 (55.6) | 12 (44.4) | |
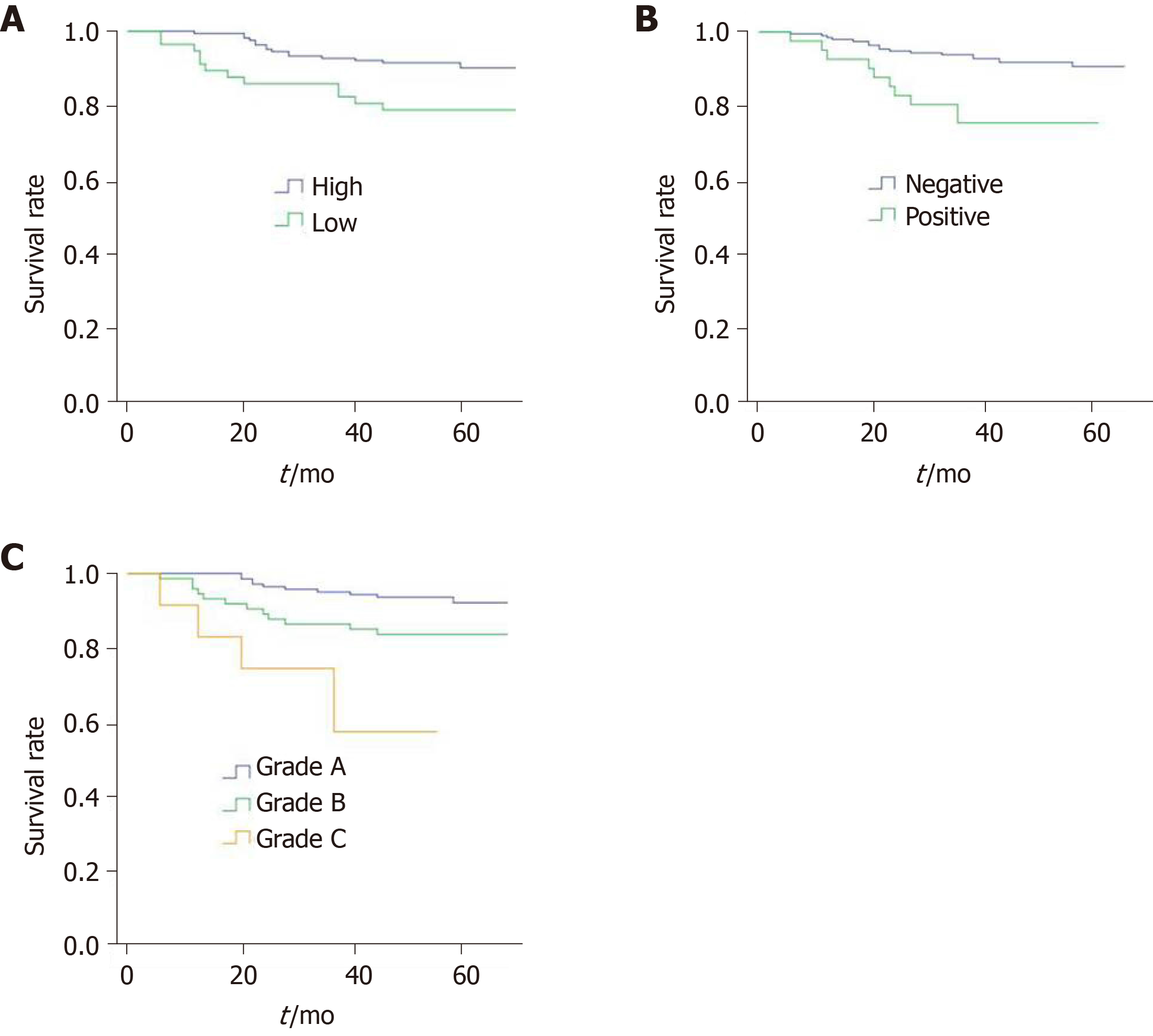
Univariate and multivariate prognostic analyses were performed to identify the factors that affected patient survival (Table 3). In univariate analysis, it was found that T stage, PNI, and claudin 7 expression levels were the most important factors influencing DFS; whereas, no statistical differences were observed for gender, age, CEA level, tumor location, postoperative adjuvant chemotherapy, tumor differentiation, and lymphovascular invasion. With regard to T stage, claudin 7 expression levels and PNI were included in the multivariate analysis. The results revealed that only PNI (HR = 2.586; 95%CI: 1.121-5.966; P = 0.026) and claudin 7 (HR = 2.366; 95%CI: 1.100-5.091; P = 0.028) were independent prognostic factors associated with DFS. Figure 3A shows the DFS curves of different claudin 7 subgroups, and DFS was significantly worse in the claudin 7 low expression group than in the high expression group (P = 0.011, log-rank test). Similarly, compared to patients with PNI, those without PNI had a better DFS (P = 0.002, log-rank test), the survival curves are shown in Figure 3B.
| Variable | Univariable analysis | Multivariate analysis | ||
| HR (95%CI) | P value | HR (95%CI) | P value | |
| Age in yr | ||||
| ≤ 60 | Reference | |||
| > 60 | 1.310 (0.616-2.788) | 0.483 | ||
| Gender | ||||
| Male | Reference | |||
| Female | 0.903 (0.413-1.971) | 0.797 | ||
| CEA level | ||||
| Negative | Reference | |||
| Positive | 1.224 (0.488-3.067) | 0.667 | ||
| Unknown | 1.133 (0.452-2.842) | 0.789 | ||
| Tumor location | ||||
| Colon | Reference | |||
| Rectum | 1.384 (0.651-2.945) | 0.399 | ||
| Tumor differentiation | ||||
| Well/moderate | Reference | |||
| Poor | 1.228 (0.370-4.080) | 0.737 | ||
| T stage | ||||
| T3 | Reference | Reference | ||
| T4 | 2.558 (1.147-5.701) | 0.022 | 1.817 (0.771-4.285) | 0.172 |
| Lymphovascular invasion | ||||
| Negative | Reference | |||
| Positive | 1.984 (0.800-4.919) | 0.139 | ||
| Perineural invasion | ||||
| Negative | Reference | Reference | ||
| Positive | 3.190 (1.457-6.985) | 0.004 | 2.586 (1.121-5.966) | 0.026 |
| Postoperative chemotherapy | ||||
| No | Reference | |||
| Yes | 0.859 (0.386-1.913) | 0.710 | ||
| Claudin 7 expression | ||||
| High | Reference | Reference | ||
| Low | 2.578 (1.206-5.509) | 0.015 | 2.366 (1.100-5.091) | 0.028 |
In order to determine whether the combined assessment of claudin 7 and PNI can better classify patient survival, we further developed a prognostic grading system based on the above two independent prognostic factors (Table 4), and the patients were classified into three prognostic grades: Grade A, grade B, and grade C. Grade A was defined as claudin 7 high expression (claudin 7-high) and PNI negative (PNI-), grade B was defined as claudin 7 low expression (claudin 7-low) and PNI negative or claudin 7 high expression and PNI positive (PNI+), and grade C was defined as claudin 7 low expression and PNI positive. Cox analysis showed that grade B (HR = 2.512; 95%CI: 1.084-5.817; P = 0.032) and grade C patients (HR = 7.963; 95%CI: 2.704-23.452; P < 0.001) had a statistically poorer DFS compared with grade A patients. Similarly, the DFS in grade C patients was also significantly worse than in grade B patients (HR = 2.987; 95%CI: 1.051-8.489; P = 0.040). Figure 3C shows the survival curves of the three grades (P < 0.001, log-rank test).
| Variable | HR | 95%CI | P value |
| Grade A (claudin 7-high and PNI-) | 1 | - | - |
| Grade B (claudin 7-high and PNI+, claudin 7-low and PNI-) | 2.512 | 1.084-5.817 | 0.032 |
| Grade C (claudin 7-low and PNI+) | 7.963 | 2.704-23.452 | < 0.001 |
More and more studies have reported the effects of claudin 7 on various cancers[11,18,21]. However, the role of claudin 7 in CRC has not been fully elucidated. Therefore, further studies are necessary to examine the potential value of claudin 7 in CRC. In the present study, we analyzed claudin 7 expression level in stage II CRC, and evaluated its relationship with clinicopathological features and prognosis. As far as we know, we are the first group to investigate the value of claudin 7 in stage II CRC.
Claudin 7 expression differs in different types of cancers. For example, in gastric carcinoma, Jun et al[20] reported that claudin 7 expression was up-regulated, while Zhou et al[19] showed that claudin 7 expression was down-regulated in laryngeal carcinoma. For CRC, Tang et al[22] revealed that claudin 7 had low expression in cancer tissues; similarly, Bornholdt et al[23] reported the same conclusion at the mRNA level, based on PCR analysis. In the present study, we analyzed the protein expression levels of claudin 7 using immunohistochemistry and found that claudin 7 expression was decreased in CRC tissues compared to that in adjacent normal tissues, which further confirmed the findings of Tang et al[22] and Bornholdt et al[23].
At present, only a limited number of articles have reported the relationship between claudin 7 and clinicopathological features in CRC. Moreover, the conclusions vary among different studies. Wang et al[24] assessed claudin 7 expression in 80 CRC patients and showed that there was a significant association between claudin 7 expression and degree of differentiation and liver metastasis. In addition, Oshima et al[25] analyzed 205 CRC patients, and revealed that claudin 7 expression was correlated with liver metastasis and venous invasion but not with the degree of differentiation. However, unlike the above studies, our study specifically focused on stage II CRC and found that low expression of claudin 7 was only related to disease recurrence, and no correlations were found with other clinicopathological features, including tumor differentiation, T stage, PNI, and lymphovascular invasion. The differences in variables and sample sizes in different studies may be the reason for the above contradictions. Therefore, more and larger studies are needed to obtain definitive conclusions in the future.
In addition to its involvement in the development of cancer, claudin 7 is also an important prognostic factor. Its prognostic value has been previously reported in different cancers, such as gastric cancer, ovarian cancer, hepatocellular cancer and so on. In the study by Li et al[21], a significant correlation was found between low expression of claudin 7 and poor prognosis in clear cell renal cell carcinoma. Additionally, Kim et al[18] also revealed that claudin 7 expression was related to progression-free survival of epithelial ovarian carcinoma patients. Jun et al[20] analyzed the survival of patients with gastric cancer, and reached the same conclusion that claudin 7 was a significant factor affecting the prognosis of patients. Similarly, Bouchagier et al[17] and Yamamoto et al[13] also emphasized the prognostic value of claudin 7 in hepatocellular carcinoma and lung cancer. However, little is known about the relationship of claudin 7 with survival outcomes in CRC due to a limited number of cases and lack of survival information in previous studies.
In order to understand whether claudin 7 has prognostic significance in CRC, we evaluated the effects of claudin 7 on DFS in stage II CRC patients and confirmed that claudin 7 was an independent predictor of DFS and that the DFS in the claudin 7 high expression group was significantly better than in the low-expression group. Our study showed that claudin 7 was a novel marker for predicting DFS in stage II CRC patients. Moreover, the protein expression level of claudin 7 can be detected using routine immunohistochemistry; therefore, claudin 7 detection can be easily applied in clinical practice to provide guidance for better prediction of DFS in stage II CRC.
In addition to claudin 7, PNI was another important predictive factor for DFS. In our study, the DFS of patients with PNI was significantly worse than those without PNI, which was consistent with the findings of Huh et al[26]. In order to assess whether claudin 7 combined with PNI could more accurately classify DFS in patients with stage II CRC, we constructed a prognostic grading system and divided the patients into three subgroups based on the above two independent predictors and found that DFS was worse in patients with low expression of claudin 7 and the presence of PNI; in addition, patients with high expression of claudin 7 and absence of PNI had the best DFS. By prognostic stratification analysis, statistical differences in DFS were found among the three subgroups, and claudin 7 combined with PNI can better stratify DFS. Therefore, in clinical practice, we can make individualized risk assessments based on this prognostic grading system.
In conclusion, the present study revealed the clinical value of claudin 7 in stage II CRC. Moreover, we also constructed a prognostic grading system using the combination of claudin 7 and PNI, which can more accurately distinguish the survival difference among different patients.
Claudin 7 has been found to be abnormally expressed in cancers and plays a critical role in the progression of some malignancies. However, there have been no studies on the clinical significance of claudin 7 in stage II colorectal cancer (CRC) to date. Therefore, it is necessary to clarify the potential value of claudin 7 in stage II CRC.
We evaluated the expression and prognostic value of claudin 7 in stage II CRC, and further constructed a prognostic grading system to accurately classify the survival difference among patients.
To assess the clinical significance of claudin 7 and construct a prognostic grading system for stage II CRC.
This retrospective study included 231 stage II CRC patients who underwent radical surgery at our hospital from 2013 to 2014. The protein expression level of claudin 7 was assessed and its relationship with clinicopathological features and prognosis was statistically analyzed. The Kaplan-Meier method was used to assess the disease-free survival, and Cox proportional hazards models were used to determine the independent prognostic factors. A prognostic grading system was constructed to stratify the patients into different subgroups, and the survival differences among different subgroups were compared.
The expression of claudin 7 was decreased in cancer tissues compared to that in normal tissues, and its low expression was related to disease recurrence. Prognostic analysis showed that claudin 7 low expression (claudin 7-low) and perineural invasion positivity (PNI+) were independent prognostic factors associated with disease-free survival. A prognostic grading system based on the above two independent prognostic factors divided the patients into three prognostic grades: grade A (claudin 7-high and PNI-), grade B (claudin 7-low and PNI-, claudin 7-high and PNI+), and grade C (claudin 7-low and PNI+). Subgroup analysis showed that the prognosis of patients with different grades differed significantly.
Our study identifies the prognostic significance of claudin 7 in stage II CRC. Furthermore, we also confirm that the prognostic grading system can better classify patient survival.
The prognostic grading system can be used as an effective prognostic predictive tool to help clinicians more accurately predict survival.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Deepak P, Li G, Pizzirusso F, Zimmerman M S-Editor: Wang JL L-Editor: Filipodia E-Editor: Xing YX
| 1. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 55776] [Article Influence: 7968.0] [Reference Citation Analysis (132)] |
| 2. | Arnold M, Sierra MS, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global patterns and trends in colorectal cancer incidence and mortality. Gut. 2017;66:683-691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3058] [Cited by in RCA: 3298] [Article Influence: 412.3] [Reference Citation Analysis (3)] |
| 3. | González-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 800] [Cited by in RCA: 837] [Article Influence: 38.0] [Reference Citation Analysis (0)] |
| 4. | Pope JL, Bhat AA, Sharma A, Ahmad R, Krishnan M, Washington MK, Beauchamp RD, Singh AB, Dhawan P. Claudin-1 regulates intestinal epithelial homeostasis through the modulation of Notch-signalling. Gut. 2014;63:622-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 111] [Cited by in RCA: 187] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 5. | Lee JW, Lee SJ, Seo J, Song SY, Ahn G, Park CS, Lee JH, Kim BG, Bae DS. Increased expressions of claudin-1 and claudin-7 during the progression of cervical neoplasia. Gynecol Oncol. 2005;97:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Günzel D, Yu AS. Claudins and the modulation of tight junction permeability. Physiol Rev. 2013;93:525-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1046] [Cited by in RCA: 1029] [Article Influence: 85.8] [Reference Citation Analysis (0)] |
| 7. | Tassi RA, Bignotti E, Falchetti M, Ravanini M, Calza S, Ravaggi A, Bandiera E, Facchetti F, Pecorelli S, Santin AD. Claudin-7 expression in human epithelial ovarian cancer. Int J Gynecol Cancer. 2008;18:1262-1271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Sauer T, Pedersen MK, Ebeltoft K, Naess O. Reduced expression of Claudin-7 in fine needle aspirates from breast carcinomas correlate with grading and metastatic disease. Cytopathology. 2005;16:193-198. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Kominsky SL, Argani P, Korz D, Evron E, Raman V, Garrett E, Rein A, Sauter G, Kallioniemi OP, Sukumar S. Loss of the tight junction protein claudin-7 correlates with histological grade in both ductal carcinoma in situ and invasive ductal carcinoma of the breast. Oncogene. 2003;22:2021-2033. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 341] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 10. | Singh AB, Dhawan P. Claudins and cancer: Fall of the soldiers entrusted to protect the gate and keep the barrier intact. Semin Cell Dev Biol. 2015;42:58-65. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | Usami Y, Chiba H, Nakayama F, Ueda J, Matsuda Y, Sawada N, Komori T, Ito A, Yokozaki H. Reduced expression of claudin-7 correlates with invasion and metastasis in squamous cell carcinoma of the esophagus. Hum Pathol. 2006;37:569-577. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 117] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 12. | Bernardi MA, Logullo AF, Pasini FS, Nonogaki S, Blumke C, Soares FA, Brentani MM. Prognostic significance of CD24 and claudin-7 immunoexpression in ductal invasive breast cancer. Oncol Rep. 2012;27:28-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Yamamoto T, Oshima T, Yoshihara K, Yamanaka S, Nishii T, Arai H, Inui K, Kaneko T, Nozawa A, Woo T, Rino Y, Masuda M, Imada T. Reduced expression of claudin-7 is associated with poor outcome in non-small cell lung cancer. Oncol Lett. 2010;1:501-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Suren D, Yildirim M, Kaya V, Elal R, Selcuk OT, Osma U, Yildiz M, Gunduz S, Sezer C. Expression patterns of claudins 1, 4, and 7 and their prognostic significance in nasopharyngeal carcinoma. J BUON. 2015;20:212-217. [PubMed] |
| 15. | Hsueh C, Chang YS, Tseng NM, Liao CT, Hsueh S, Chang JH, Wu IC, Chang KP. Expression pattern and prognostic significance of claudins 1, 4, and 7 in nasopharyngeal carcinoma. Hum Pathol. 2010;41:944-950. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Melchers LJ, Bruine de Bruin L, Schnell U, Slagter-Menkema L, Mastik MF, de Bock GH, van Dijk BA, Giepmans BN, van der Laan BF, van der Wal JE, Roodenburg JL, Schuuring E. Lack of claudin-7 is a strong predictor of regional recurrence in oral and oropharyngeal squamous cell carcinoma. Oral Oncol. 2013;49:998-1005. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 29] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 17. | Bouchagier KA, Assimakopoulos SF, Karavias DD, Maroulis I, Tzelepi V, Kalofonos H, Karavias DD, Kardamakis D, Scopa CD, Tsamandas AC. Expression of claudins-1, -4, -5, -7 and occludin in hepatocellular carcinoma and their relation with classic clinicopathological features and patients' survival. In Vivo. 2014;28:315-326. [PubMed] |
| 18. | Kim CJ, Lee JW, Choi JJ, Choi HY, Park YA, Jeon HK, Sung CO, Song SY, Lee YY, Choi CH, Kim TJ, Lee JH, Kim BG, Bae DS. High claudin-7 expression is associated with a poor response to platinum-based chemotherapy in epithelial ovarian carcinoma. Eur J Cancer. 2011;47:918-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Zhou S, Piao X, Wang C, Wang R, Song Z. Identification of claudin‑1, ‑3, ‑7 and ‑8 as prognostic markers in human laryngeal carcinoma. Mol Med Rep. 2019;20:393-400. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 20. | Jun KH, Kim JH, Jung JH, Choi HJ, Chin HM. Expression of claudin-7 and loss of claudin-18 correlate with poor prognosis in gastric cancer. Int J Surg. 2014;12:156-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 21. | Li Y, Gong Y, Ning X, Peng D, Liu L, He S, Gong K, Zhang C, Li X, Zhou L. Downregulation of CLDN7 due to promoter hypermethylation is associated with human clear cell renal cell carcinoma progression and poor prognosis. J Exp Clin Cancer Res. 2018;37:276. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 41] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 22. | Tang W, Dou T, Zhong M, Wu Z. Dysregulation of Claudin family genes in colorectal cancer in a Chinese population. Biofactors. 2011;37:65-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 23. | Bornholdt J, Friis S, Godiksen S, Poulsen SS, Santoni-Rugiu E, Bisgaard HC, Lothe IM, Ikdahl T, Tveit KM, Johnson E, Kure EH, Vogel LK. The level of claudin-7 is reduced as an early event in colorectal carcinogenesis. BMC Cancer. 2011;11:65. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Wang K, Li T, Xu C, Ding Y, Li W, Ding L. Claudin-7 downregulation induces metastasis and invasion in colorectal cancer via the promotion of epithelial-mesenchymal transition. Biochem Biophys Res Commun. 2019;508:797-804. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Oshima T, Kunisaki C, Yoshihara K, Yamada R, Yamamoto N, Sato T, Makino H, Yamagishi S, Nagano Y, Fujii S, Shiozawa M, Akaike M, Wada N, Rino Y, Masuda M, Tanaka K, Imada T. Reduced expression of the claudin-7 gene correlates with venous invasion and liver metastasis in colorectal cancer. Oncol Rep. 2008;19:953-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Huh JW, Kim HR, Kim YJ. Prognostic value of perineural invasion in patients with stage II colorectal cancer. Ann Surg Oncol. 2010;17:2066-2072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 88] [Article Influence: 5.9] [Reference Citation Analysis (0)] |