Published online May 26, 2020. doi: 10.12998/wjcc.v8.i10.1848
Peer-review started: January 10, 2020
First decision: February 20, 2020
Revised: March 1, 2020
Accepted: April 22, 2020
Article in press: April 22, 2020
Published online: May 26, 2020
Processing time: 135 Days and 21.9 Hours
Receptor interacting protein kinase 1 (RIPK1)-mediated cell death, including apoptosis and necroptosis, belongs to programmed cell death. It has been reported that RIPK1-mediated necroptosis exists in lesions of cerebral hemorrhage (CH). Electroacupuncture, a treatment derived from traditional Chinese medicine, could improve neurological impairment in patients with brain injury.
To investigate the protective role of cross electro-nape acupuncture (CENA) in CH, and clarify the potential mechanism.
CH rat models were established, and CENA was applied to the experimental rats. Neurological functions and encephaledema were then measured. Necrotic cells in the brain of rats with CH were evaluated by propidium iodide staining. Necroptosis was assessed by immunofluorescence. Activation of the necroptosis-related pathway was detected by western blot. Extraction of brain tissue, cerebrospinal fluid and serum samples was conducted to measure the expression and secretion of inflammatory cytokines by quantitative real-time polymerase chain reaction and enzyme-linked immunosorbent assay.
The necroptotic marker p-MLKL was detectable in the brains of rats with CH. Next, we found that CENA could ameliorate neurological functions in rat models of CH. Moreover, the upregulation of RIPK1-mediated necroptosis-related molecules in the brains of rats with CH were inhibited by CENA. Further investigation revealed that CENA partially blocked the interaction between RIPK1 and RIPK3. Finally, in vivo assays showed that CENA decreased the expression of the inflammatory cytokines tumor necrosis factor-α, interleukin-6 and interleukin-8 in CH rat models.
These findings revealed that CENA exerts a protective role in CH models by inhibiting RIPK1-mediated necroptosis.
Core tip: This study demonstrated that necroptosis existed in the brain of rats with cerebral hemorrhage (CH). Further examination revealed that receptor interacting protein kinase 1 (RIPK1) mediated necroptosis in CH, and cross electro-nape acupuncture (CENA) suppressed RIPK1 to ameliorate brain damage in rats with CH. Moreover, CENA inhibited inflammation in CH. Therefore, these results suggested a neuroprotective role of CENA by targeting RIPK1-mediated necroptosis in rats with CH.
- Citation: Cai GF, Sun ZR, Zhuang Z, Zhou HC, Gao S, Liu K, Shang LL, Jia KP, Wang XZ, Zhao H, Cai GL, Song WL, Xu SN. Cross electro-nape-acupuncture ameliorates cerebral hemorrhage-induced brain damage by inhibiting necroptosis. World J Clin Cases 2020; 8(10): 1848-1858
- URL: https://www.wjgnet.com/2307-8960/full/v8/i10/1848.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i10.1848
Cerebral hemorrhage (CH) is one of the most common cerebrovascular emergencies with high mortality worldwide[1-3]. Although surgery, especially minimally invasive surgery, has led to some progress in the treatment of CH, the long-term outcomes including inflammatory response and encephaledema after CH are not completely resolved. Moreover, there is a lack of a systematic therapeutic mode in the treatment of CH. Therefore, it is of necessity to determine the pathophysiological pathogenesis of CH and develop more effective treatments.
Necroptosis, a new-found programmed cell death, was originally nominated by Degterev et al[4], who found that this type of cell death could be triggered by the tumor necrosis factor receptor (TNFR) signaling pathway. Generally, once the TNFR signaling pathway is activated, receptor interacting protein kinase 1 (RIPK1) is then recruited to the intracellular domain of TNFR to activate the downstream signaling pathway[5,6]. When modified by ubiquitination, RIPK1 interacts with the IκB kinase (IKK) complex and transforming growth factor β-activated kinase-1 (TAK1) to induce activation of the nuclear factor-kappa B (NF-κB) signaling pathway and the mitogen-activated protein kinases (MAPKs) signaling pathway[7,8]. In the absence of caspase-8, RIPK1 interacts with RIPK3 to form a necrosome, resulting in auto-phosphorylation of RIPK3 (p-RIPK3)[9]. Furthermore, p-RIPK3 activates and phosphorylates mixed lineage kinase domain like pseudokinase (MLKL), which translocates to the cell membrane and induces rupture of the cell membrane[10]. Therefore, RIPK1 functions at the crossroad. Although the morphological features of necroptosis are indistinct from necrosis, genetic and pharmacological interventions could block necroptosis, which belongs to programmed cell death[11]. It has been demonstrated that necroptosis is involved in a variety of diseases, including tumors[12], inflammatory diseases[13] and ischemic injury[14]. Recently, Su et al[15] reported that targeting RIPK1 using the chemical necrostatin-1 could mitigate brain damage after intracerebral hemorrhage in mice. In addition, inhibiting necroptosis contributes to the improvement of synaptic injury induced by subarachnoid hemorrhage in the hippocampus[16]. Therefore, overcoming necroptosis may be a novel approach for the treatment of CH.
Acupuncture, a traditional Chinese medicine for multiple diseases, acts on specific acupoints of the body to exert its functions, and is extensively used worldwide[17,18]. It has been demonstrated that acupuncture ameliorates neurological impairments in rats with CH[19]. A randomized controlled trial was carried out in patients with CH and showed that acupuncture improved neurological functions[20]. Based on the effectiveness of acupuncture in CH, researchers have applied electric stimulus into acupuncture to assess the effects of electroacupuncture (EA) in CH, and in vivo experiments show that EA inhibits expression of apoptosis-related proteins to reduce brain damage in rats with CH[21]. Cross electro-nape-acupuncture (CENA) is a modified EA, and our previous clinical trial demonstrated that CENA promoted recovery of lung infection in patients with CH by remodeling the cough reflex[22], which suggested a potential therapy for CH. However, the underlying mechanism is still unknown.
In the present study, we determined the protective effects of CENA on brain damage in rats with CH and investigated the underlying mechanism, in order to provide a theoretical foundation to better understand the pathogenesis of CH and to develop optimized treatments.
A total of one hundred and eight male Sprague-Dawley (SD) rats, weighing 300 ± 15 g, were obtained from Heilongjiang University of Chinese Medicine. The procedures were approved by the Animal Use and Care Committee of Heilongjiang University of Chinese Medicine. The rats were randomized into four groups: Sham group (n = 27), CENA group (n = 27), CH group (n = 27) and CH+CENA group (n = 27). To obtain brain tissues, we randomly chose 36 rats in total with 9 rats from each group on the third day after surgery and treatment. The remaining 72 rats continued to be treated with the experimental procedures.
The rats were anesthetized with pentobarbital sodium (40 mg/kg) and placed in stereotaxic apparatus. A hole was drilled through the skull and a microsyringe was injected into the basal ganglia region (5 mm to the left side of the bregma and 0.2 mm in front of the coronal suture) with a depth of 5 mm. A total of 50 μL autogenous femoral arterial blood was transfused at a speed of 20 μL/min. After 10 min, the microsyringe was slowly withdrawn. The hole was covered with Self Curing Denture Acrylic (Pearson, United States). Rats in the sham group underwent the same procedures without the transfusion.
After surgery, all the rats were fixed. Rats in the CENA group and CH+CENA group were treated with CENA. The acupuncture needles (Hwato, China) were placed in the left Fengchi acupoint (GB20) and the right Yifeng acupoint (TE17), and connected to the EA therapeutic apparatus (Hwato, China). Rats were treated with CENA for 30 min/d after the surgical procedures. Rats in the sham group and CH group were fixed without treatment.
Propidium iodide (PI, Abcam, United States) was used to monitor the necrotic cells in brain tissues of the experimental rats. The brain tissues were embedded with O.C.T. Compound (Sakura, United States) and then cut into sections 30 μm thick. The sections were incubated with PI solution for 30 min. After washing three times with PBS, images of the sections were captured by fluorescence microscopy (Olympus, Japan).
Brain sections were incubated with p-MLKL primary antibodies (Abcam, United States) overnight at 4°C. NeuN (Abcam, United States) was used to label neurons. After washing, the sections were incubated with goat anti-mouse IgG-Cy3 Conjugated secondary antibodies (Beyotime, China) and goat anti-rabbit IgG-Alexa Fluor 488 Conjugated secondary antibodies (Beyotime, China) for 2 h. DAPI (Beyotime, China) was used to label cell nuclei. Images of the sections were visualized and captured by confocal microscopy.
Neurological functions were determined by estimating the neurological scores[23] and behavioral scores[24] as previously reported. For detection of neurological scores, the contributing factors consisted of six parts: Autonomic movement, tail-suspension four-limb movement, forelimb stretching, climbing and grasping ability, somatosensory response, and beard-touching response. Each factor was graded from 0 (no response) to 3 (normal). Lower neurological scores in the experimental rats represented impaired neurological function. For evaluation of the behavioral scores, the contributing factors included ipsilateral circling, bilateral grasp, and beam walking. Each factor was graded from 0 (normal behavior) to 4 (no response). Higher behavioral scores in the experimental rats represented damaged behavioral function.
On the third day after surgery and treatment, the brain tissues of experimental rats (n = 36) were harvested. Water content (edema) was measured as reported previously[23]. Water content (%) = (wet weight - dry weight)/wet weight.
Western blot analysis was carried out as reported previously[15]. Total protein was extracted from brain tissues of experimental rats using RIPA Lysis Buffer (MultiSciences, China). The BCA Protein Quantification Kit (MultiSciences, China) was used to detect protein concentration. The proteins were separated by SDS-PAGE gel (MultiSciences, China). Primary antibodies (RIPK1, RIPK3, p-RIPK3, MLKL, p-MLKL, GAPDH) were purchased from Abcam (United States). ChemDocTM XRS+ System (Bio-Rad, United States) was used to detect the protein bands. GAPDH was the internal reference protein.
The immunoprecipitation (IP) assay was conducted using the Pierce Co-Immunoprecipitation Kit (Thermo Fisher Scientific, United States) according to the manufacturer’s instructions. The brain tissues were cut into pieces and lysed by IP Lysis Buffer. Next, the RIPK1 primary antibody (Abcam, United States) was added to the lysis solution containing total protein, Sodium cyanoborohydride, Coupling Buffer, Elution Buffer and Loading Buffer were used in sequence to obtain the samples. The interaction between RIPK1 and RIPK3 was evaluated by western blot analysis.
Total RNA was extracted from the freshly isolated brains of rats using TRIzol (Ambion, United States). The acquisition of cDNA and the following quantitative real-time polymerase chain reaction (qRT-PCR) were performed using SuperScript III (Invitrogen, United States) and SYBR Premix Ex Taq II (Takara, Japan), respectively. The set procedures were as follow: 95 °C, 30 s; 55 °C, 30 s; 72 °C, 30 s. A total of 40 cycles were performed. The primers were obtained from Sangon (China). Interleukin (IL)-6 forward: 5’-CCACTTGGATGTAACTGGCCT-3’; IL-6 reverse: 5’-GGAAAAAGTGCTGCTACCCTG-3’. IL-8 forward: 5’-CATTAATATTTAACGATGTGGATGC-3’; IL-8 reverse: 5’-TAACACGTCAAATTTCTACCATCCG-3’. Tumor necrosis factor-α (TNF-α) forward: 5’-TATCCGTCCAACCTCAGCAT-3’; TNF-α reverse: 5-’GCGAATGAACGAACAAGCGT-3’. GAPDH forward: 5’-TGAAATGTGCACGCACCAAG-3’; GAPDH reverse: 5’-GGGAAGCAGCATTCAGGTCT-3’. GAPDH was the internal reference.
On the seventh day after surgery and treatment, 300 μL of cerebrospinal fluid (CSF) from each rat and 1.2 mL of fresh blood were collected. After centrifugation, the CSF samples and serum samples were added into plates to measure the concentration of IL-6, IL-8 and TNF-α using a Rat IL-6 enzyme-linked immunosorbent assay (ELISA) Kit (Beyotime, China), Rat IL-8 ELISA Kit (NeoScientific, United Kingdom) and Rat TNF-α ELISA Kit (Beyotime, China), respectively, in accordance with the manufacturers’ guidelines.
All data are shown as mean ± SD, and were analyzed using GraphPad Prism 6.01 Software (GraphPad, United States). The statistical analysis methods used were t-test and two-way ANOVA. A P value less than 0.05 was considered statistically significant.
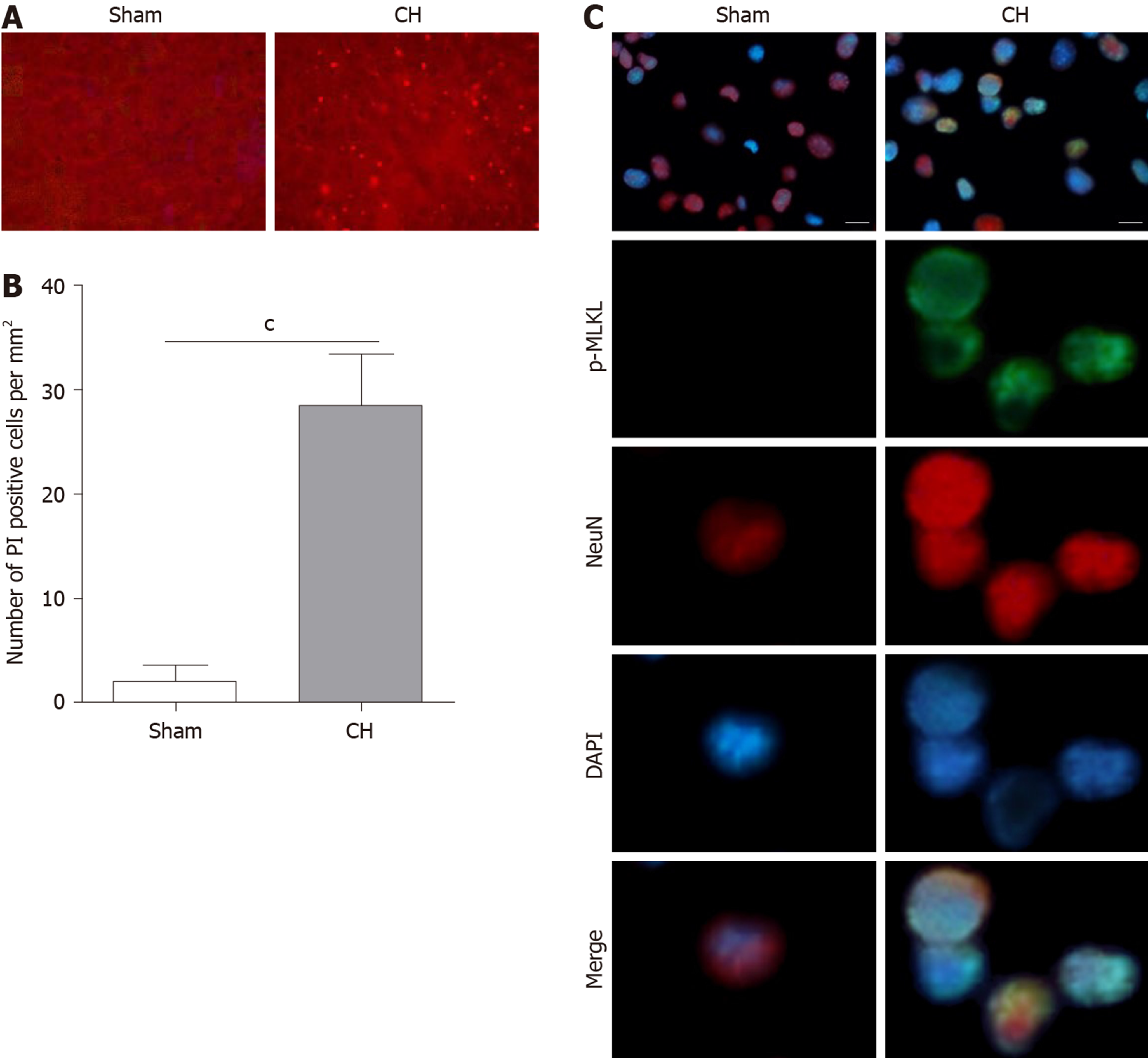
To investigate the involvement of necroptosis in CH, we first monitored the necrotic cells in brains by evaluating the PI positive rate. As shown in Figure 1A and B, the number of PI positive cells was significantly increased in rats with CH (cP < 0.001), indicating that CH can induce necrosis. It has been reported that p-MLKL could be regarded as an indicator of necroptosis[25,26]. Next, we performed immunofluorescence to determine the expression of p-MLKL in situ. The results showed that p-MLKL was not detectable in the neurons of rats in the sham group, whereas p-MLKL was positively expressed in the neurons of rats with CH (Figure 1C). These results demonstrated that necroptosis existed in CH.
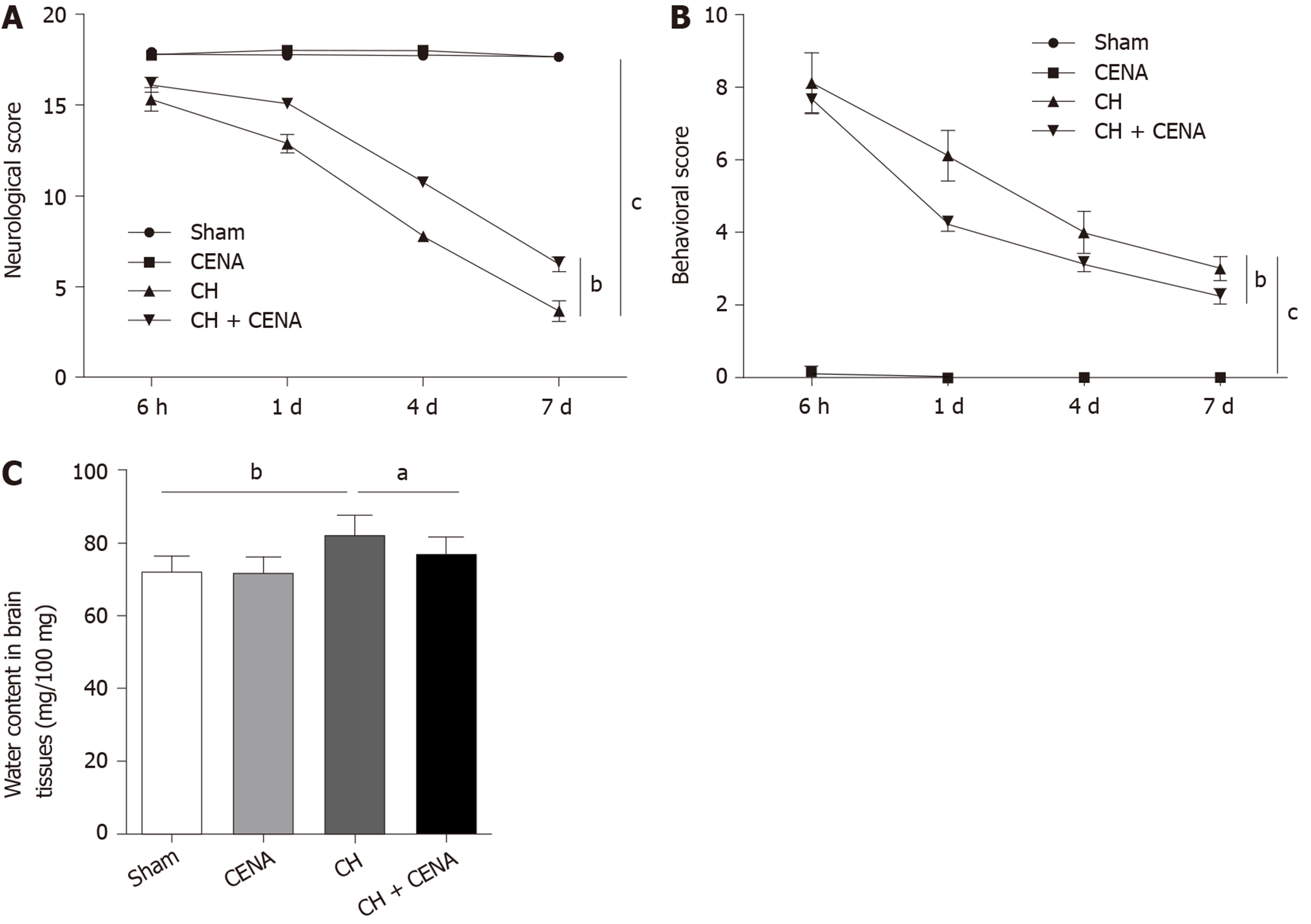
To determine whether CENA exerted protective effects in rats with CH, we performed the following experiments. The neurological scores of rats in the CENA group showed a similar trend to that in the sham group; however, CENA reversed the decreased neurological score in rats with CH (Figure 2A, bP < 0.01). In parallel, CENA ameliorated the damaging behavior of rats with CH by evaluating the behavioral score (Figure 2B, bP < 0.01). Next, we measured brain tissue edema in the different groups. On the third day after surgery and treatment, rat brain tissues were collected, and it was found that the water content in the brain tissues of rats with CH was increased compared with that in the sham group, while CENA partially reduced water content in the brain tissues of rats with CH (Figure 2C, aP < 0.05). These results suggested that CENA can alleviate brain damage.
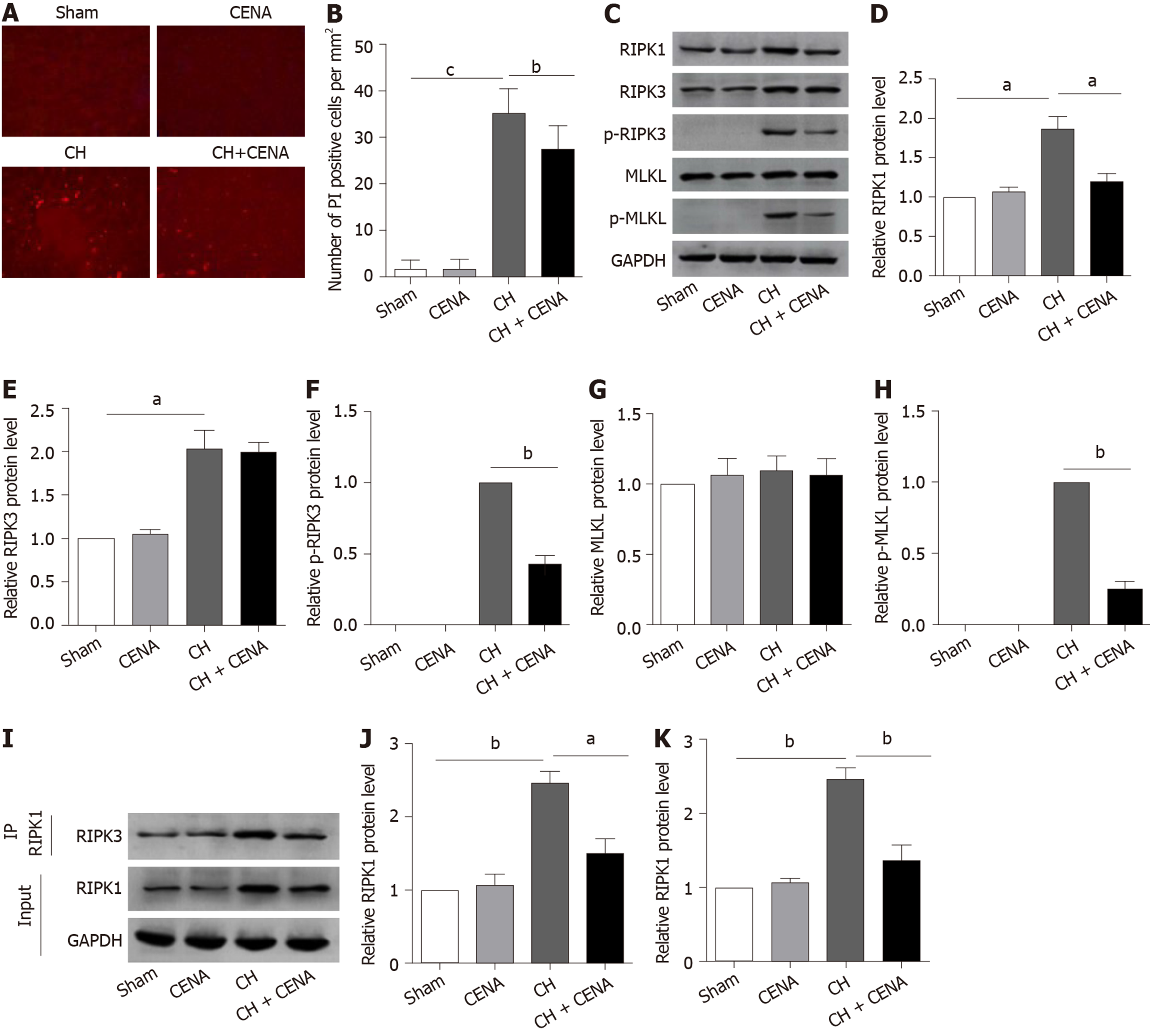
Based on the above findings, we assessed whether CENA reduced cell death in the brains of rats with CH, especially necroptosis. Immunofluorescence analysis in situ showed that the number of PI positive cells in the CENA group was similar as that in the sham group (Figure 3A and B). Importantly, the increased PI positive cells in the CH group were reduced by CENA in the brains of rats with CH (Figure 3A and B, bP < 0.01). As necroptosis is mediated by RIPK1, and executed by RIPK3 and MLKL, it is still unclear whether CENA affected the expression of necroptosis-related molecules in the brain tissues of these rats. Western blot analysis revealed that CENA treatment did not upregulate the expression of RIPK1, RIPK3 and MLKL, and did not activate RIPK3 and MLKL in normal brain tissues (Figure 3C-3H). It was also found that rat brain tissues in the CH group displayed a dramatically higher protein level of RIPK1, RIPK3, p-RIPK3 and p-MLKL, which was reversed by CENA treatment (Figure 3C-F and H, aP < 0.05). However, the expression of MLKL showed no obvious change (Figure 3C and G). In addition, an IP assay was carried out to evaluate necrosome formation. The results showed that RIPK3 interacting with RIPK1 increased markedly in rat brain tissues after CH, and CENA treatment partially disturbed this interaction between RIPK1 and RIPK3 (Figure 3I-K, aP < 0.05). These results indicated that CENA reduced cell death by inhibiting necrosome formation in rat brain tissues after CH.
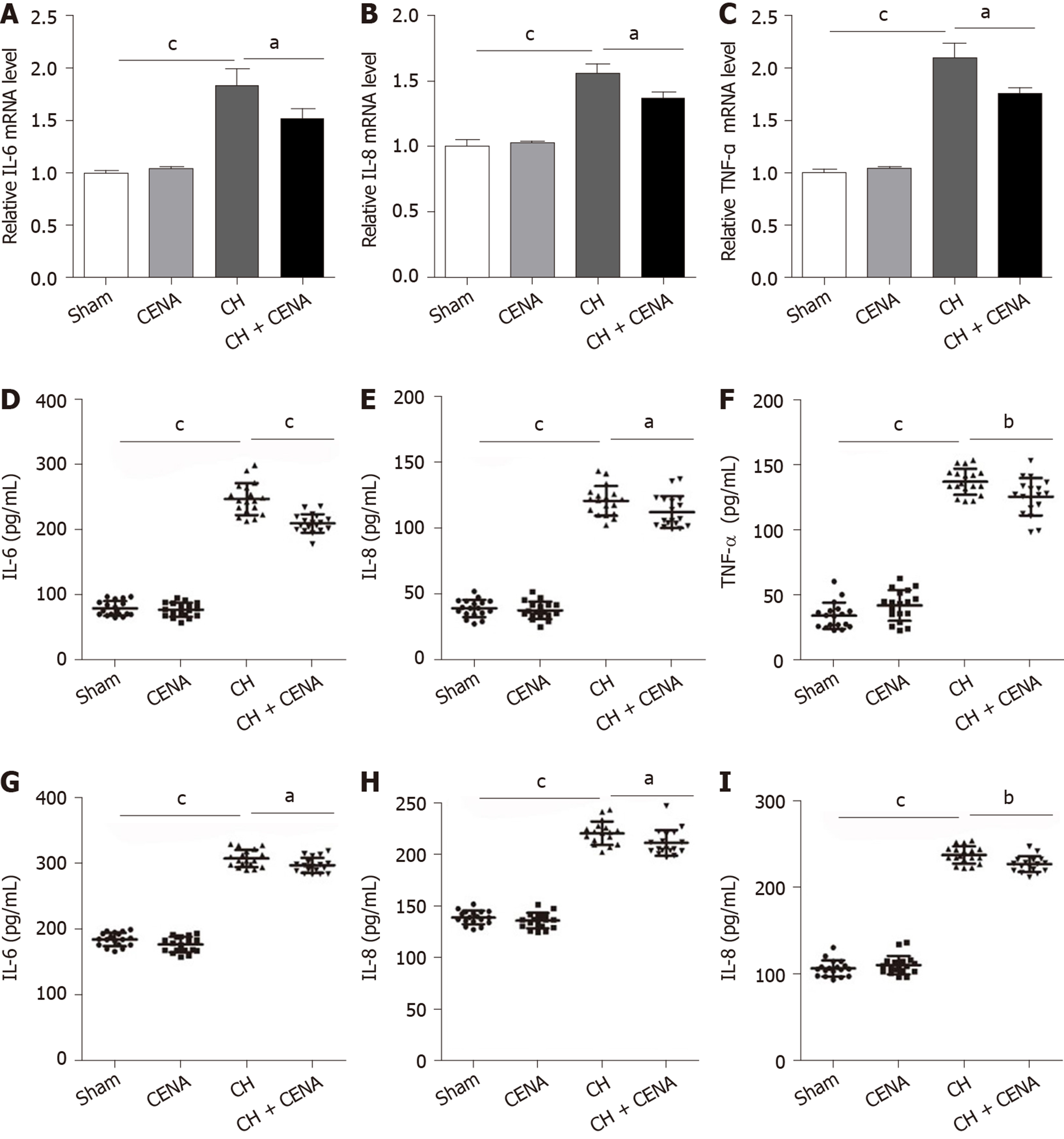
It has been demonstrated that CH is accompanied by inflammation[27,28]. We first detected the expression of inflammatory factors IL-6, IL-8 and TNF-α in the brain tissues of rats. qRT-PCR showed that rat brain tissues in the CH group displayed a significantly higher mRNA level of IL-6, IL-8 and TNF-α, and CENA treatment partially decreased these three inflammatory factors (Figure 4A-C, aP < 0.05). Next, we extracted CSF and measured the content of IL-6, IL-8 and TNF-α using ELISA. Consistent with the results of qRT-PCR, increased concentrations of IL-6, IL-8 and TNF-α were found in the CSF of CH model rats, which was reduced by CENA treatment (Figure 4D-F, aP < 0.05). In parallel, the secretion of IL-6, IL-8 and TNF-α in rat serum showed a similar trend to that detected in CSF (Figure 4G-I, aP < 0.05). Thus, CENA can ameliorate local and systemic inflammation caused by CH.
In the current study, we investigated the neuroprotective role of CENA in rats with CH and investigated the underlying mechanism. First, we found that a necroptotic marker was detectable in the brain of a CH rat model. The impaired neurological functions induced by CH were reversed by CENA. Furthermore, our results showed that necroptosis mediator RIPK1 was overexpressed in the brain tissues of the CH rat model. In addition, CH induced phosphorylation of RIPK3 and MLKL. Notably, CENA reduced the upregulation of RIPK1, p-RIPK3 and p-MLKL, and partially blocked the interaction between RIPK1 and RIPK3. Finally, CENA contributed to the amelioration of CH-induced inflammatory response in rats.
Although a variety of studies have focused on the pathogenesis of brain damage after CH, the complex mechanisms make it difficult to overcome the problem of ineffective treatments. Increasing evidence has demonstrated that apoptosis and necrosis play leading roles in neuron death after CH[29,30]. Apoptosis belongs to programmed cell death; while necrosis is passive cell death and accompanied by inflammatory response[31]. As necrosis cannot be regulated, there is little research on the role of necrosis in CH. A previous investigation reported that a new-found necrotic cell death, namely necroptosis, exists in ischemic brain damage, which could be suppressed by small molecule inhibitor necrostatin-1[4]. Subsequently, RIPK1 was identified as the key mediator of necroptosis and the target of necrostatin-1[32]. Accumulating evidence indicates that RIPK1 interacts with RIPK3 to form a necrosome, which phosphorylates the substrate molecule MLKL to break the integrity of the plasm membrane and cause cell death[5,8,10,26]. In addition, genetic intervention of RIPK3[33] or MLKL inhibition with small interfering RNA and small molecule inhibitor necrosulfonamide[34] can prevent necroptosis. Therefore, targeting RIPK1, RIPK3 or MLKL is a promising strategy to treat necroptosis-related diseases.
It has been demonstrated that necroptosis is an alternative cell death in CH. Initially, Laird et al[35] reported that the hemoglobin metabolite hemin resulted in oxidative injury, elevation of inflammatory cytokines and necroptosis of astrocytes in vitro, inferring that necroptosis might contribute to brain damage after CH. The application of RIPK1 inhibitor in mice after CH reduced the necrotic rate of neurons and ameliorated neurological impairment[15]. Both in vivo and in vitro experiments further elucidated the crucial role of RIPK1 in necroptosis-mediated brain damage after CH, as RIPK1 overexpression in cultured neurons promoted necroptosis[36]. In addition, necrostatin-1 treatment ameliorated blood-brain barrier damage in rats with subarachnoid hemorrhage by inhibiting activation of the RIPK1-RIPK3-MLKL cascade[37]. As shown by Yuan et al[38], RIPK1 and RIPK3 were involved in brain damage after subarachnoid hemorrhage in rats. In our study, we found that the neuroprotective effects of CENA targeted RIPK1, but not RIPK3 in rats after CH.
Similar to necrosis, necroptosis is accompanied by the release of inflammatory cytokines. Mechanistically, RIPK1 can induce an inflammatory response by activating the NF-κB signaling pathway and MAPKs signaling pathway[5,13]. Accumulating evidence has shown that RIPK1 intervention using genetic and pharmacological approaches prevents severe inflammatory response during pathogen infection[39] and autoimmune diseases[40]. In addition, NF-κB activation-induced production of IL-6 and TNF-α promotes neuroinflammation[41]. It has been demonstrated that inflammation can aggravate brain damage after CH, and targeting inhibition of inflammation is a potential treatment for CH[42]. Zhou et al[43] reported that patients with CH displayed a significantly higher level of IL-6 and IL-8, and ameliorating inflammation could reduce brain edema and improve outcomes. Our results suggested that CH-induced elevation of inflammatory cytokines (IL-6, IL-8 and TNF-α) in brain tissue, serum and CSF of rats with CH could be partially controlled by CENA. Moreover, CENA suppressed the expression of RIPK1 in the brain tissues of rats with CH. Combined with the findings from other research groups, our study indicated that CENA inhibited RIPK1 to ameliorate necroptosis and inflammation in rats with CH.
Taken together, our findings suggested a potential protective effect of CENA on brain damage after CH, which involves the suppression of necroptosis. Further investigations are needed to determine the effectiveness of CENA in patients with CH, which might provide a novel treatment for CH.
Cerebral hemorrhage (CH) is a severe disease worldwide. Although accumulating evidence has demonstrated that cell death is the dominant event in the pathogenesis of CH, there is still a lack of preferred treatment. Therefore, developing novel methods to treat CH is currently an urgent issue.
Our previous study showed that cross electro-nape acupuncture (CENA), a modified electroacupuncture, could ameliorate lung infection in patients with CH. However, the role of CENA in brain damage in patients with CH and the underlying mechanism are still unclear.
The aim of this work was to investigate the exact effect of CENA on rats with CH and its underlying mechanism.
Rats were surgically treated to mimic CH and received CENA treatment. Propidium iodide staining and immunofluorescence analysis were performed to determine cell death. Neurological score, behavioral score and brain water content were calculated to evaluate brain damage. Western blot, immunoprecipitation assay, quantitative real-time polymerase chain reaction and enzyme-linked immunosorbent assay were conducted to determine the underlying mechanism.
The present study identified the presence of necroptotic marker p-MLKL in the brain tissues of rats with CH. CENA decreased neurological score, behavioral score and brain water content in rats with CH. Further investigation revealed that CENA inhibited necrosome formation and the expression of IL-6, IL-8 and TNF-α in rats with CH.
Our research found that receptor interacting protein kinase 1 (RIPK1)-mediated necroptosis was involved in brain damage in rats with CH. CENA treatment suppressed necroptosis and the inflammatory response to ameliorate brain damage in rats with CH, providing a novel strategy for CH treatment.
Based on the clinical findings and in vivo experiments, RIPK1 could be a novel therapeutic target for CH-induced brain damage, and CENA improved brain damage by targeting RIPK1 and inhibiting the expression of inflammatory factors. Further in vivo assays in primates are necessary to clarify the protective effects of CENA on CH-induced brain damage, which is very meaningful and would contribute to the clinical application of CENA.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Inchauspe AA S-Editor: Wang YQ L-Editor: Webster JR E-Editor: Liu JH
| 1. | Cordonnier C, Demchuk A, Ziai W, Anderson CS. Intracerebral haemorrhage: current approaches to acute management. Lancet. 2018;392:1257-1268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 464] [Article Influence: 66.3] [Reference Citation Analysis (0)] |
| 2. | Sun L, Clarke R, Bennett D, Guo Y, Walters RG, Hill M, Parish S, Millwood IY, Bian Z, Chen Y, Yu C, Lv J, Collins R, Chen J, Peto R, Li L, Chen Z; China Kadoorie Biobank Collaborative Group; International Steering Committee; International Co-ordinating Centre, Oxford; National Co-ordinating Centre, Beijing; Regional Co-ordinating Centres. Causal associations of blood lipids with risk of ischemic stroke and intracerebral hemorrhage in Chinese adults. Nat Med. 2019;25:569-574. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 229] [Article Influence: 38.2] [Reference Citation Analysis (0)] |
| 3. | Tatlisumak T, Cucchiara B, Kuroda S, Kasner SE, Putaala J. Nontraumatic intracerebral haemorrhage in young adults. Nat Rev Neurol. 2018;14:237-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 61] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 4. | Degterev A, Huang Z, Boyce M, Li Y, Jagtap P, Mizushima N, Cuny GD, Mitchison TJ, Moskowitz MA, Yuan J. Chemical inhibitor of nonapoptotic cell death with therapeutic potential for ischemic brain injury. Nat Chem Biol. 2005;1:112-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1858] [Cited by in RCA: 2394] [Article Influence: 119.7] [Reference Citation Analysis (0)] |
| 5. | Ofengeim D, Yuan J. Regulation of RIP1 kinase signalling at the crossroads of inflammation and cell death. Nat Rev Mol Cell Biol. 2013;14:727-736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 406] [Cited by in RCA: 483] [Article Influence: 40.3] [Reference Citation Analysis (0)] |
| 6. | Yuan J, Amin P, Ofengeim D. Necroptosis and RIPK1-mediated neuroinflammation in CNS diseases. Nat Rev Neurosci. 2019;20:19-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 665] [Article Influence: 110.8] [Reference Citation Analysis (0)] |
| 7. | de Almagro MC, Goncharov T, Newton K, Vucic D. Cellular IAP proteins and LUBAC differentially regulate necrosome-associated RIP1 ubiquitination. Cell Death Dis. 2015;6:e1800. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 90] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 8. | Newton K. RIPK1 and RIPK3: critical regulators of inflammation and cell death. Trends Cell Biol. 2015;25:347-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 268] [Article Influence: 26.8] [Reference Citation Analysis (0)] |
| 9. | Wu XN, Yang ZH, Wang XK, Zhang Y, Wan H, Song Y, Chen X, Shao J, Han J. Distinct roles of RIP1-RIP3 hetero- and RIP3-RIP3 homo-interaction in mediating necroptosis. Cell Death Differ. 2014;21:1709-1720. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 339] [Article Influence: 30.8] [Reference Citation Analysis (0)] |
| 10. | Sun L, Wang H, Wang Z, He S, Chen S, Liao D, Wang L, Yan J, Liu W, Lei X, Wang X. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell. 2012;148:213-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1601] [Cited by in RCA: 2188] [Article Influence: 168.3] [Reference Citation Analysis (0)] |
| 11. | Shan B, Pan H, Najafov A, Yuan J. Necroptosis in development and diseases. Genes Dev. 2018;32:327-340. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 167] [Cited by in RCA: 273] [Article Influence: 39.0] [Reference Citation Analysis (0)] |
| 12. | Qin X, Ma D, Tan YX, Wang HY, Cai Z. The role of necroptosis in cancer: A double-edged sword? Biochim Biophys Acta Rev Cancer. 2019;1871:259-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 93] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 13. | Dhuriya YK, Sharma D. Necroptosis: a regulated inflammatory mode of cell death. J Neuroinflammation. 2018;15:199. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 483] [Cited by in RCA: 462] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 14. | Galluzzi L, Kepp O, Chan FK, Kroemer G. Necroptosis: Mechanisms and Relevance to Disease. Annu Rev Pathol. 2017;12:103-130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 308] [Cited by in RCA: 521] [Article Influence: 57.9] [Reference Citation Analysis (0)] |
| 15. | Su X, Wang H, Kang D, Zhu J, Sun Q, Li T, Ding K. Necrostatin-1 ameliorates intracerebral hemorrhage-induced brain injury in mice through inhibiting RIP1/RIP3 pathway. Neurochem Res. 2015;40:643-650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 80] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 16. | Yang C, Li T, Xue H, Wang L, Deng L, Xie Y, Bai X, Xin D, Yuan H, Qiu J, Wang Z, Li G. Inhibition of Necroptosis Rescues SAH-Induced Synaptic Impairments in Hippocampus via CREB-BDNF Pathway. Front Neurosci. 2018;12:990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 17. | Armour M, Smith CA, Wang LQ, Naidoo D, Yang GY, MacPherson H, Lee MS, Hay P. Acupuncture for Depression: A Systematic Review and Meta-Analysis. J Clin Med. 2019;8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 99] [Article Influence: 16.5] [Reference Citation Analysis (2)] |
| 18. | Chon TY, Lee MC. Acupuncture. Mayo Clin Proc. 2013;88:1141-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 93] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 19. | Liu XY, Dai XH, Zou W, Yu XP, Teng W, Wang Y, Yu WW, Ma HH, Chen QX, Liu P, Guan RQ, Dong SS. Acupuncture through Baihui (DU20) to Qubin (GB7) mitigates neurological impairment after intracerebral hemorrhage. Neural Regen Res. 2018;13:1425-1432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Wang HQ, Bao CL, Jiao ZH, Dong GR. Efficacy and safety of penetration acupuncture on head for acute intracerebral hemorrhage: A randomized controlled study. Medicine (Baltimore). 2016;95:e5562. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Zhu Y, Deng L, Tang H, Gao X, Wang Y, Guo K, Kong J, Yang C. Electroacupuncture improves neurobehavioral function and brain injury in rat model of intracerebral hemorrhage. Brain Res Bull. 2017;131:123-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 22. | Cai Gf, Shang L, Liu K, Zhao H, Quan A, Yan C, Sun H, Li X, Zhuang Z. [Remodeling of cross electro-nape-acupuncture on cough reflex in patients with tracheotomy after cerebral hemorrhage: a randomized controlled trial]. Zhongguo Zhen Jiu. 2015;35:3-6. [PubMed] |
| 23. | Zhang CY, Ren XM, Li HB, Wei W, Wang KX, Li YM, Hu JL, Li X. Simvastatin alleviates inflammation and oxidative stress in rats with cerebral hemorrhage through Nrf2-ARE signaling pathway. Eur Rev Med Pharmacol Sci. 2019;23:6321-6329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 24. | Rosenberg GA, Mun-Bryce S, Wesley M, Kornfeld M. Collagenase-induced intracerebral hemorrhage in rats. Stroke. 1990;21:801-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 443] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 25. | Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z. A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci USA. 2015;112:5017-5022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 139] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 26. | Wang H, Sun L, Su L, Rizo J, Liu L, Wang LF, Wang FS, Wang X. Mixed lineage kinase domain-like protein MLKL causes necrotic membrane disruption upon phosphorylation by RIP3. Mol Cell. 2014;54:133-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 1330] [Article Influence: 120.9] [Reference Citation Analysis (0)] |
| 27. | Li X, Wang T, Zhang D, Li H, Shen H, Ding X, Chen G. Andrographolide ameliorates intracerebral hemorrhage induced secondary brain injury by inhibiting neuroinflammation induction. Neuropharmacology. 2018;141:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 57] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 28. | Ye L, Gao L, Cheng H. Inflammatory Profiles of the Interleukin Family and Network in Cerebral Hemorrhage. Cell Mol Neurobiol. 2018;38:1321-1333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 29. | Bobinger T, Burkardt P, B Huttner H, Manaenko A. Programmed Cell Death after Intracerebral Hemorrhage. Curr Neuropharmacol. 2018;16:1267-1281. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 85] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 30. | Duan X, Wen Z, Shen H, Shen M, Chen G. Intracerebral Hemorrhage, Oxidative Stress, and Antioxidant Therapy. Oxid Med Cell Longev. 2016;2016:1203285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 217] [Article Influence: 24.1] [Reference Citation Analysis (0)] |
| 31. | Zhang Y, Chen X, Gueydan C, Han J. Plasma membrane changes during programmed cell deaths. Cell Res. 2018;28:9-21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 420] [Cited by in RCA: 704] [Article Influence: 88.0] [Reference Citation Analysis (0)] |
| 32. | Degterev A, Hitomi J, Germscheid M, Ch'en IL, Korkina O, Teng X, Abbott D, Cuny GD, Yuan C, Wagner G, Hedrick SM, Gerber SA, Lugovskoy A, Yuan J. Identification of RIP1 kinase as a specific cellular target of necrostatins. Nat Chem Biol. 2008;4:313-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1393] [Cited by in RCA: 1723] [Article Influence: 101.4] [Reference Citation Analysis (0)] |
| 33. | Ni HM, Chao X, Kaseff J, Deng F, Wang S, Shi YH, Li T, Ding WX, Jaeschke H. Receptor-Interacting Serine/Threonine-Protein Kinase 3 (RIPK3)-Mixed Lineage Kinase Domain-Like Protein (MLKL)-Mediated Necroptosis Contributes to Ischemia-Reperfusion Injury of Steatotic Livers. Am J Pathol. 2019;189:1363-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 34. | Ding Y, He C, Lu S, Wang X, Wang C, Wang L, Zhang J, Piao M, Chi G, Luo Y, Sai K, Ge P. MLKL contributes to shikonin-induced glioma cell necroptosis via promotion of chromatinolysis. Cancer Lett. 2019;467:58-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 43] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 35. | Laird MD, Wakade C, Alleyne CH, Dhandapani KM. Hemin-induced necroptosis involves glutathione depletion in mouse astrocytes. Free Radic Biol Med. 2008;45:1103-1114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 97] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 36. | Shen H, Liu C, Zhang D, Yao X, Zhang K, Li H, Chen G. Role for RIP1 in mediating necroptosis in experimental intracerebral hemorrhage model both in vivo and in vitro. Cell Death Dis. 2017;8:e2641. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 91] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 37. | Zhang S, Hu ZW, Luo HY, Mao CY, Tang MB, Li YS, Song B, Wang YH, Zhang ZX, Zhang QM, Fan LY, Zhang Y, Yu WK, Shi CH, Xu YM. AAV/BBB-Mediated Gene Transfer of CHIP Attenuates Brain Injury Following Experimental Intracerebral Hemorrhage. Transl Stroke Res. 2020;11:296-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 38. | Yuan S, Yu Z, Zhang Z, Zhang J, Zhang P, Li X, Li H, Shen H, Chen G. RIP3 participates in early brain injury after experimental subarachnoid hemorrhage in rats by inducing necroptosis. Neurobiol Dis. 2019;129:144-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 39. | Saleh D, Najjar M, Zelic M, Shah S, Nogusa S, Polykratis A, Paczosa MK, Gough PJ, Bertin J, Whalen M, Fitzgerald KA, Slavov N, Pasparakis M, Balachandran S, Kelliher M, Mecsas J, Degterev A. Kinase Activities of RIPK1 and RIPK3 Can Direct IFN-β Synthesis Induced by Lipopolysaccharide. J Immunol. 2017;198:4435-4447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 40. | Jhun J, Lee SH, Kim SY, Ryu J, Kwon JY, Na HS, Jung K, Moon SJ, Cho ML, Min JK. RIPK1 inhibition attenuates experimental autoimmune arthritis via suppression of osteoclastogenesis. J Transl Med. 2019;17:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 42] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 41. | Wen J, Yang CY, Lu J, Wang XY. Ptprj-as1 mediates inflammatory injury after intracerebral hemorrhage by activating NF-κB pathway. Eur Rev Med Pharmacol Sci. 2018;22:2817-2823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 42. | de Oliveira Manoel AL, Macdonald RL. Neuroinflammation as a Target for Intervention in Subarachnoid Hemorrhage. Front Neurol. 2018;9:292. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 116] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 43. | Zhou X, Chen J, Wang C, Wu L. Anti-inflammatory effects of Simvastatin in patients with acute intracerebral hemorrhage in an intensive care unit. Exp Ther Med. 2017;14:6193-6200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |