Published online Jan 6, 2020. doi: 10.12998/wjcc.v8.i1.179
Peer-review started: October 15, 2019
First decision: November 13, 2019
Revised: November 22, 2019
Accepted: November 30, 2019
Article in press: November 30, 2019
Published online: January 6, 2020
Processing time: 83 Days and 12.7 Hours
Neuroendocrine tumors mainly occur in the stomach, intestine, pancreas, and lung and are rarely detected in the thyroid. Thyroid neuroendocrine tumors, designated medullary thyroid carcinoma, generally present with elevated calcitonin. Calcitonin-negative neuroendocrine tumors of the thyroid are extremely rare.
Here, we present a case report of a 56-year-old female patient with a neck pain complaint. Total thyroidectomy was conducted after comprehensive evaluation, and diagnosis was confirmed as calcitonin-negative neuroendocrine tumor of the thyroid. Two months later, liver metastasis was detected, and transcatheter arterial chemoembolization was subsequently performed to control growth. However, the curative effect was unsatisfactory and multiple intrahepatic metastases occurred after 3 mo.
Owing to the rarity of this disease, no clear guidelines are available for treatment. In addition to reporting this rare case, we have reviewed and summarized associated medical literature with an aim to provide a comprehensive reference platform for subsequent research.
Core tip: Calcitonin-negative neuroendocrine tumor of the thyroid is an extremely rare disorder, with past literature mostly documenting case reports. Medical imaging has revealed no specific manifestations, and current diagnosis mainly depends on pathological biopsy findings. The prognosis of thyroid neuroendocrine tumors is poorer than that of other thyroid tumors. Transcatheter arterial chemoembolization is not effective in cases of liver metastasis, and no improved treatments have been developed to date. This article provides a complete introduction of this case report with an aim to provide a meaningful reference for future research and trials.
- Citation: Cai HJ, Wang H, Cao N, Huang B, Kong FL, Lu LR, Huang YY, Wang W. Calcitonin-negative neuroendocrine tumor of the thyroid with metastasis to liver-rare presentation of an unusual tumor: A case report and review of literature. World J Clin Cases 2020; 8(1): 179-187
- URL: https://www.wjgnet.com/2307-8960/full/v8/i1/179.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i1.179
Medullary thyroid carcinoma (MTC) is one of the most common neuroendocrine tumors (NETs) of the thyroid with an incidence of 1%-2% according to the revised guidelines for the treatment of MTC issued by the American Thyroid Association[1]. In general, calcitonin is produced by thyroid parafollicular cells and its elevation in serum is the main criterion for clinical diagnosis of MTC. Calcitonin is additionally an important indicator for prognosis of the disease[2,3].
Clinical diagnosis of calcitonin-negative neuroendocrine tumors of the thyroid (CNNET) is confusing[4]. Owing to the extreme rarity of these cases, standardized management protocols are yet to be formulated. Prognosis of CNNET is poorer than that of other thyroid tumors. CNNET features on ultrasound are indistinguishable from those of papillary thyroid carcinoma, and diagnosis is mainly dependent on pathological findings. In cases where morphological identification is difficult, positivity of TTF-1, CgA, Syn, Ki-67, and other specific markers may aid in further diagnosis.
In terms of treatment and prognosis, surgical resection of the thyroid gland is the preferred option, while transcatheter arterial chemoembolization (TACE) has limited efficacy for liver metastasis. In this study, the ultrasonic features and immunohistochemical characteristics of CNNET with liver metastases have been investigated in detail. Furthermore, comparative analysis of the current case with previous reports of CNNET has provided a deeper understanding of the underlying factors and integration of case information with the objective of facilitating standardization of diagnosis and effective disease management.
On October 29, 2018, a 56-year-old female patient was admitted to our hospital due to goiter and pain for over a month.
Radical resection of rectal carcinoma 1 year earlier.
The family history was unremarkable.
Normalities were noted on physical examination with the exception of slight goiter with pain, and multiple enlarged lymph nodes were detected in the bilateral neck.
Normalities were detected in laboratory tests, including calcitonin and CEA levels and thyroid and parathyroid function.
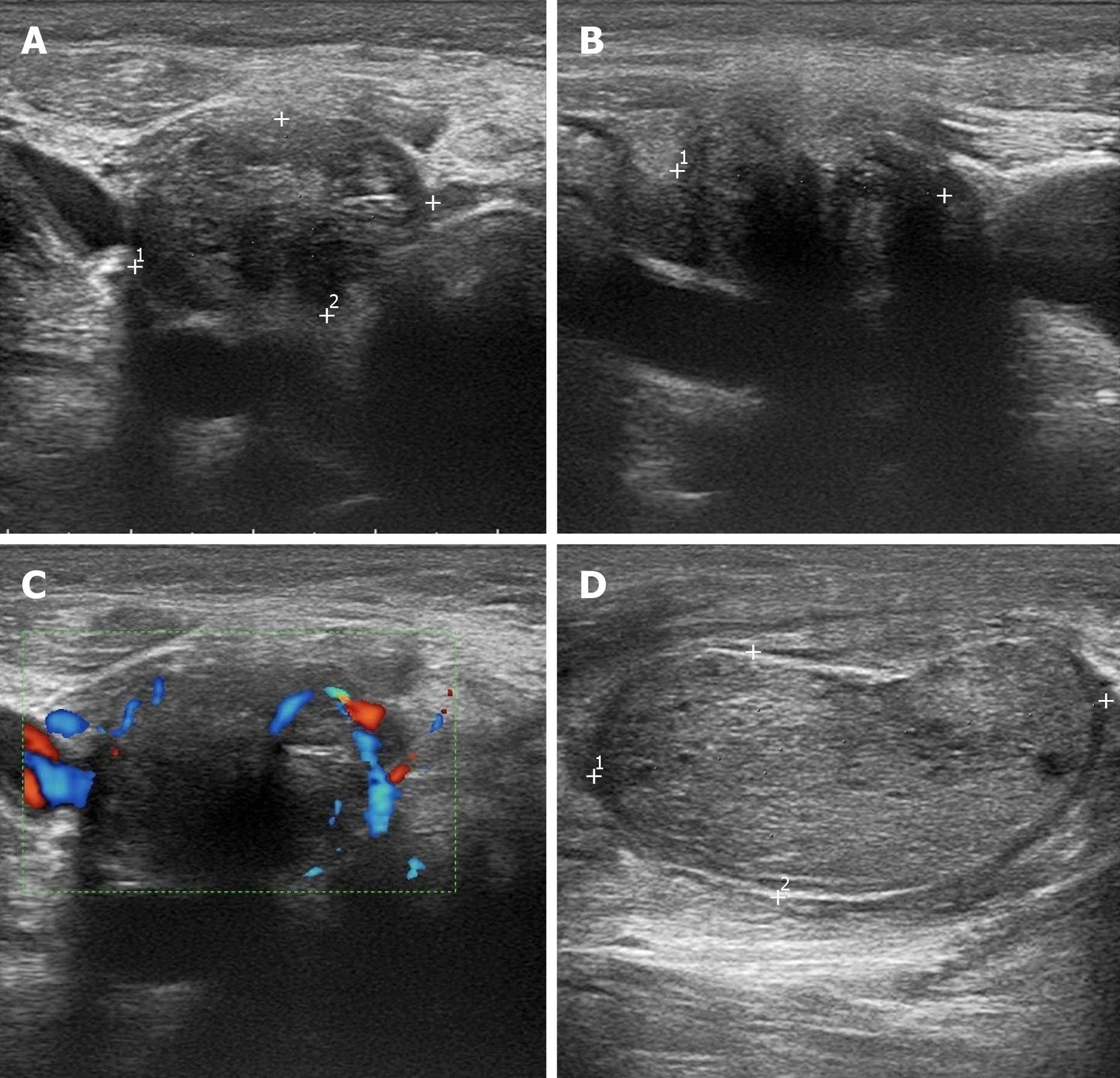
Ultrasound examination revealed that the thyroid gland was full and irregular in shape, and the envelope was not smooth. Multiple nodules were detected in bilateral lobes; the larger about 2.6 cm × 2.1 cm × 1.7 cm in size located in the right thyroid. The internal echo was uneven. Color Doppler flow imaging indicated abundant blood supply. Multiple enlarged lymph nodes were detected in the bilateral neck; the largest about 4.2 cm × 2.6 cm × 2.0 cm in size located on the right side (Figure 1).
Routine blood parameters, coagulation function, thyroid function, parathyroid function, calcitonin, and tumor indices were within the normal range. Pathological diagnosis of whole tissue disclosed that the thyroid gland was beam-shaped with significant cell atypia, thick and granular nuclear chromatin, obvious karyokinesis, and patchy necrosis. Immunohistochemical analyses showed calcitonin-, TG-, CEA-, PAX-8-, TTF-1+, CgA+, Syn+, and Ki-67+, suggestive of CNNET with cervical lymph node metastasis (Figure 2).
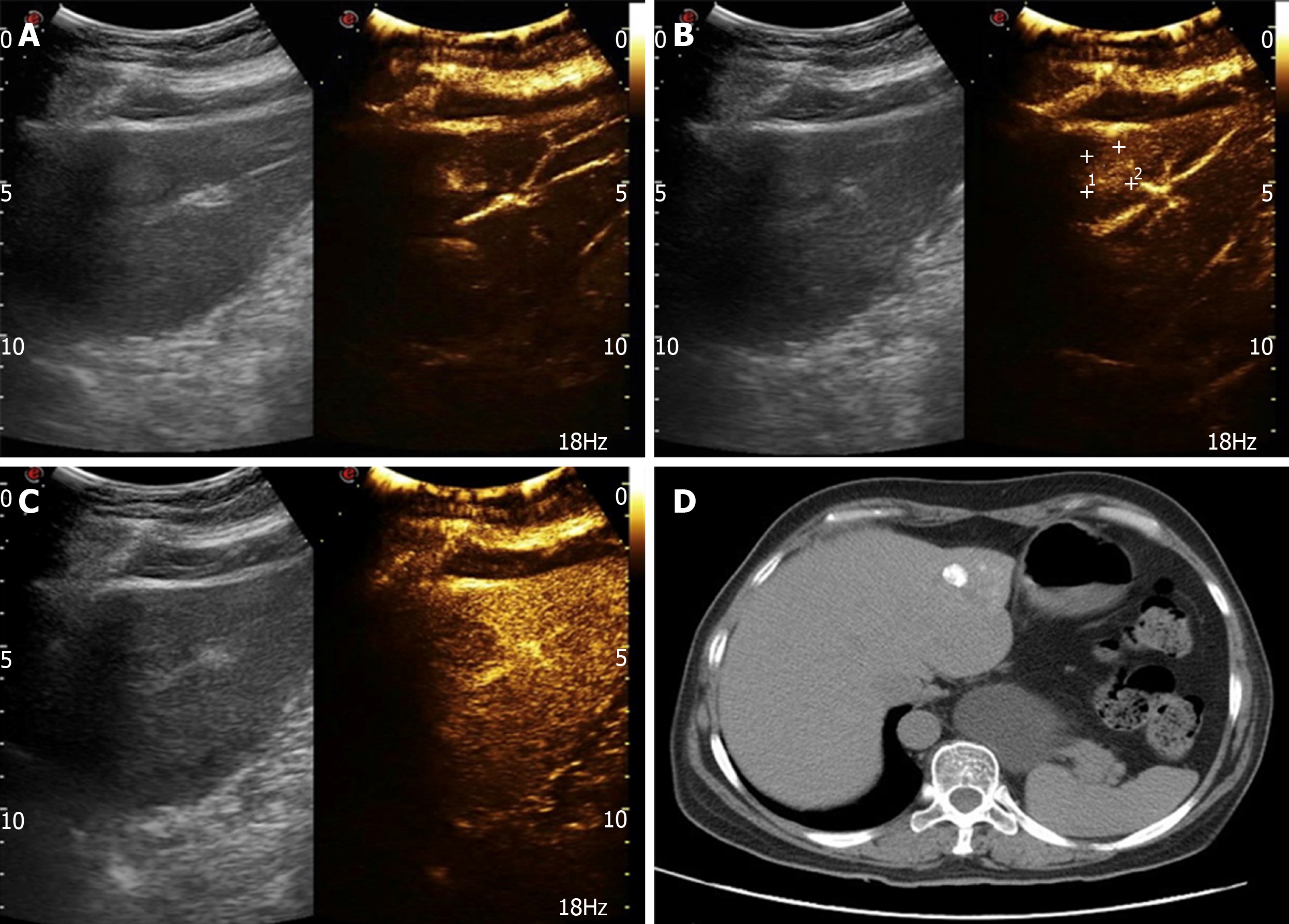
After 2 mo, the patient was readmitted due to dull upper abdominal pain. Ultrasound findings suggested an equal echo nodule in the left liver (S2) measuring 1.9 cm × 1.8 cm. The boundary was clear with a surrounding sound halo similar to the bull’s eye sign. Contrast-enhanced ultrasonography (CEUS) showed that the mass was enhanced rapidly after 13 s, reached a peak in 17 s, and showed a "fast in and fast out" performance pattern, compared with normal liver parenchyma (Figure 3A-C). In magnetic resonance images (MRI), we observed low T1 signal mass and high T2 and DWI signal mass, with a ring-shaped edge of enhancement.
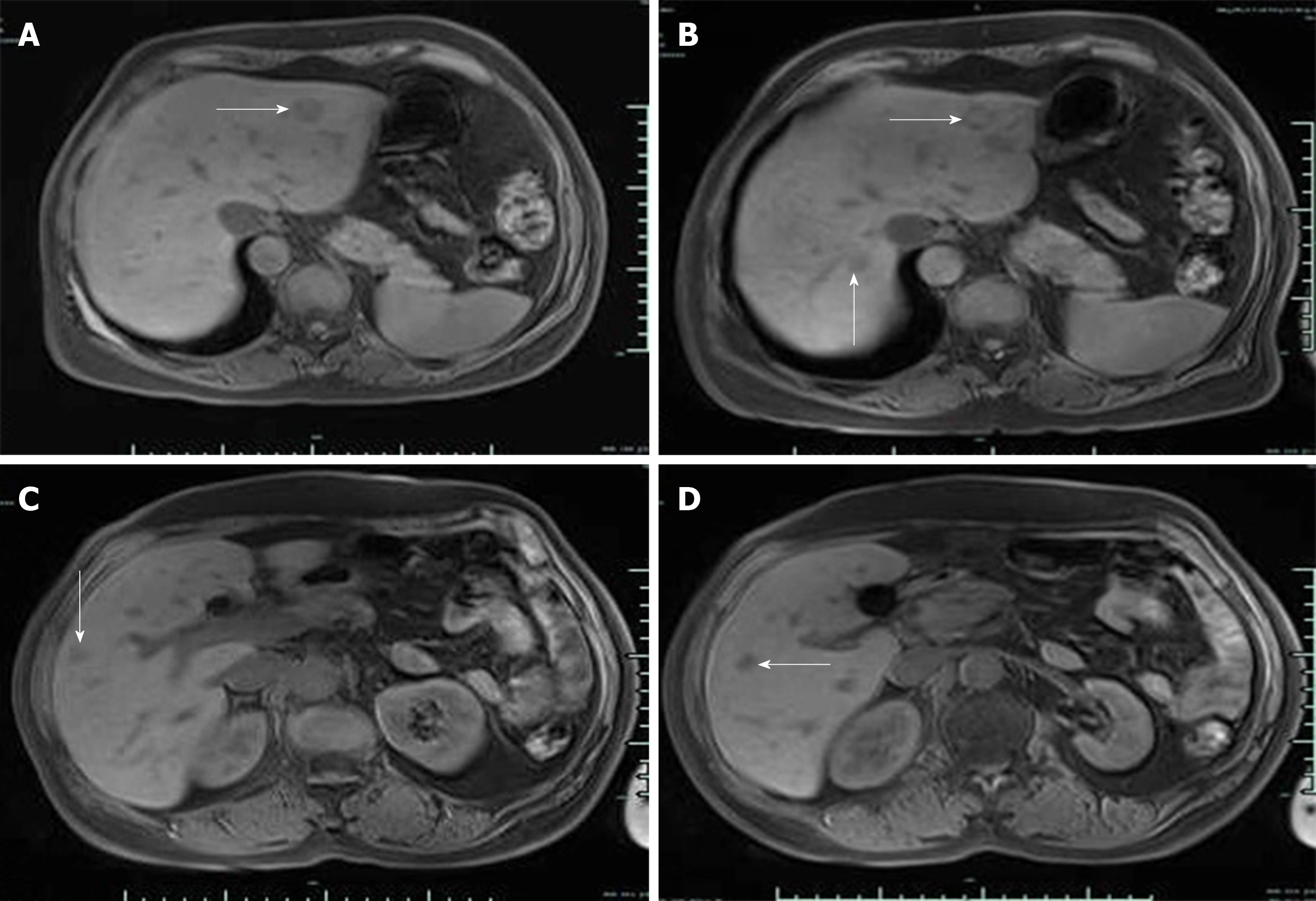
Based on the combined medical history and imaging findings, metastasis was suspected. Biopsy findings confirmed neurosecretory liver metastasis. After TACE, review of computed tomography (CT) findings showed iodized oil perfusion of the entire mass (Figure 3D). MRI performed 3 mo later revealed no significant reduction in the previous mass and multiple new metastases in the liver; the largest of which was 1.0 cm × 0.7 cm in size (Figure 4).
Calcitonin-negative neuroendocrine tumor of the thyroid with metastasis to liver.
According to the 2015 American Thyroid Association management guidelines, the patient had puncture indications, and fine needle biopsy was performed on the right lobe nodules and right lymph nodes. The results suggested that right nodules were suspicious for tumor cells and right lymph nodes should be considered for metastasis. Consequently, complete thyroidectomy was performed after exclusion of contraindication, followed by clearance of lymphoid and adipose tissue of bilateral neck in areas II, III, IV, and V. Intraoperatively, bilateral nodules with a hard texture and unclear boundary occupied almost the entire thyroid gland. Bilateral neck exploration revealed multiple enlarged lymph nodes of hard texture, although the boundary remained clear. Two months after operation, TACE treatment was elected to control metastatic tumor growth of the left liver (S2) after consultation with the patient. The tumor blood supply artery was injected with epirubicin (20 mg) and cisplatin (60 mg) through a microcatheter, followed by 5 mL super-liquefied lipiodol suspension, and the puncture site pressurized after the operation.
MRI performed 3 mo later revealed no significant reduction in the previous mass and multiple new metastases in the liver, and the patient was referred to a specialist hospital to seek treatment.
MTC is a rare thyroid NET with clinical features mainly characterized by proliferation of thyroid parafollicular cells with substantial levels of peptide hormones and calcitonin, which facilitate its differentiation from other thyroid tumors. Mutation of the RET proto-oncogene on chromosome 10 is the main underlying cause of pathogenesis in MTC[5]. CNNET is an even rarer condition, with only 17 cases documented in PubMed and no identified pathogenic factors. Early CNNET generally has no clinical symptoms, and its presence is difficult to detect until the tumor is further enlarged or accompanied by lymph node metastases.
Because the current case had a history of rectal cancer, we should also consider the possibility of rectal cancer origin. While the likelihood is low, rectal cancer metastasis to the thyroid gland has been documented[6]. Diagnosis of CNNET mainly relies on the presence of pathological immunomarkers, such as CgA, Syn, CEA, and TTF-1. In particular, TTF-1 is a transcription factor that primarily regulates expression of thyroid-specific genes, including thyroglobulin and thyroid peroxidase, which may be highly suggestive of tumor origin in the thyroid gland and often detected in MTC or CNNET[7].
CNNET has no specific manifestations in imaging and is difficult to distinguish from other tumors. Discrimination from a number of diseases is required for confirmation of diagnosis. One is thyroid paraganglioma, a rare primary NET of the thyroid gland. Histologically, this tumor presents as a solid nest, organ-like structure, with no expression of calcitonin and TTF-1, distinct from CNNETs. The value of malignant small cell tumors in differential diagnosis has also been demonstrated. Poorly differentiated small-cell medullary carcinoma with low amyloid load is similar in cell size to small cell carcinoma, malignant lymphoma, Ewing sarcoma, and small-cell malignant melanoma. However, these tumors have unique morphological characteristics, and immunohistochemistry is helpful for differentiation. The third tumor type is metastasis of lung origin. TTF-1 is expressed not only in normal thyroid C cells, follicular epithelial cells, and tumors formed by these cells but also in lung adenocarcinoma. However, cells of lung adenocarcinoma are more heterogeneous and may have adenoid structure or glandular cavity. Combined pulmonary CT and histological analyses may be applied to exclude this tumor type[7]. The characteristics of CNNET based on earlier literature are summarized in Table 1[8-22].
| Patient | Ref. | Sex/age | Tumor size in cm | Immunohistochemistry | Metastasis | ||||
| CGA | SYN | TTF-1 | CEA | TG | |||||
| 1 | Redding et al[8], 2000 | F/31 | 4.5 | NA | + | NA | NA | - | NA |
| 2 | Giovanella et al[9], 2008 | F/43 | NA | - | NA | NA | + | NA | NO |
| 3 | Wang et al[10], 2008 | M/68 | 6.5 | NA | + | + | - | NA | Lymph nodes |
| 4 | Dora et al[11], 2008 | M/43 | 1.7 | + | NA | NA | + | - | Lymph nodes |
| 5 | Alapat et al[12], 2011 | F/16 | 3.0 | + | NA | NA | + | NA | NO |
| 6 | Chernyavsky et al[13], 2011 | F/40 | 1.5 | + | + | NA | NA | + | NO |
| 7 | Nakazawa et al[14], 2014 | M/76 | 6.0 | + | - | - | - | NA | NO |
| 8 | Mussazhanova et al[15], 2014 | M/64 | 5.4 | + | + | - | - | + | NO |
| 9 | Kim et al[16], 2014 | M/34 | 0.6 | + | + | + | - | + | NO |
| 10 | Ismi et al[17], 2014 | M/57 | 15.0 | + | + | - | - | NA | Lung, peritoneum |
| 11 | Brutsaert et al[18], 2015 | F/39 | 2.6 | + | NA | + | - | NA | NO |
| 12 | Kasajima et al[19], 2016 | M/48 | 2.8 | + | + | + | + | NA | NO |
| 13 | Samà et al[20], 2016 | M/60 | 3.8 | + | NA | NA | + | NA | NO |
| 14 | Samà et al[20], 2016 | M/53 | 1.2 | - | NA | NA | - | NA | NO |
| 15 | Samà et al[20], 2016 | M/62 | 4.5 | + | NA | NA | - | NA | NO |
| 16 | Parmer et al[21], 2017 | F/74 | 1.6 | + | + | + | + | NA | NA |
| 17 | Sukpanich et al[22], 2019 | F/60 | 3.8 | + | + | + | NA | + | NO |
The 17 cases reported to date include 10 males and 7 females aged 16-76 years (mean age: 51 ± 16 years) with tumor sizes of 0.6-15 cm (average size: 4.0 ± 3.4 cm). Expression of CgA and Syn was detected in almost all cases. TTF-1, a specific marker of thyroid follicular cells, has a high positive rate in common benign and malignant thyroid lesions and is specifically expressed in the nucleus. TTF-1 results were collected in 9 cases, among which 3 cases were negative, and 6 were positive. Expression of TTF-1 was not significantly correlated with patient age or tumor size. TG, the main glycoprotein produced by the thyroid gland, is generally used as a template for thyroid hormone synthesis and storage of thyroid iodine. Differentiated thyroid carcinoma is often positive for TG. As shown in Table 1, TG data were obtained in 6 cases (2 negative and 4 positive). Further studies on a larger number of cases are required to ascertain whether TG is specific for CNNET. Owing to the lack of unique pathological characteristics, no distinctive prognostic biomarkers have been identified. Lymph node metastasis occurred in two of the cases, one of which was peritoneal metastasis with a higher degree of malignancy. The primary thyroid mass had a diameter of 15 cm. The lower boundary reached the hilar level, with Ki increment index reaching 70%. The tumor rapidly metastasized and could only be controlled by palliative chemotherapy. The mean survival rate of CNNET has not been determined due to limited information.
For smaller CNNETs, surgery is the preferred treatment of choice. According to previous reports, surgical treatment achieved good results. However, in this case, liver metastasis was detected 2 mo after surgery. Because the patient had a history of radical rectal cancer surgery a year earlier, it was necessary to exclude the possibility of rectal cancer origin. Metastases from rectal cancer usually present as well-defined hyperechoic nodules, some of which may calcify with acoustic shadows in the rear. In this case, the ultrasound was isoechoic and CEUS characterized as "fast in, fast out, high overall enhancement", similar to the performance of pancreatic NET reported by Takada et al[23]. The imaging findings were additionally more consistent with those of NET origin. The liver mass was punctured, and the thyroid confirmed as the tumor source via pathology.
The rapid development of CEUS in recent years has provided significant advantages in diagnosis of tumors with a rich blood supply. Ishikawa et al[24] reported 87.8% accuracy, 85.0% specificity, and 90.5% sensitivity of ultrasound for NET. Kitano et al[25] further assessed the sensitivity (78.9%) and specificity (98.7%) of contrast-enhanced ultrasound in diagnosis of NET with a rich blood supply. Experiments by Palazzo et al[26] indicated that multimodal ultrasound can be effectively applied to evaluate the texture of NET and predict invasiveness of tumors with high specificity (82%), accuracy (86%), sensitivity (96%), positive predictive value (71%), and negative predictive value (98%). Moreover, Takada et al[23] showed that CEUS and time-intensity curve could effectively aid in pathological classification of NET. However, metastasis of CNNET to the liver has not been reported until now, and no unified standards exist for CEUS characteristics of CNNET, which requires further investigation.
Liver metastasis could be attributed to three potential factors. First, preoperative CNNET had metastasized when the mass was small and could not be detected using ultrasound. Second, surgical excision led to hemorrhagic metastasis. Third, the degree of malignancy itself was higher. The third option was the most feasible because the CNNET blood supply in this case was extremely rich, and Ki increment index was 20%. In a study by Grozinsky-Glasberg et al[27], single treatment of eight cases of MTC metastatic tumors to the liver with TACE achieved a degree of therapeutic efficacy. However, in the current case, the mass did not shrink significantly after TACE, and several new lesions were detected. According to the guidelines of the American Association for the Study of Liver Diseases[28], efficacy evaluation for this patient was assessed as progressive disease, confirmed by the high degree of malignancy.
For CNNET, poor prognostic factors include distant or lymph node metastasis, infiltration of the capsule, advanced age, and significant elevation of Ki. Based on the limited existing knowledge, it is difficult to prevent its occurrence, and early stages often develop with no symptoms, presenting a significant challenge for clinicians. Some MTCs do not secrete calcitonin at the early stage, which may be related to low secretory behavior of the tumor. Therefore, regular review of calcitonin and CEA levels after CNNET surgery is necessary. Follow-up thyroid ultrasound, lung CT, abdominal MRI, and other examinations are critical to improve patient prognosis. Fluorine-18-fluorodeoxyglucose positron emission tomography/computed tomography may be used as an effective complementary method to detect smaller tumors.
In conclusion, our understanding of the etiology and pathogenesis of CNNET is limited owing to the rarity of the disease. Further information on clinical progression and accumulating experience of the disease is essential to improve therapeutic outcomes.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Coskun A, Handra-Luca A, Papadakis M S-Editor: Gong ZM L-Editor: Filipodia E-Editor: Qi LL
| 1. | Wells SA, Asa SL, Dralle H, Elisei R, Evans DB, Gagel RF, Lee N, Machens A, Moley JF, Pacini F, Raue F, Frank-Raue K, Robinson B, Rosenthal MS, Santoro M, Schlumberger M, Shah M, Waguespack SG; American Thyroid Association Guidelines Task Force on Medullary Thyroid Carcinoma. Revised American Thyroid Association guidelines for the management of medullary thyroid carcinoma. Thyroid. 2015;25:567-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1802] [Cited by in RCA: 1463] [Article Influence: 146.3] [Reference Citation Analysis (0)] |
| 2. | Storani ME, Bostico ST, Subies FA, Musich M, Oneto A. [Routine serum calcitonin measurement in thyroid nodules]. Medicina (B Aires). 2019;79:271-275. [PubMed] |
| 3. | Trimboli P, Lauretta R, Barnabei A, Valabrega S, Romanelli F, Giovanella L, Appetecchia M. Procalcitonin as a postoperative marker in the follow-up of patients affected by medullary thyroid carcinoma. Int J Biol Markers. 2018;33:156-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 4. | Schmid KW, Ensinger C. "Atypical" medullary thyroid carcinoma with little or no calcitonin expression. Virchows Arch. 1998;433:209-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Ting S, Synoracki S, Schmid KW. [Thyroid C cells and their pathology: Part 1: normal C cells, - C cell hyperplasia, - precursor of familial medullary thyroid carcinoma]. Pathologe. 2015;36:246-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Minami S, Inoue K, Irie J, Mine T, Tada N, Hirabaru M, Noda K, Ito S, Haraguchi M. Metastasis of colon cancer to the thyroid and cervical lymph nodes: a case report. Surg Case Rep. 2016;2:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Katoh R, Miyagi E, Nakamura N, Li X, Suzuki K, Kakudo K, Kobayashi M, Kawaoi A. Expression of thyroid transcription factor-1 (TTF-1) in human C cells and medullary thyroid carcinomas. Hum Pathol. 2000;31:386-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 72] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 8. | Redding AH, Levine SN, Fowler MR. Normal preoperative calcitonin levels do not always exclude medullary thyroid carcinoma in patients with large palpable thyroid masses. Thyroid. 2000;10:919-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 9. | Giovanella L, Crippa S, Cariani L. Serum calcitonin-negative medullary thyroid carcinoma: role of CgA and CEA as complementary markers. Int J Biol Markers. 2008;23:129-131. [PubMed] |
| 10. | Wang TS, Ocal IT, Sosa JA, Cox H, Roman S. Medullary thyroid carcinoma without marked elevation of calcitonin: a diagnostic and surveillance dilemma. Thyroid. 2008;18:889-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Dora JM, Canalli MH, Capp C, Puñales MK, Vieira JG, Maia AL. Normal perioperative serum calcitonin levels in patients with advanced medullary thyroid carcinoma: case report and review of the literature. Thyroid. 2008;18:895-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 12. | Alapat DV, Ain KB, Sloan DA, Monaghan KG, Karabakhtsian RG. Disparity between tissue and serum calcitonin and carcinoembryonic antigen in a patient with medullary thyroid carcinoma. Endocrine. 2011;39:148-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Chernyavsky VS, Farghani S, Davidov T, Ma L, Barnard N, Amorosa LF, Trooskin SZ. Calcitonin-negative neuroendocrine tumor of the thyroid: a distinct clinical entity. Thyroid. 2011;21:193-196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Nakazawa T, Cameselle-Teijeiro J, Vinagre J, Soares P, Rousseau E, Eloy C, Sobrinho-Simões M. C-cell-derived calcitonin-free neuroendocrine carcinoma of the thyroid: the diagnostic importance of CGRP immunoreactivity. Int J Surg Pathol. 2014;22:530-535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 28] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 15. | Mussazhanova Z, Miura S, Stanojevic B, Rougounovitch T, Saenko V, Shiraishi T, Kurashige T, Shichijo K, Kaneko K, Takahashi H, Ito M, Nakashima M. Radiation-associated small cell neuroendocrine carcinoma of the thyroid: a case report with molecular analyses. Thyroid. 2014;24:593-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 16. | Kim GY, Park CY, Cho CH, Park JS, Jung ED, Jeon EJ. A Calcitonin-Negative Neuroendocrine Tumor Derived from Follicular Lesions of the Thyroid. Endocrinol Metab (Seoul). 2015;30:221-225. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 17. | Ismi O, Arpaci RB, Berkesoglu M, Dag A, Sezer E, Bal KK, Vayısoğlu Y. Calcitonin-negative neuroendocrine tumor of thyroid gland mimicking anaplastic carcinoma: an unusual entity. Gland Surg. 2015;4:344-349. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 18. | Brutsaert EF, Gersten AJ, Tassler AB, Surks MI. Medullary thyroid cancer with undetectable serum calcitonin. J Clin Endocrinol Metab. 2015;100:337-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 19. | Kasajima A, Cameselle-Teijeiro J, Loidi L, Takahashi Y, Nakashima N, Sato S, Fujishima F, Watanabe M, Nakazawa T, Naganuma H, Kondo T, Kato R, Sasano H. A Calcitonin Non-producing Neuroendocrine Tumor of the Thyroid Gland. Endocr Pathol. 2016;27:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 20. | Samà MT, Rossetto Giaccherino R, Gallo M, Felicetti F, Maletta F, Bonelli N, Piovesan A, Palestini N, Ghigo E, Arvat E. Clinical challenges with calcitonin-negative medullary thyroid carcinoma. J Cancer Res Clin Oncol. 2016;142:2023-2029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 21. | Parmer M, Milan S, Torabi A. Calcitonin-Negative Neuroendocrine Tumor of the Thyroid. Int J Surg Pathol. 2017;25:191-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 22. | Sukpanich R, Khanafshar E, Suh I, Gosnell J. Case report of a neuroendocrine tumor of the thyroid gland with limited calcitonin expression: a diagnostic challenge. AME Case Rep. 2019;3:12. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Takada S, Kato H, Saragai Y, Muro S, Uchida D, Tomoda T, Matsumoto K, Horiguchi S, Tanaka N, Okada H. Contrast-enhanced harmonic endoscopic ultrasound using time-intensity curve analysis predicts pathological grade of pancreatic neuroendocrine neoplasm. J Med Ultrason (2001). 2019;46:449-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 20] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 24. | Ishikawa T, Itoh A, Kawashima H, Ohno E, Matsubara H, Itoh Y, Nakamura Y, Nakamura M, Miyahara R, Hayashi K, Ishigami M, Katano Y, Ohmiya N, Goto H, Hirooka Y. Usefulness of EUS combined with contrast-enhancement in the differential diagnosis of malignant versus benign and preoperative localization of pancreatic endocrine tumors. Gastrointest Endosc. 2010;71:951-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 25. | Kitano M, Kudo M, Yamao K, Takagi T, Sakamoto H, Komaki T, Kamata K, Imai H, Chiba Y, Okada M, Murakami T, Takeyama Y. Characterization of small solid tumors in the pancreas: the value of contrast-enhanced harmonic endoscopic ultrasonography. Am J Gastroenterol. 2012;107:303-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 241] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 26. | Palazzo M, Napoléon B, Gincul R, Pioche M, Pujol B, Lefort C, Fumex F, Hautefeuille V, Fabre M, Cros J, Felce M, Couvelard A, Sauvanet A, Lévy P, Ruszniewski P, Palazzo L. Contrast harmonic EUS for the prediction of pancreatic neuroendocrine tumor aggressiveness (with videos). Gastrointest Endosc. 2018;87:1481-1488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 27. | Grozinsky-Glasberg S, Bloom AI, Lev-Cohain N, Klimov A, Besiso H, Gross DJ. The role of hepatic trans-arterial chemoembolization in metastatic medullary thyroid carcinoma: a specialist center experience and review of the literature. Eur J Endocrinol. 2017;176:463-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 28. | Lencioni R, Llovet JM. Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis. 2010;30:52-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3353] [Cited by in RCA: 3301] [Article Influence: 220.1] [Reference Citation Analysis (36)] |