Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1932
Peer-review started: January 31, 2020
First decision: April 8, 2020
Revised: April 24, 2020
Accepted: April 27, 2020
Article in press: April 27, 2020
Published online: May 26, 2020
Processing time: 115 Days and 7.7 Hours
Neurofibromas are tumors comprised of peripheral nerve sheath and connective tissue components. They can occur sporadically or as part of familial syndromes such as neurofibromatosis type 1. Isolated colonic neurofibroma without systemic manifestations is a rarely reported clinical entity. Here we present a case of a 51 years old male with an isolated colonic neurofibroma seen on a screening colonoscopy.
Fifty-one years old male who was otherwise healthy without a significant family history of cancer underwent a screening colonoscopy and was found have a 2.3 cm × 1.4 cm lesion in the colon. Tissue biopsy revealed a spindle cell tumor. Magnetic resonance imaging of the pelvis was negative for adenopathy. He underwent an endoscopic ultrasound that showed an ill-defined avascular lesion of mixed echogenicity measuring 2.8 cm × 15.2 cm in the submucosa with no communication with muscularis mucosa or propria. Immunohistochemistry staining of the tumor was strongly positive for S100, with rare penetrating axons deep within the tumor. Tumor cells were negative for c-kit and desmin and had low Ki-67 index. These findings were consistent with a solitary colonic submucosal neurofibroma. A detailed history and physical examination did not reveal any evidence of extraintestinal neurofibromatosis. He underwent transanal surgical resection of the tumor. The patient tolerated the procedure well without any complications.
While neurofibromas have been well described in literature, an isolated colonic neurofibroma is a rare pathological entity. Malignant transformation of neurofibromas has been reported in patients with neurofibromatosis syndromes. We report a case of isolated colonic neurofibroma and highlight the importance of resection due to the increased risk of tumorigenesis.
Core tip: Neurofibromas are composed of neural and connective tissue components. They are associated with hereditary syndromes such as neurofibromatosis type 1 and 2. They have systemic manifestations and may involve multiple organs. Malignant transformation of this tumor has been reported in literature. Isolated colonic neurofibroma is a rarely reported pathological entity. Clinical follow-up after surgical resection is recommended as solitary colonic neurofibromas might be the first sign of neurofibromatosis type 1.
- Citation: Ghoneim S, Sandhu S, Sandhu D. Isolated colonic neurofibroma, a rare tumor: A case report and review of literature. World J Clin Cases 2020; 8(10): 1932-1938
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1932.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1932
Neurofibromas are peripheral nerve sheath tumors that consist of Schwan cells, perineural cells and myofibroblasts[1-3]. They occur sporadically or as part of familial syndromes such as neurofibromatosis type 1 (NF1) (von Recklinghausen’s disease), NF2 (central or bilateral neurofibromatosis), multiple endocrine neoplasia type IIb, or intestinal neurofibromatosis[2]. NF1 and 2 are both autosomal dominant syndromes with variable clinical manifestations[1-3]. The genes for NF1 and NF2 are found on chromosomes 17 and 22, respectively[3]. Both genes encode tumor suppressor proteins. NF1 has no sex predilection and occurs with a prevalence of 1 in every 3000 individuals[3,4]. About 50% of patients have a positive family history. In the remaining 50% of patients, the disorder represents a sporadic new mutation[3,4]. The diagnosis of NF1 is largely based on clinical criteria established by the National Institutes of Health and involves the presence of two or more of the following features: Six or more café au lait patches, two or more neurofibromas, one plexiform neurofibroma, freckling in the axillary or groin region, Lisch nodules, optic glioma, a first degree relative with NF1 and/or the presence of osseous dysplasia[3].
Gastrointestinal involvement is seen in NF1 but not NF2[4]. Abdominal neoplasms in NF1 can be divided into five categories: Neurogenic tumors, neuroendocrine tumors, gastrointestinal mesenchymal tumors, embryonal tumors and miscellaneous tumors. Neurogenic neoplasms seen in the abdomen include neurofibroma, plexiform neurofibroma, malignant sheath peripheral nerve sheath tumor, triton tumor and ganglioneuroma[3-4]. Sporadic intestinal neurofibromas mainly arise in the small or large intestine and affect middle age adults[5]. In the gastrointestinal tract, neurofibromas have been reported in the following order of decreasing frequency: the jejunum, stomach, ileum, duodenum and colon[3-7]. Isolated colonic neurofibromas are rare with a few cases reported in literature (Table 1)[8-23]. Here we present a case of an isolated colonic neurofibroma seen on a routine screening colonoscopy.
| Ref. | Age | Sex | Location |
| Keith et al[8], 1937 | 50 | Female | Rectum |
| Woolf et al[9], 1938 | 70 | Male | Rectum |
| Butler and Hanna[10], 1959 | 45 | Female | Rectum |
| Suzuki and Yamashita[11], 1966 | NA | NA | Rectum |
| Geboes et al[12], 1978 | NA | NA | Rectum |
| Abramsone et al[13], 1997 | 53 | Male | Transverse colon |
| Bononi et al[14], 2000 | 68 | Female | Diffuse involvement |
| Panteris et al[15], 2005 | 65 | Female | Descending colon |
| Carter and Laurini[16], 2008 | 52 | Female | Rectum, transverse colon |
| Hindy et al[17], 2012 | 59 | Male | Transverse colon |
| Chelimilla et al[18], 2013 | 70 | Female | Ascending colon |
| Ahn et al[19], 2016 | 26 | Female | Sigmoid colon |
| Bilal et al[20], 2016 | 52 | Male | Descending colon |
| Adioui et al[21], 2018 | 29 | Female | Sigmoid colon |
| Miao et al[22], 2018 | 24 | Female | Ileocecal valve |
| Sun et al[23], 2020 | 33 | Female | Ascending colon |
A 51-year-old Caucasian male was referred to our gastroenterology clinic for a routine screening colonoscopy. He had no complaints.
He was otherwise healthy and denied having bloody stools, fevers, chills or unintentional weight loss.
He had no significant past medical history or surgical history and was not taking any medications.
Father had a history of hypertension. No family member had a history of cancer.
Temperature was 37 °C, heart rate was 75 beats per minute, respiratory rate was 18 breaths per minute, blood pressure was 129/72 mmHg and body mass index was 23.4 kg/m2. Physical examination was normal. His abdomen was soft with no tenderness or palpable masses. He had no skin lesions, pigmented or hypopigmented spots.
Laboratory testing including complete blood count, basic metabolic panel, liver function tests and coagulation profile were all within normal limits. Hepatic serology was also negative.
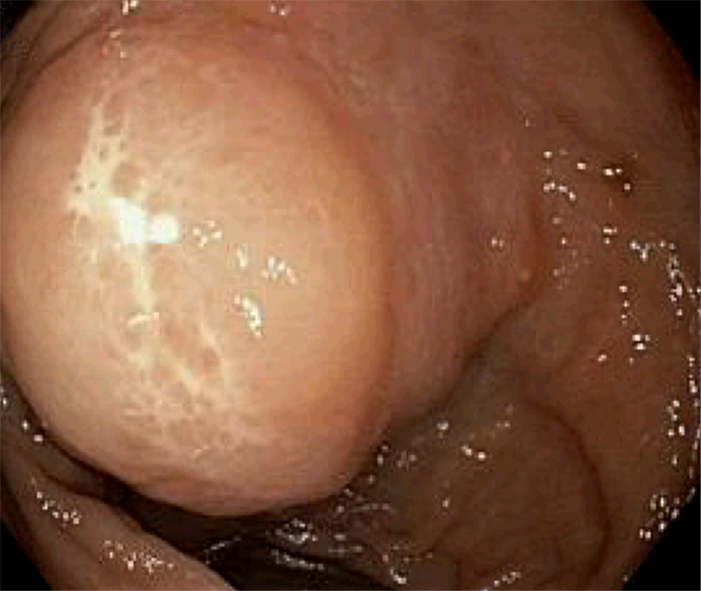
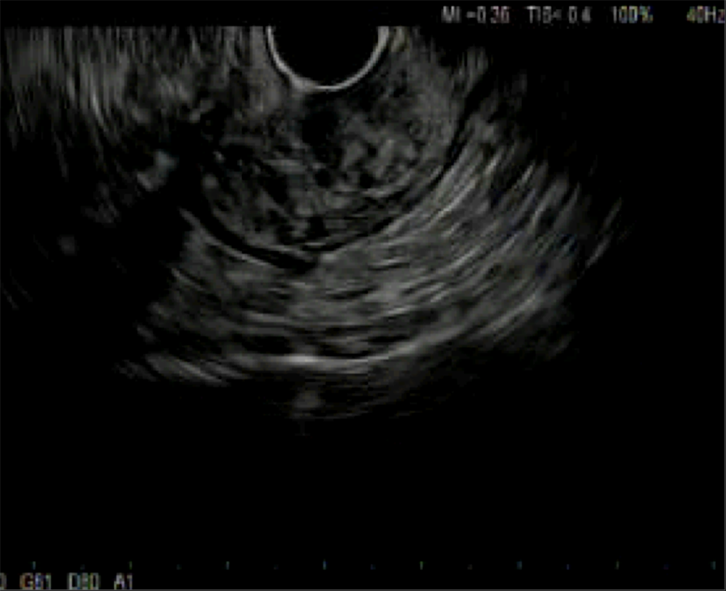
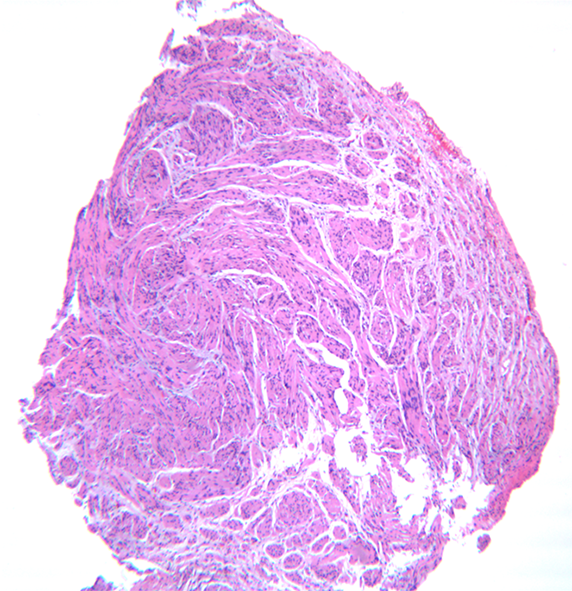
He underwent a screening colonoscopy and was found to have a 3-cm submucosal pedunculated polyp about 15 cm from anal verge adjacent to the second rectal fold (Figure 1). Mucosal biopsies were obtained during the procedure. The specimen included fragments of colonic mucosa and a spindle cell tumor. As the mucosal specimens were unrevealing, the patient underwent magnetic resonance imaging of the pelvis which showed a 2.3 cm × 1.4 cm lesion in the submucosa with no adenopathy. A subsequent endoscopic ultrasound revealed an ill-defined avascular lesion of mixed echogenicity measuring 2.8 cm × 15.2 cm in the submucosa with no communication with muscularis mucosa or propria (Figure 2). Fine needle biopsy, with 19G SharkCore™ needle (Covidien), was obtained for pathology assessment (Figures 2 and 3). The tumor showed bland spindle cell proliferation in a uniform fascicular pattern with edematous stroma (Figure 3). The cells were elongated, with oval/wavy nuclei. No mitotic figures, necrosis, or tumoral hemorrhage were seen. The tumor showed strong and diffuse S100 staining, with rare penetrating axons deep within the tumor (Figure 4). Tumor cells were negative for c-kit, desmin and had low Ki-67 index.
These findings were consistent with a colonic submucosal neurofibroma. A detailed history and physical examination did not reveal any evidence of extraintestinal manifestations.
Our patient opted for transanal surgical resection.
The patient tolerated the procedure well without any complications. He will undergo a repeat colonoscopy in one year to evaluate for recurrence.
Our patient was diagnosed with a localized colonic neurofibroma without any clinical signs of NF1. The presence of such lesions in the intestinal tract without associated systemic manifestations is a rare finding.
Gastrointestinal involvement in NF1 has been reported in up to 25% of cases, with most lesions localizing to the upper gastrointestinal tract and rarely to the lower intestinal tract[24,25]. The pathological forms seen in the gastrointestinal tract of patients with NF1 are characterized by hyperplasia and hypertrophy of the nerve plexus and ganglionic cells in the mucosa, submucosa, muscularis propria or even serosa[20,26]. In addition to neurogenic tumors, patients may develop neuroendocrine and mesenchymal tumors such as gangliocytic paragangliomas, pheochromocytomas and gastrointestinal stromal tumors (GIST)[2-4]. Intestinal neurofibromas predominately originate from the plexus of Meissner in the submucosa or the plexus of Auerbach[9]. Dense growths known as plexiform neurofibromatosis may infiltrate the mesentery and lead to arterial compression or nerve injury[25]. Endoscopically, neurofibromas are often sessile and wide-based or, as seen in this patient, pedunculated polyps[23]. Clinically patients may present with abdominal pain, palpable masses, rectal bleeding, or obstruction due to intussusception or extra-luminal pressure, although most cases are found incidentally in asymptomatic patients.
Since there is no recommendation about modalities to explore these patients and because of the nonspecific clinical presentation of gastrointestinal lesion manifestations and limitations of endoscopic techniques, cross-sectional imaging may be used to aid in diagnosis. MR enterography is a non-invasive technique that has high sensitivity for detecting small bowel lesions and is more preferable than computed topography (CT) enterography for follow up of these patients because of concerns over exposure to ionizing radiation[24]. CT colonography (virtual colonoscopy) is a potential alternative to conventional colonoscopy for the detection of colorectal tumors especially in poor risk patients[27]. It is a non-invasive technique that requires no sedation and is well tolerated by most patients. CT colonography allows for complete visualization of the entire large bowel in almost all patients even following incomplete endoscopy and for colorectal examination proximal to an obstructing lesion. The use of multidector technology improves the detection of many colorectal lesions[27]. Potential limitations of this modality include the inability to obtain tissue biopsy and exposure to ionizing radiation[27,28].
Tissue biopsy remains the gold standard for diagnosis[26]. The differential diagnosis for neurofibroma of the abdomen includes schwannoma, myxoma, inflammatory fibroid polyp and GIST[2,25]. Unlike myxomas, neurofibromas are diffusely positive for S-100[2]. The absence of calretinin and a peripheral lymphoid cuff help distinguish these tumors from schwannomas and inflammatory fibroid polyps, respectively. In contrast to schwannomas, neurofibromas stain positively for CD34 and factor XIIIa[2]. On the other hand, GIST stain positive for c-kit while neurofibromas do not[2]. In our patient, histopathology revealed bland spindle cell proliferation in a fascicular pattern. Immunohistochemical staining was positive for S100, negative for c-kit and desmin. These features are consistent with a diagnosis of neurofibroma.
Malignant transformation of neurofibromas has been reported in patients with NF1[3,27]. Malignant peripheral nerve sheath tumors are highly aggressive tumors associated with low survival rates (34%-52%)[29-31]. The lifetime risk of these tumors in patients with NF1 is 8%-13%[3]. Plexiform and larger neurofibromas are more likely to undergo malignant transformation[30-32]. Malignant sheath peripheral nerve sheath tumors are often clinically silent when they arise in the abdomen and are often diagnosed at advanced stage. The prognosis remains poor and depends on the tumor size and location, resection margins, adjuvant chemotherapy, distant metastasis, stage and site[29-32].
The clinical significance of solitary neurofibromas is currently unknown, hence a thorough search should be made to exclude the possibility of colonic neurofibromas presenting as initial sign of NF1. Unfortunately, due to the rarity of this condition there is no consensus regarding management of these patients. Given the low incidence, and very few cases reported, long term follow up and screening goals are also unknown but close follow up is recommended to exclude neurofibromatosis and the associated risk of malignant transformation[29-32].
An isolated colonic neurofibroma without associated signs of neurofibromatosis or systemic disease is a rare pathological finding. The clinical significance lies in the need to follow these patients as bowel involvement might be the first systemic manifestation of syndromic diseases such as NF1.
We would like to thank Dr. Dan X Cai for providing the pathology slides.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cuocolo R, de Melo FF, El-Razek AA S-Editor: Tang JZ L-Editor: A E-Editor: Li X
| 1. | Jett K, Friedman JM. Clinical and genetic aspects of neurofibromatosis 1. Genet Med. 2010;12:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 321] [Article Influence: 20.1] [Reference Citation Analysis (0)] |
| 2. | Hechtman JF, Harpaz N. Neurogenic polyps of the gastrointestinal tract: a clinicopathologic review with emphasis on differential diagnosis and syndromic associations. Arch Pathol Lab Med. 2015;139:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Razek AAKA. MR imaging of neoplastic and non-neoplastic lesions of the brain and spine in neurofibromatosis type I. Neurol Sci. 2018;39:821-827. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 4. | Levy AD, Patel N, Dow N, Abbott RM, Miettinen M, Sobin LH. From the archives of the AFIP: abdominal neoplasms in patients with neurofibromatosis type 1: radiologic-pathologic correlation. Radiographics. 2005;25:455-480. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 5. | Mann NS, Mann SK, Alam I. The safety of hot biopsy forceps in the removal of small colonic polyps. Digestion. 1999;60:74-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Pinsk I, Dukhno O, Ovnat A, Levy I. Gastrointestinal complications of von Recklinghausen's disease: two case reports and a review of the literature. Scand J Gastroenterol. 2003;38:1275-1278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Parmar ZB, Chudasama B, Rajpura H, Desai S, Nilkanthe R, Jetly D. The isolated rectal neurofibroma: a rare case report. OMCIS J Radiol. 2016;5:3. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Keith AR. A case of neurofibromatosis of the rectal wall. Trans Am Protocol Soc. 1937;38:68. |
| 9. | Woolf MS. Neurofibroma of the Rectum. Cal West Med. 1938;49:463-464. [PubMed] |
| 10. | Butler DB, Hanna E. Neurogenic tumor of the rectum. Dis Colon Rectum. 1959;2:291-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 11. | Suzuki H, Yamashita E. A case report of intestinal neurofibroma. Nihon Geka Hokan. 1966;35:948-950. [PubMed] |
| 12. | Geboes K, De Wolf-Peeters C, Rutgeerts P, Vantrappen G, Desmet V. Submucosal tumors of the colon: experience with twenty-five cases. Dis Colon Rectum. 1978;21:420-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 20] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Abramson LP, Orkin BA, Schwartz AM. Isolated colonic neurofibroma manifested by massive lower gastrointestinal bleeding and intussusception. South Med J. 1997;90:952-954. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Bononi M, De Cesare A, Stella MC, Fiori E, Galati G, Atella F, Angelini M, Cimitan A, Lemos A, Cangemi V. Isolated intestinal neurofibromatosis of colon. Single case report and review of the literature. Dig Liver Dis. 2000;32:737-742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 15. | Panteris V, Vassilakaki T, Vaitsis N, Elemenoglou I, Mylonakou I, Karamanolis DG. Solitary colonic neurofibroma in a patient with transient segmental colitis: case report. World J Gastroenterol. 2005;11:5573-5576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 24] [Cited by in RCA: 25] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Carter JE, Laurini JA. Isolated intestinal neurofibromatous proliferations in the absence of associated systemic syndromes. World J Gastroenterol. 2008;14:6569-6571. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 25] [Cited by in RCA: 23] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Hindy P, Parvin R, Hanna K, Andrawes S, Gress F, Goodman A. An isolated neurofibromal polyp of the colon. Case Rep Gastroenterol. 2012;6:58-62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Chelimilla H, Chandrala CK, Niazi M, Kumbum K. Incidental finding of isolated colonic neurofibroma. Case Rep Gastroenterol. 2013;7:369-375. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 19. | Ahn S, Chung CS, Kim KM. Neurofibroma of the Colon: A Diagnostic Mimicker of Gastrointestinal Stromal Tumor. Case Rep Gastroenterol. 2016;10:674-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Bilal M, Bilimoria F, Clarke K. An isolated colonic neurofibroma. Ann Gastroenterol. 2016;29:381. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 21. | Adioui T, Tamzaourte M, Nejjari F, Chakkor A, Elkaoui H, Boudhas A, Oukabli M, Rouibaa F, Aourarh A, Zentar A. Isolated Neurofibroma of the Sigmoid Colon: a Case Report and Review of the Literature. J Gastrointest Cancer. 2018;49:195-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Miao Y, Wang JJ, Chen ZM, Zhu JL, Wang MB, Cai SQ. Neurofibroma discharged from the anus with stool: A case report and review of literature. World J Clin Cases. 2018;6:455-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 4] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 23. | Sun WY, Pandey A, Lee M, Wasilenko S, Karmali S. Isolated colonic neurofibroma in the setting of Lynch syndrome: A case report and review of literature. World J Gastrointest Surg. 2020;12:28-33. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 3] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Murphey MD, Smith WS, Smith SE, Kransdorf MJ, Temple HT. From the archives of the AFIP. Imaging of musculoskeletal neurogenic tumors: radiologic-pathologic correlation. Radiographics. 1999;19:1253-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 417] [Cited by in RCA: 358] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 25. | Kumar BS, Gopal M, Talwar A, Ramesh M. Diffuse neurofibroma of the scalp presenting as circumscribed alopecic patch. Int J Trichology. 2010;2:60-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Lefere I, Dalle I, Thieren H, Decock S, Ramboer K. Diffuse intestinal ganglioneuromatosis of the ileum. JBR-BTR. 2012;95:152-153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Abd-El Khalek Abd-ALRazek A, Fahmy DM. Diagnostic Value of Diffusion-Weighted Imaging and Apparent Diffusion Coefficient in Assessment of the Activity of Crohn Disease: 1.5 or 3 T. J Comput Assist Tomogr. 2018;42:688-696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 28. | Abdel Razek AA, Abu Zeid MM, Bilal M, Abdel Wahab NM. Virtual CT colonoscopy versus conventional colonoscopy: a prospective study. Hepatogastroenterology. 2005;52:1698-1702. [PubMed] |
| 29. | Boldorini R, Tosoni A, Leutner M, Ribaldone R, Surico N, Comello E, Min KW. Multiple small intestinal stromal tumours in a patient with previously unrecognised neurofibromatosis type 1: immunohistochemical and ultrastructural evaluation. Pathology. 2001;33:390-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 30. | Hirbe AC, Gutmann DH. Neurofibromatosis type 1: a multidisciplinary approach to care. Lancet Neurol. 2014;13:834-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 336] [Article Influence: 30.5] [Reference Citation Analysis (0)] |
| 31. | Basile U, Cavallaro G, Polistena A, Giustini S, Orlando G, Cotesta D, Petramala L, Letizia C, Calvieri S, De Toma G. Gastrointestinal and retroperitoneal manifestations of type 1 neurofibromatosis. J Gastrointest Surg. 2010;14:186-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Hwang IK, Hahn SM, Kim HS, Kim SK, Kim HS, Shin KH, Suh CO, Lyu CJ, Han JW. Outcomes of Treatment for Malignant Peripheral Nerve Sheath Tumors: Different Clinical Features Associated with Neurofibromatosis Type 1. Cancer Res Treat. 2017;49:717-726. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |