Published online Jun 6, 2020. doi: 10.12998/wjcc.v8.i11.2150
Peer-review started: January 2, 2020
First decision: February 26, 2020
Revised: April 17, 2020
Accepted: May 1, 2020
Article in press: May 1, 2020
Published online: June 6, 2020
Processing time: 157 Days and 10.8 Hours
Vitamin D deficiency is common in patients with chronic pain and healthy people, but the difference between the two has not been reported; thus, whether there is a relationship between vitamin D deficiency and chronic pain remains to be confirmed. Osteoporosis is a common disease in chronic pain disorders. Understanding the relationship between vitamin D and osteoporosis will provide a basis for the rational supplementation of vitamin D to prevent osteoporosis, and to understand the risk factors of bone mass change to provide a new treatment plan for early prevention of osteoporosis.
To determine 25 hydroxy vitamin D (25OHD) level in patients with chronic pain to clarify its clinical significance. The relationship between vitamin D and bone mineral density (BMD) and the risk factors for bone mass change were also evaluated.
In this study, 184 patients with chronic pain were included in the study group, and 104 healthy individuals who underwent routine health checkups during the same period were included in the control group. 25OHD level was detected in both groups by enzyme-linked immunosorbent assay. According to the BMD test results, the patients in the study group were further classified into three subgroups: Normal BMD group, reduced BMD group, and osteoporosis group. Age, sex, ethnicity, living altitude, body mass index, 25OHD level, parathyroid hormone (PTH), calcium (Ca) and phosphorus levels were analyzed statistically in both groups.
The vitamin D level in the study group was lower than that in the control group at 53.8% vs 57.7%, with no significant difference between the two groups. The proportion of patients with severe vitamin D deficiency in the study group was higher than that in the control group. The mean age was greater in the osteoporosis subgroup, and the youngest in the normal BMD subgroup. Vitamin D level in the osteoporosis subgroup was lower than that in the other two subgroups, and was not specific for the diagnosis of bone mass reduction and osteoporosis. The above results were analyzed statistically and showed significant differences (P < 0.05). There was a positive correlation between age and BMD in patients with chronic pain (R = 0.567, P < 0.001). Age, PTH and Ca were risk factors for bone mass reduction, while age, ethnicity and altitude were risk factors for osteoporosis.
Vitamin D deficiency is a common phenomenon in patients with chronic pain, and severe vitamin D deficiency is not uncommon. Vitamin D level is not a risk factor for bone mass reduction and osteoporosis. Bone mass reduction is correlated with age, PTH and Ca, while osteoporosis is correlated with age, ethnicity and altitude.
Core tip: Vitamin D deficiency is common in chronic pain patients and healthy people, and its level is not different between the two, but severe deficiency is more common in patients with chronic pain. Vitamin D is not a risk factor for bone loss and osteoporosis. Age, parathyroid hormone, and calcium are risk factors for bone loss. Age, ethnicity, and altitude are risk factors for osteoporosis.
- Citation: Duan BL, Mao YR, Xue LQ, Yu QY, Liu MY. Determination of vitamin D and analysis of risk factors for osteoporosis in patients with chronic pain. World J Clin Cases 2020; 8(11): 2150-2161
- URL: https://www.wjgnet.com/2307-8960/full/v8/i11/2150.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i11.2150
Chronic pain is one of the most challenging clinical problems for clinicians. Patients with chronic pain not only have to endure somatic pain but also bear a huge psychological burden due to behavioral responses. Some studies have demonstrated that vitamin D deficiency is associated with non-specific musculoskeletal pain[1], knee osteoarthritis[2], migraine[3], chronic cervical pain[4], and other multiple chronic pains. It is also a risk factor for various chronic diseases such as osteoporosis, hypertension and cardiovascular disease[5].
Vitamin D deficiency is not only present in patients with chronic pain but also a common phenomenon in healthy populations. It is estimated that more than 50% of the world’s population have vitamin D deficiency[6]. A recent multi-center survey in China showed that the prevalence of vitamin D deficiency in Chinese urban residents is approximately 55.9%[7]. Vitamin D is a pro-hormone that regulates the calcium (Ca) and phosphorus (P) balance and skeletal structures. Vitamin D deficiency increases the risk of osteoporosis[8]. The occurrence of osteoporosis is a silent and imperceptible process, and is often ignored until fragile fracture occurs. For osteoporotic patients, early detection, diagnosis and treatment are of primary importance. Currently, the diagnosis of osteoporosis mainly depends on bone mineral density (BMD) measured by dual-energy X-ray absorptiometry. Many studies have reported the relationship between vitamin D and BMD, but the conclusions are controversial.
The aim of the present study was to determine the changes in vitamin D level in patients with chronic pain, clarify the relationship between vitamin D and BMD, and explore the risk factors for bone mass reduction and osteoporosis in patients with chronic pain, in an attempt to provide experimental clues for the prevention and treatment of osteoporosis.
In this study, 184 patients with chronic pain who were admitted to the Pain Department of Qinghai People’s Hospital (Xining, China) between May and September 2017 were enrolled. These patients consisted of 49 males and 135 females with a mean age of 57.62 ± 13.79 years. An additional 104 healthy individuals who underwent routine health checkups in the same hospital during the same period were enrolled as controls, including 25 males and 79 females with a mean age of 56.52 ± 13.66 years. According to the BMD test results, the patients in the study group were further classified into three subgroups: Normal BMD group (n = 55), reduced BMD group (n = 74) and osteoporosis group (n = 55). The exclusion criteria were as follows: Patients with endocrine and autoimmune diseases; malignant tumors; chronic liver disease, chronic obstructive pulmonary disease and chronic kidney disease; sequelae of cardio-cerebrovascular disease affecting extremity function; skin diseases who could not be exposed to sunlight; those using active vitamin D, steroids, sex hormones, parathyroid hormone (PTH), calcitonin, diphosphate and other drugs that may affect bone metabolism within 6 mo before initiation of the study.
The research protocol was approved by Qinghai People’s Hospital, and informed consent was obtained from all participants in the study on the principle of voluntary participation. Of the initially recruited 346 patients with chronic pain, 103 patients with complicated autoimmune, cardiovascular, endocrine and chronic kidney diseases, malignant tumors, and those who had a history of using anti-osteoporosis drugs or Ca supplements, and 59 patients who lacked BMD, PTH and other key data were excluded from the study. In total, 184 patients were included in this study for analysis.
Criteria for test index evaluation: Venous blood samples were collected at 7:00-8:00 AM from all participants under fasting conditions and sent to our laboratory for analysis. 25 hydroxy vitamin D (25OHD) and PTH were measured by a Cobas 8000 electrochemiluminescence immunoassay kit. Ca and P levels were detected using an automatic biochemical analyzer and the required kit (Hitachi. Japan). BMD of the L-1-4 and femoral neck, Ward’s triangle and trochanter was measured by dual-energy X-ray absorptiometry (Hologic Q DR 2000, United States). Other general data of the participants including age, height, weight, body mass index (BMI), living altitude, past medical history and drug administration were also recorded for analysis.
The vitamin D status was assessed by measuring the serum level of 25OHD, and was classified as severe vitamin D deficiency (< 10 ng/mL), vitamin D deficiency (≥ 10 < 20 ng/mL), vitamin D insufficiency (≥ 20 < 30 ng/mL), and vitamin D sufficiency (≥ 30 ng/mL).
The criteria for BMD assessment were as follows: Patients with a BMD T value > 1 in any of the L1-4, total hip and femoral neck were included in the normal BMD group, -1 to 2.5 were included in the reduced BMD group, and ≤ -2.5 were included in the osteoporosis group.
The criteria for PTH assessment were as follows: Serum PTH < 15 pg/mL was considered reduced, ≥ 15 pg/mL ≤ 65 pg/mL was considered normal, and > 65 pg/mL was considered increased.
The criteria for P assessment were as follows: Serum P < 0.85 mmol/L was considered reduced, ≥ 0.85 mmol/L ≤ 1.51 mmol/L was considered normal, and > 1.51 mol/L was considered increased.
The criteria for Ca assessment were as follows: Serum Ca < 2.2 mmol/L was considered reduced, ≥ 2.2 mmol/L ≤ 2.7 mmol/L was considered normal, and > 2.7 mol/L was considered increased.
All statistical analyses were performed using SPSS 17.0. Quantitative data are expressed as x ± s. Comparisons between two groups were performed using the independent sample t test; comparisons between three groups were performed by one way ANOVA; and comparisons between multiple groups were performed by LSD. Counting data are expressed as c2. Two sets of ordered counting data were analyzed by Mantel-Haenszel test for linear trend. Risk factors were analyzed by binary logistic regression. Values of P < 0.05 were considered statistically significant.
Comparisons of the general data indicated no significant difference in age and sex between the two groups. The vitamin D concentration in the study group was significantly lower than that in the control group (P < 0.05) (Table 1).
| Group | n | Age (yr) | Sex (M/F) | Vitamin D (ng/mL) |
| Study | 184 | 57.62 ± 13.79 | 49/135 | 13.76 ± 7.15 |
| Control | 104 | 56.52 ± 13.66 | 25/79 | 20.12 ± 7.59 |
| t (c2) | 0.656 | 0.234 | 7.085 | |
| P value | 0.512 | 0.629 | 0.000 |
Vitamin D deficiency was predominant in both groups, accounting for 53.8% and 57.7%, respectively, with no significant difference between the two groups. The proportion of patients with severe vitamin D deficiency in the study group was significantly higher than that in the control group (P < 0.05) (Table 2).
| Vitamin D level | Study group (n = 184), n (%) | Control group (n = 104), n (%) | c2 | P value |
| < 10 | 55 (29.9) | 1 (1.0) | 35.502 | 0.000 |
| 10-20 | 99 (53.8) | 60 (57.7) | 0.406 | 0.524 |
| 20-30 | 25 (13.6) | 33 (31.7) | 13.600 | 0.000 |
| ≥ 30 | 5 (2.7) | 10 (9.6) | 6.404 | 0.011 |
Two-group comparisons showed a significant difference in age between the normal BMD, reduced BMD and osteoporosis groups, with the oldest patients in the osteoporosis group and the youngest in the normal BMD group. There was no significant difference in sex between the three groups. The vitamin D level in the osteoporosis group was lower than that in the other two groups (Table 3).
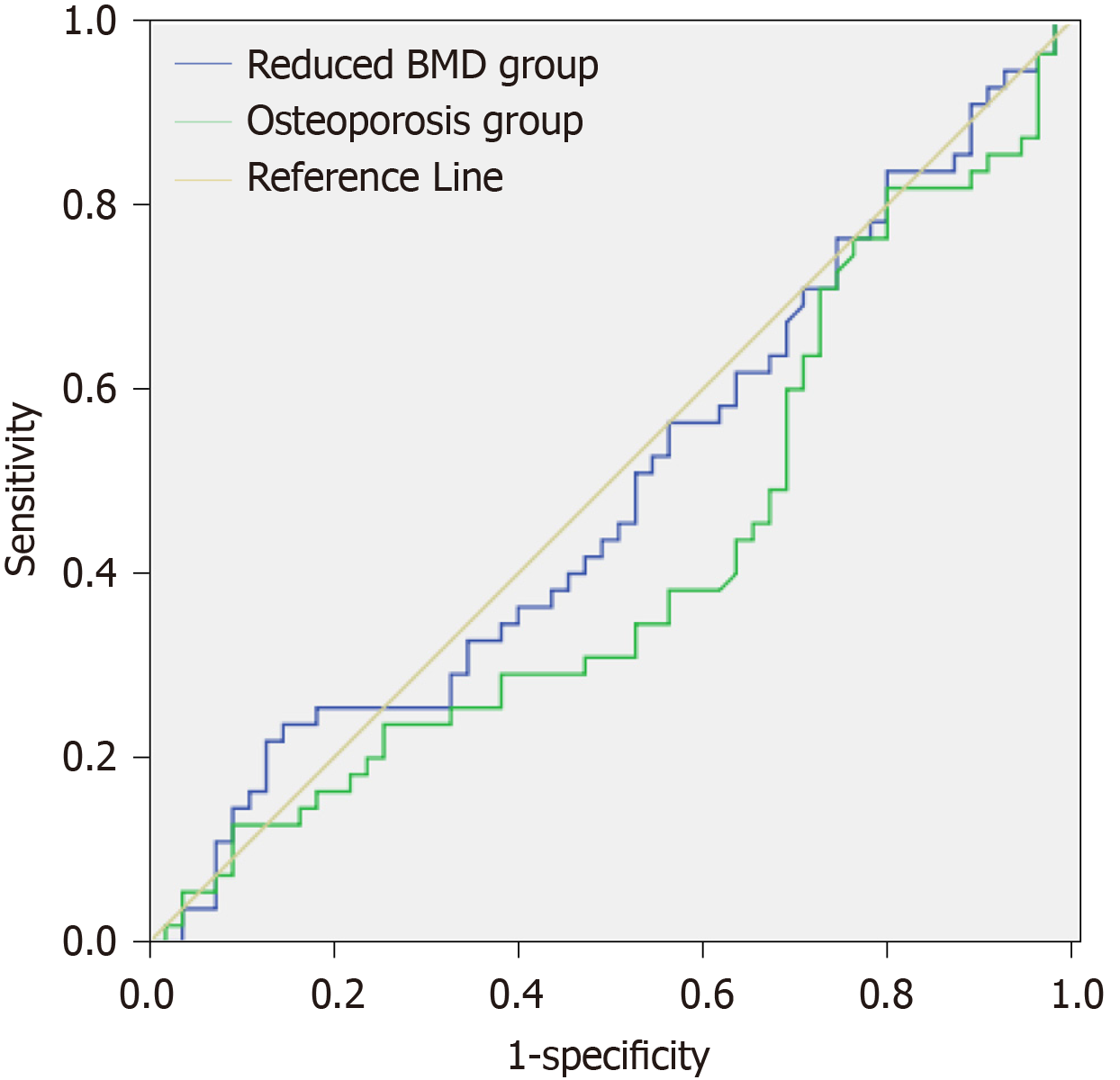
The area under the receiver operating characteristic curve of vitamin D concentration in the reduced BMD and osteoporosis groups was 0.487 and 0.417, respectively, suggesting that it was significant for the diagnosis of bone mass reduction and osteoporosis (Table 4, Figure 1).
| Group | n | AUC | SD | P value | 95%CI |
| Reduced BMD | 74 | 0.487 | 0.056 | 0.820 | 0.379-0.596 |
| Osteoporosis | 55 | 0.417 | 0.055 | 0.134 | 0.309-0.525 |
The relationship between age and BMD was analyzed by the Mantel-Haenszel chi-square test, and the results showed a linear correlation between age and BMD in patients with chronic pain (c2 = 58.933, P < 0.001). Pearson correlation analysis showed that R = 0.567 and P < 0.001, indicating that BMD decreased gradually with increased age in chronic pain patients (Table 5).
| Age group (yr) | n | Normal BMD (%) | Reduced BMD (%) | Osteoporosis (%) | c2 | P value |
| < 45 | 32 | 27 (49.09) | 4 (5.41) | 1 (1.82) | ||
| 45-64 | 86 | 24 (43.63) | 43 (58.11) | 19 (34.55) | 58.933 | 0.000 |
| ≥ 65 | 66 | 4 (7.27) | 27 (36.49) | 35 (63.64) | ||
| Total | 184 | 55 (100) | 74 (100) | 55 (100) |
There was no significant difference in vitamin D change between the different age groups in the study group (P > 0.05) (Table 6).
| Age group (yr) | n | Vitamin D (ng/mL) |
| < 45 | 32 | 13.23 ± 5.58 |
| 45-64 | 86 | 1.64 ± 7.06 |
| ≥ 65 | 66 | 14.15 ± 8.00 |
| F | 0.189 | |
| P value | 0.28 |
Ten factors including age, sex, ethnicity and BMI in the normal and reduced BMD groups were analyzed by univariate analysis and the results showed that age, sex, ethnicity, PTH and Ca showed statistical significance (P < 0.05) (Table 7).
| Factor | Reduced BMD (%) | Normal BMD (%) | c2 | P value |
| Age (yr) | 37.533 | 0.000 | ||
| < 45 | 4 | 27 | ||
| 45-64 | 43 | 24 | ||
| ≥ 65 | 27 | 4 | ||
| Sex | 23.334 | 0.000 | ||
| M | 19 | 20 | ||
| F | 55 | 35 | ||
| Ethnicity | 8.407 | 0.038 | ||
| Tibetan | 3 | 10 | ||
| Han | 60 | 37 | ||
| Hui | 7 | 7 | ||
| Others | 4 | 1 | ||
| BMI | 2.302 | 0.512 | ||
| < 18.5 | 3 | 4 | ||
| 18.5-22.9 | 38 | 33 | ||
| 24-27 | 22 | 11 | ||
| ≥ 28 | 11 | 7 | ||
| Occupation | 0.144 | 0.704 | ||
| Farmer | 18 | 15 | ||
| Non-farmer | 56 | 40 | ||
| Vitamin D | 1.323 | 0.724 | ||
| < 10 | 21 | 15 | ||
| 10-20 | 38 | 32 | ||
| 20-30 | 13 | 6 | ||
| ≥ 30 | 2 | 2 | ||
| PTH | 7.503 | 0.023 | ||
| < 15 | 1 | 0 | ||
| 15-65 | 41 | 43 | ||
| > 65 | 32 | 12 | ||
| Ca | 21.396 | 0.000 | ||
| < 2.2 | 39 | 26 | ||
| 2.2-2.7 | 17 | 29 | ||
| > 2.7 | 18 | 0 | ||
| P | 1.312 | 0.519 | ||
| < 0.85 | 5 | 3 | ||
| 0.85-1.51 | 61 | 49 | ||
| > 1.51 | 8 | 3 | ||
| Altitude | 2.988 | 0.224 | ||
| 1500-2500 | 58 | 40 | ||
| 2500-3500 | 15 | 11 | ||
| 3500-5800 | 1 | 4 |
Of the above results, age, sex, ethnicity, PTH, and Ca were entered into binary logistic analysis, and the results showed that age, PTH and Ca were risk factors for bone mass reduction (Table 8).
| Factor | B value | SD (S.E.) | Wald value | Variance | P value | OR value | OR, 95%CI |
| Age | 2.670 | 0.473 | 31.868 | 1 | 0.000 | 14.440 | 5.714-36.488 |
| PTH | 2.210 | 0.546 | 16.387 | 1 | 0.000 | 9.119 | 3.127-26.592 |
| Ca | 0.717 | 0.363 | 3.906 | 1 | 0.048 | 2.048 | 1.006-4.171 |
| constant | -1.298 | 2.135 | 28.008 | 1 | 0.000 | 0.000 |
Univariate analysis of 10 factors including age, sex, ethnicity and BMI in the osteoporosis and normal BMD subgroups showed significant differences in age, sex, ethnicity and altitude between these two subgroups (P < 0.05) (Table 9).
| Factor | Osteoporosis, (%) | Normal BMD (%) | c2 | P value |
| Age (yr) | 49.365 | 0.000 | ||
| < 45 | 1 | 27 | ||
| 45-64 | 19 | 24 | ||
| ≥ 65 | 35 | 4 | ||
| Sex | 4.583 | 0.032 | ||
| M | 10 | 20 | ||
| F | 45 | 35 | ||
| Ethnicity | 9.913 | 0.019 | ||
| Tibetan | 2 | 10 | ||
| Han | 38 | 37 | ||
| Hui | 8 | 7 | ||
| Others | 7 | 1 | ||
| BMI | 0.777 | 0.855 | ||
| < 18.5 | 5 | 4 | ||
| 18.5-23.9 | 36 | 33 | ||
| 24-27 | 9 | 11 | ||
| > 28 | 5 | 7 | ||
| Occupation | ||||
| Farmer | 10 | 15 | 1.294 | 0.255 |
| Non-farmer | 45 | 40 | ||
| Vitamin D | 0.959 | 0.811 | ||
| < 10 | 19 | 15 | ||
| 10-20 | 29 | 32 | ||
| 20-30 | 6 | 6 | ||
| ≥ 30 | 1 | 2 | ||
| PTH | 0.767 | 0381 | ||
| < 15 | 0 | 0 | ||
| 15-65 | 39 | 43 | ||
| > 65 | 16 | 12 | ||
| Ca | 0.146 | 0.703 | ||
| < 2.2 | 28 | 26 | ||
| 2.2-2.7 | 27 | 29 | ||
| > 2.7 | 0 | 0 | ||
| P | 1.942 | 0.379 | ||
| < 0.85 | 7 | 3 | ||
| 0.85-1.51 | 46 | 49 | ||
| > 1.51 | 2 | 3 | ||
| Altitude | 6.381 | 0.041 | ||
| 1500-2500 | 49 | 40 | ||
| 2500-3500 | 6 | 11 | ||
| 3500-5800 | 0 | 4 |
Of the above results, age, sex, ethnicity and altitude were entered into binary logistic analysis, and the results showed that age, ethnicity and altitude were risk factors for osteoporosis (Table 10).
| Factor | B value | SD (S.E.) | Wald value | Variance | P value | OR value | OR 95%CI |
| Age | 3.976 | 0.846 | 22.062 | 1 | 0.000 | 53.305 | 10.144-280.095 |
| Ethnicity | 1.262 | 0.521 | 5.871 | 1 | 0.015 | 5.531 | 1.273-9.797 |
| Altitude | -2.049 | 0.514 | 15.871 | 1 | 0.000 | 0.129 | 0.047-0.353 |
| Constant | -8.343 | 2.141 | 15.186 | 1 | 0.000 | 0.000 |
Vitamin D is a hormone synthesized in the skin during sunlight exposure. It not only participates in bone and cellular metabolism but plays a role in the regulation of autoimmune disease, inflammation, neuromuscular and other immune functions. During the process of vitamin D metabolism, serum 25OHD concentration is the optimal marker for evaluating vitamin D status[9]. A number of studies have demonstrated the deficiency or insufficiency of vitamin D in multiple chronic pain disorders including non-specific musculoskeletal pain, chronic generalized pain, fibromuscular pain, lower back pain, headache and lumbar canal stenosis. In addition, the results of vitamin D surveys in healthy populations worldwide are not optimistic, with the prevalence of vitamin insufficiency reaching 30%-50%[10]. In the present study, it was found that vitamin deficiency was generally present in both chronic pain patients and healthy individuals, although the overall vitamin D level in chronic pain patients was lower than that in healthy individuals, especially in those with vitamin D deficiency or severe vitamin D insufficiency. Some studies showed that vitamin D supplements could alleviate pain[11-14]. However, three meta-analyses found no significant correlation between vitamin D supplementation and pain relief in their randomized study and placebo control groups[15-17]. Serum 25OHD concentration is affected by multiple host and environmental factors, including age, sex, diet, sunlight exposure, physical activity, body weight, skin pigmentation, and genetic factors. Whether there is a relationship between vitamin D deficiency/severe insufficiency and chronic pain requires further studies.
Vitamin D is a pro-hormone that regulates Ca and P balance and skeletal structures, and has an adverse impact on skeletal health and neuromuscular function. Vitamin D deficiency increases the risk of osteoporosis. Serum 25OHD level lower than 20 ng/mL was reported to be associated with defective bone mineralization[8,18]. In our study, it was found that vitamin D concentration was lowest in osteoporotic patients but was not a risk factor for bone mass reduction and osteoporosis, suggesting that they are not correlated. This finding is consistent with the results of a large-scale cross-sectional study conducted in Northwest China[19]. However, the role of vitamin D in the basic treatment of osteoporosis should not be questioned. A large number of studies have confirmed that vitamin D alone cannot reduce the risk of bone fracture, but combined use of vitamin D and Ca supplements can reduce the risk of hip and non-vertebral fractures[20-22]. The anti-osteoporosis treatment guidelines from multiple different countries and regions maintain that sufficient Ca uptake plus the vitamin D status is a precondition of any regimen for the prevention and treatment of osteoporosis regardless of which drug is used[23-25]. Thus, questions arise as to what amount of vitamin D supplementation can maximally increase the serum 25OHD concentration and at the same time ensure that the concentration will not be excessive? In other words, what is the optimal vitamin D supplement concentration required for the 25OGD target for general populations? One study set up the general practical criteria and the latest suggestions for preventive administration of vitamin D for newborns, infants, children, adolescents and adults (including pregnant women, lactating mothers and older people) in central European areas, in which the vitamin D concentration < 20 ng/mL is defined as deficiency, 20-30 ng/mL as the sub-healthy state, and 30-50 ng/mL as the optimal target concentration of vitamin D action and the range of serum 25OHD concentration. It also suggests that all individuals with serum 25OHD lower than 20 ng/mL should be supplemented or corrected for vitamin D, and 30 ng/mL is the target most suitable for infirm, osteoporotic and senile patients[26].
Osteoporosis is one of the main health concerns worldwide. With increasing age, bone mass decreases gradually, leading to bone mass reduction and osteoporosis. The differential diagnosis of bone mass reduction and osteoporosis is based on the BMD level. The main goal of osteoporosis screening and treatment is to prevent fracture by reducing bone mass loss. It is therefore extremely important to recognize risk factors associated with bone mass change in order to identify high risk populations and take early preventive strategies to reduce the occurrence of osteoporosis. With increasing age, the risk of bone mass reduction and fracture is generally increased, especially in menopausal women whose estrogen level and liver/kidney function in synthesizing active vitamin D are both reduced, leading to reduced absorption of Ca in the gut. In addition, a reduction in estrogen level activates PTH, thus increasing the activity of osteoclasts, increasing bone absorption, and quickening bone turnover and bone mass loss. As a result, the bone trabecula becomes thinner, the bone cortex is structurally damaged and becomes progressively thinner, and the bone density is decreased. Undoubtedly, age is an important risk factor for bone mass change, which is consistent with related reports[23]. What is different is that we failed to find a significant correlation between bone mass loss and BMI. Lifestyle factors associated with the risk of osteoporosis include alcohol consumption, diet, hormones, sports and smoking. In addition, Ca and vitamin D are extremely important in age-related BMD and skeletal muscle mass reduction[27]. It was observed in our study that PTH and Ca were risk factors contributing to bone mass reduction, while ethnicity and altitude were risk factors contributing to osteoporosis. Whether this phenomenon is due to the relatively small sample size or reflects intrinsic differences remains to be confirmed in future studies.
Multiple individual factors should be considered in the treatment of osteoporosis. Personalized treatment strategies should be built on disease severity, patient sex, complications and adverse effects that the drug(s) may produce[28]. Differences in related risk factors should also be included in the selection of therapies.
Vitamin D deficiency is common in chronic pain patients and healthy people, but the difference between the two has not been reported. The relationship between vitamin D, chronic pain and bone mineral density (BMD) is controversial. The aim of the present study was to observe changes in vitamin D levels in patients with chronic pain, clarify the relationship between vitamin D and BMD, and explore risk factors for bone mass reduction and osteoporosis.
To understand the relationship between vitamin D and chronic pain, clarify the relationship between vitamin D and bone density, analyze the risk factors of bone mass reduction and osteoporosis, and provide a basis for early treatment of osteoporosis.
Vitamin D deficiency is very common in patients with chronic pain and healthy people, but severe vitamin D deficiency is more likely to occur in patients with chronic pain. Vitamin D is not a risk factor for bone mass changes. Age, parathyroid hormone (PTH) and calcium (Ca) were risk factors for bone mass reduction, while age, ethnicity and altitude were risk factors for osteoporosis.
25 hydroxy vitamin D and PTH were determined by a Cobas 8000 electrochemical luminescence immunoassay kit. Ca and P levels were detected using a fully automated biochemical analyzer and the relevant kit (Hitachi). BMD of l-1-4, femoral neck, Ward's triangle, and femoral trochanter was measured by dual-energy X-ray absorptiometry. General patient information was collected including age, height, weight, body mass index, altitude of residence, previous medical history and medication use. All statistical analyses were performed using SPSS 17.0. Quantitative data are expressed as x ± s. Comparisons between two groups were performed using the independent sample t test; comparisons between three groups were performed by one way ANOVA; and comparisons between multiple groups were performed by LSD. Counting data are expressed as c2. Two sets of ordered counting data were analyzed by the Mantel-Haenszel test for linear trend. Risk factors were analyzed by binary logistic regression. Values of P < 0.05 were considered statistically significant.
Both chronic pain patients and healthy people were mainly deficient in vitamin D, but the proportion of severe vitamin D deficiency in chronic pain patients was significantly higher than that in the healthy group. Patients with chronic pain were more likely to suffer from vitamin D deficiency or severe deficiency, the causes of which require further study. The levels of vitamin D in the osteoporosis group were lower than those in the groups with normal bone mass and reduced bone mass, but vitamin D levels were not a risk factor for changes in bone mass. Age, PTH and Ca were risk factors for bone mass loss. Age, sex, ethnicity, and altitude were risk factors for osteoporosis.
Vitamin D deficiency is common among patients with chronic pain, and severe vitamin D deficiency is more likely to occur. Although vitamin D levels are lowest during osteoporosis, they are not a risk factor for changes in bone mass. According to the different risk factors of bone mass change, independent treatment methods can be adopted clinically.
Chronic pain patients are more likely to have severe vitamin D deficiency, and the reason for this is not clear. Vitamin D is not a risk factor for osteoporosis. Whether vitamin D supplementation is beneficial requires further study. Whether early monitoring of PTH and Ca levels has an effect on the prevention of bone mass change, and which age group can effectively prevent osteoporosis requires further investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Poturoglu S, Yu C S-Editor: Tang JZ L-Editor: Webster JR E-Editor: Qi LL
| 1. | Knutsen KV, Brekke M, Gjelstad S, Lagerløv P. Vitamin D status in patients with musculoskeletal pain, fatigue and headache: a cross-sectional descriptive study in a multi-ethnic general practice in Norway. Scand J Prim Health Care. 2010;28:166-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 103] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Al-Jarallah KF, Shehab D, Al-Awadhi A, Nahar I, Haider MZ, Moussa MA. Are 25(OH)D levels related to the severity of knee osteoarthritis and function? Med Princ Pract. 2012;21:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Song TJ, Chu MK, Sohn JH, Ahn HY, Lee SH, Cho SJ. Effect of Vitamin D Deficiency on the Frequency of Headaches in Migraine. J Clin Neurol. 2018;14:366-373. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 4. | Eloqayli H, Al-Yousef A, Jaradat R. Vitamin D and ferritin correlation with chronic neck pain using standard statistics and a novel artificial neural network prediction model. Br J Neurosurg. 2018;32:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Ortega Anta RM, González-Rodríguez LG, Jiménez Ortega AI, Estaire Gómez P, Rodríguez-Rodríguez E, Perea Sánchez JM, Aparicio Vizuete A, Grupo de Investigación Nº 920030. [Insufficient intake of vitamin D in spanish schoolchildren: determinants of the problem and basis for its improvement]. Nutr Hosp. 2012;27:1437-1443. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Wimalawansa SJ, Razzaque MS, Al-Daghri NM. Calcium and vitamin D in human health: Hype or real? J Steroid Biochem Mol Biol. 2018;180:4-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 62] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 7. | Yan X, Thomson JS, Zhao R, Zhu R, Wang Z, Zhang N, Coad J. Vitamin D Status of Residents in Taiyuan, China and Influencing Factors. Nutrients. 2017;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Yuan R, Ma S, Zhu X, Li J, Liang Y, Liu T, Zhu Y, Zhang B, Tan S, Guo H, Guan S, Ao P, Zhou G. Core level regulatory network of osteoblast as molecular mechanism for osteoporosis and treatment. Oncotarget. 2016;7:3692-3701. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Tsugawa N. [Vitamin D and osteoporosis: current topics from epidemiological studies]. Rinsho Byori. 2010;58:244-253. [PubMed] |
| 10. | Emini-Sadiku M, Morina-Kuqi N. Concealing Clothing Leading to Severe Vitamin D Deficiency, Osteomalacia and Muscle Weakness. Open Access Maced J Med Sci. 2019;7:2146-2149. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Bergman P, Sperneder S, Höijer J, Bergqvist J, Björkhem-Bergman L. Low vitamin D levels are associated with higher opioid dose in palliative cancer patients--results from an observational study in Sweden. PLoS One. 2015;10:e0128223. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 12. | Björkhem-Bergman L, Bergman P. Vitamin D and patients with palliative cancer. BMJ Support Palliat Care. 2016;6:287-291. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Gendelman O, Itzhaki D, Makarov S, Bennun M, Amital H. A randomized double-blind placebo-controlled study adding high dose vitamin D to analgesic regimens in patients with musculoskeletal pain. Lupus. 2015;24:483-489. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 14. | Ovesjö ML, Skilving I, Bergman P, Rane A, Ekström L, Björkhem-Bergman L. Low Vitamin D Levels and Genetic Polymorphism in the Vitamin D Receptor are Associated with Increased Risk of Statin-Induced Myopathy. Basic Clin Pharmacol Toxicol. 2016;118:214-218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Shipton EE, Shipton EA. Vitamin D Deficiency and Pain: Clinical Evidence of Low Levels of Vitamin D and Supplementation in Chronic Pain States. Pain Ther. 2015;4:67-87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 61] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Straube S, Andrew Moore R, Derry S, McQuay HJ. Vitamin D and chronic pain. Pain. 2009;141:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 101] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 17. | Straube S, Derry S, Straube C, Moore RA. Vitamin D for the treatment of chronic painful conditions in adults. Cochrane Database Syst Rev. 2015;CD007771. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 18. | Rizzoli R, Stevenson JC, Bauer JM, van Loon LJ, Walrand S, Kanis JA, Cooper C, Brandi ML, Diez-Perez A, Reginster JY; ESCEO Task Force. The role of dietary protein and vitamin D in maintaining musculoskeletal health in postmenopausal women: a consensus statement from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Maturitas. 2014;79:122-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 182] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 19. | Zhen D, Liu L, Guan C, Zhao N, Tang X. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: its relationship to osteoporosis and lifestyle factors. Bone. 2015;71:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 82] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 20. | Tang BM, Eslick GD, Nowson C, Smith C, Bensoussan A. Use of calcium or calcium in combination with vitamin D supplementation to prevent fractures and bone loss in people aged 50 years and older: a meta-analysis. Lancet. 2007;370:657-666. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 989] [Cited by in RCA: 811] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 21. | Avenell A, Mak JC, O'Connell D. Vitamin D and vitamin D analogues for preventing fractures in post-menopausal women and older men. Cochrane Database Syst Rev. 2014;CD000227. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 142] [Cited by in RCA: 215] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 22. | Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, Liu S, Looker AC, Wallace TC, Wang DD. Calcium plus vitamin D supplementation and risk of fractures: an updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int. 2016;27:367-376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 331] [Cited by in RCA: 374] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 23. | Tarantino U, Iolascon G, Cianferotti L, Masi L, Marcucci G, Giusti F, Marini F, Parri S, Feola M, Rao C, Piccirilli E, Zanetti EB, Cittadini N, Alvaro R, Moretti A, Calafiore D, Toro G, Gimigliano F, Resmini G, Brandi ML. Clinical guidelines for the prevention and treatment of osteoporosis: summary statements and recommendations from the Italian Society for Orthopaedics and Traumatology. J Orthop Traumatol. 2017;18:3-36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 132] [Cited by in RCA: 130] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 24. | Compston J, Cooper A, Cooper C, Gittoes N, Gregson C, Harvey N, Hope S, Kanis JA, McCloskey EV, Poole KES, Reid DM, Selby P, Thompson F, Thurston A, Vine N; National Osteoporosis Guideline Group (NOGG). UK clinical guideline for the prevention and treatment of osteoporosis. Arch Osteoporos. 2017;12:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 581] [Cited by in RCA: 550] [Article Influence: 68.8] [Reference Citation Analysis (0)] |
| 25. | Al-Saleh Y, Sulimani R, Sabico S, Raef H, Fouda M, Alshahrani F, Al Shaker M, Al Wahabi B, Sadat-Ali M, Al Rayes H, Al Aidarous S, Saleh S, Al Ayoubi F, Al-Daghri NM. 2015 Guidelines for Osteoporosis in Saudi Arabia: Recommendations from the Saudi Osteoporosis Society. Ann Saudi Med. 2015;35:1-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Al-Daghri NM, Al-Saleh Y, Aljohani N, Sulimani R, Al-Othman AM, Alfawaz H, Fouda M, Al-Amri F, Shahrani A, Alharbi M, Alshahrani F, Tamimi W, Sabico S, Rizzoli R, Reginster JY. Vitamin D status correction in Saudi Arabia: an experts' consensus under the auspices of the European Society for Clinical and Economic Aspects of Osteoporosis, Osteoarthritis, and Musculoskeletal Diseases (ESCEO). Arch Osteoporos. 2017;12:1. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 56] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 27. | Erem S, Atfi A, Razzaque MS. Anabolic effects of vitamin D and magnesium in aging bone. J Steroid Biochem Mol Biol. 2019;193:105400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 28. | Schulz K, Kalscheuer H, Lehnert H. Personalized Therapy In Osteoporosis. Dtsch Med Wochenschr. 2019;144:1111-1119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |