Published online Jan 6, 2020. doi: 10.12998/wjcc.v8.i1.126
Peer-review started: October 26, 2019
First decision: November 21, 2019
Revised: November 22, 2019
Accepted: November 30, 2019
Article in press: November 30, 2019
Published online: January 6, 2020
Processing time: 72 Days and 22.2 Hours
Sarcomas of the head and neck region are rare tumors, constituting less than 1% of malignant neoplasms in this area, of which few cases (20%) originate from bone or cartilage. Chondrosarcoma is a malignant neoplasm that develops in bone, with a predilection for the pelvis, chest wall, and scapula, and is uncommon in the maxilla and jaw. Although this type of lesion has locally aggressive behavior, destroying the affected bone, it can metastasize when it is not diagnosed early and compromise the patient's life.
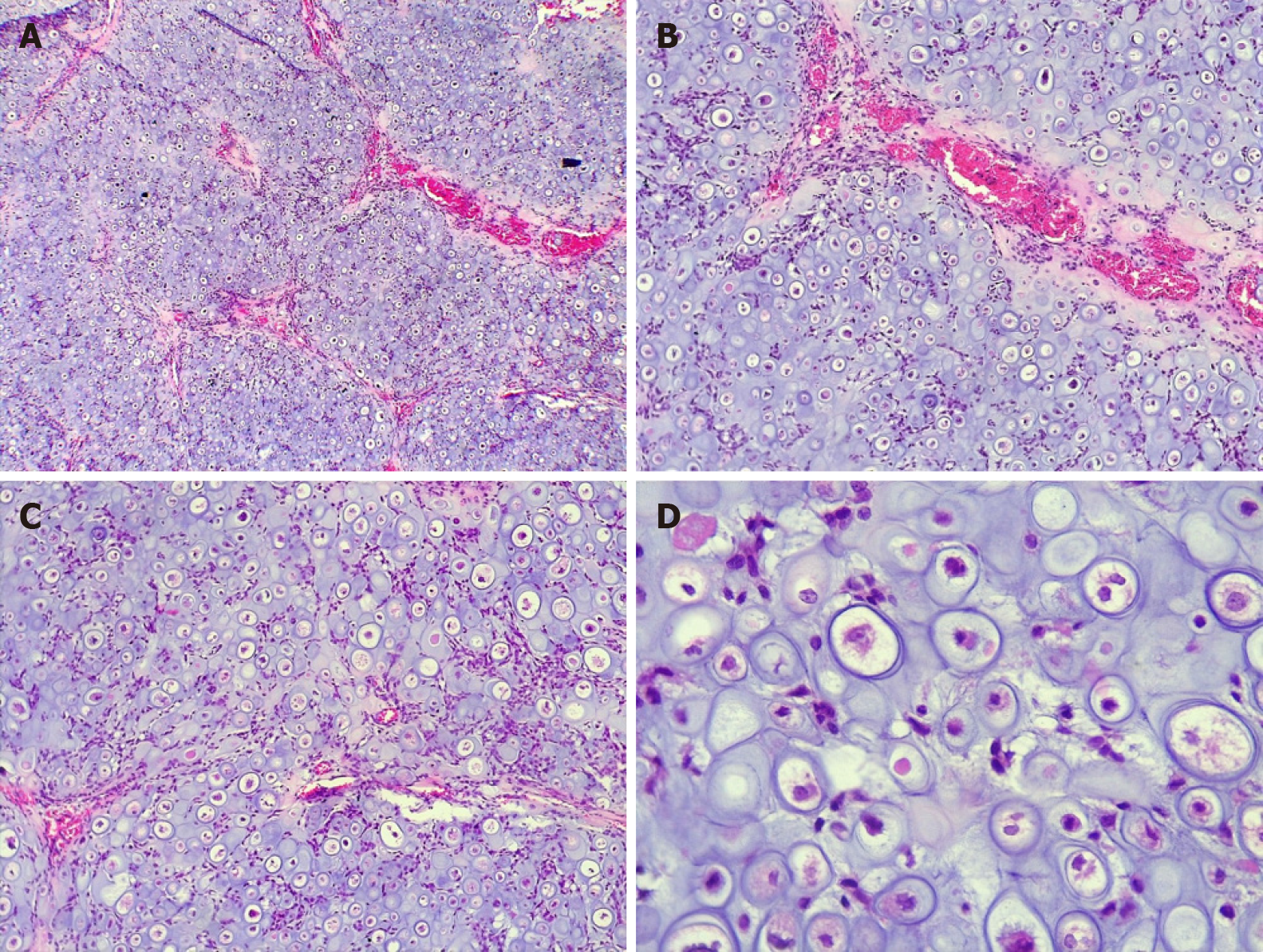
On intraoral examination of a 32-year-old female with a tumor in the middle third of the face, a well-defined rise in volume of approximately 3 cm in diameter was observed. Computed tomography with 3-dimensional reconstruction was performed, and we observed that the osteolytic lesion affected the vestibular cortex as the palatal bone. Hematoxylin and eosin staining revealed an appearance that was similar to mature hyaline cartilage, hypercellularity, nuclear and cellular pleomorphism, and multinucleated cells, with significant vacuolization.
Determination of the clinical and histopathological characteristics of rare neoplasms in the maxillofacial region, such as chondrosarcomas, allows the pathologist and surgeon to make the appropriate therapeutic decisions, optimizing the patient’s prognosis.
Core tip: Sarcomas of the head and neck region are rare tumors, constituting less than 1% of malignant neoplasms in this area. Although this type of lesion has locally aggressive behavior, destroying the affected bone, it can metastasize when it is not diagnosed early and compromise the patient’s life. Our clinical case is consistent with the age at which chondrosarcoma usually occurs, but this diagnosis was not considered, due its apparently indolent and asymptomatic evolution and lack of pathological changes in the lining mucosa.
- Citation: Cuevas-González JC, Reyes-Escalera JO, González JL, Sánchez-Romero C, Espinosa-Cristóbal LF, Reyes-López SY, Tovar Carrillo KL, Donohue Cornejo A. Primary maxillary chondrosarcoma: A case report. World J Clin Cases 2020; 8(1): 126-132
- URL: https://www.wjgnet.com/2307-8960/full/v8/i1/126.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i1.126
Sarcomas of the head and neck region are rare tumors, constituting less than 1% of malignant neoplasms in this area, of which few cases (20%) originate from bone or cartilage[1].
Chondrosarcoma is a malignant neoplasm that develops in bone, with a predilection for the pelvis, chest wall, and scapula, and is uncommon in the maxilla and jaw[2]. Although this type of lesion has locally aggressive behavior, destroying the affected bone, it can metastasize when it is not diagnosed early and compromise the patient's life[3]. Thus, we present this clinical case, highlighting the main clinical, radiographic, and histopathological characteristics, to improve the identification and diagnosis of this neoplasm.
A 32-year-old female visited our maxillofacial surgery service due to facial asymmetry affecting the middle third of the face, as well as an increase in volume in the palatal and vestibular regions of the left side.
The patient was under orthodontic treatment and had noticed and complained of intraoral bulking for at least 9 mo. The orthodontist overlooked the clinical and radiographic alterations and continued the treatment. Within this time, the patient consulted a periodontist, who stated that the lesion was reactive in nature, with no further examination or treatment needed. The tumor increased progressively, causing facial asymmetry, for which the patient sought a third opinion and visited our maxillofacial surgery service.
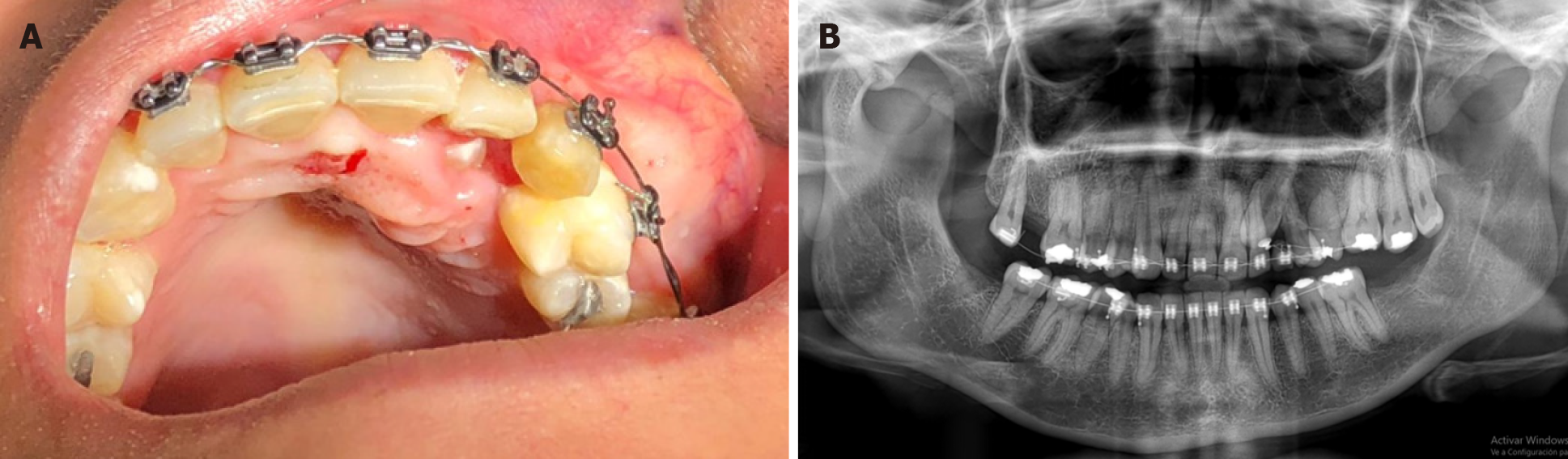
On intraoral examination, a well-defined rise in volume of approximately 3 cm in diameter affecting the left palatal and vestibular regions was observed. The mucosa was normochromic, and the canine was retained in the palatal region, and the vestibular mucosa presented with a yellowish and telangiectatic surface, with an underlying multilobulated tumor mass (Figure 1A).
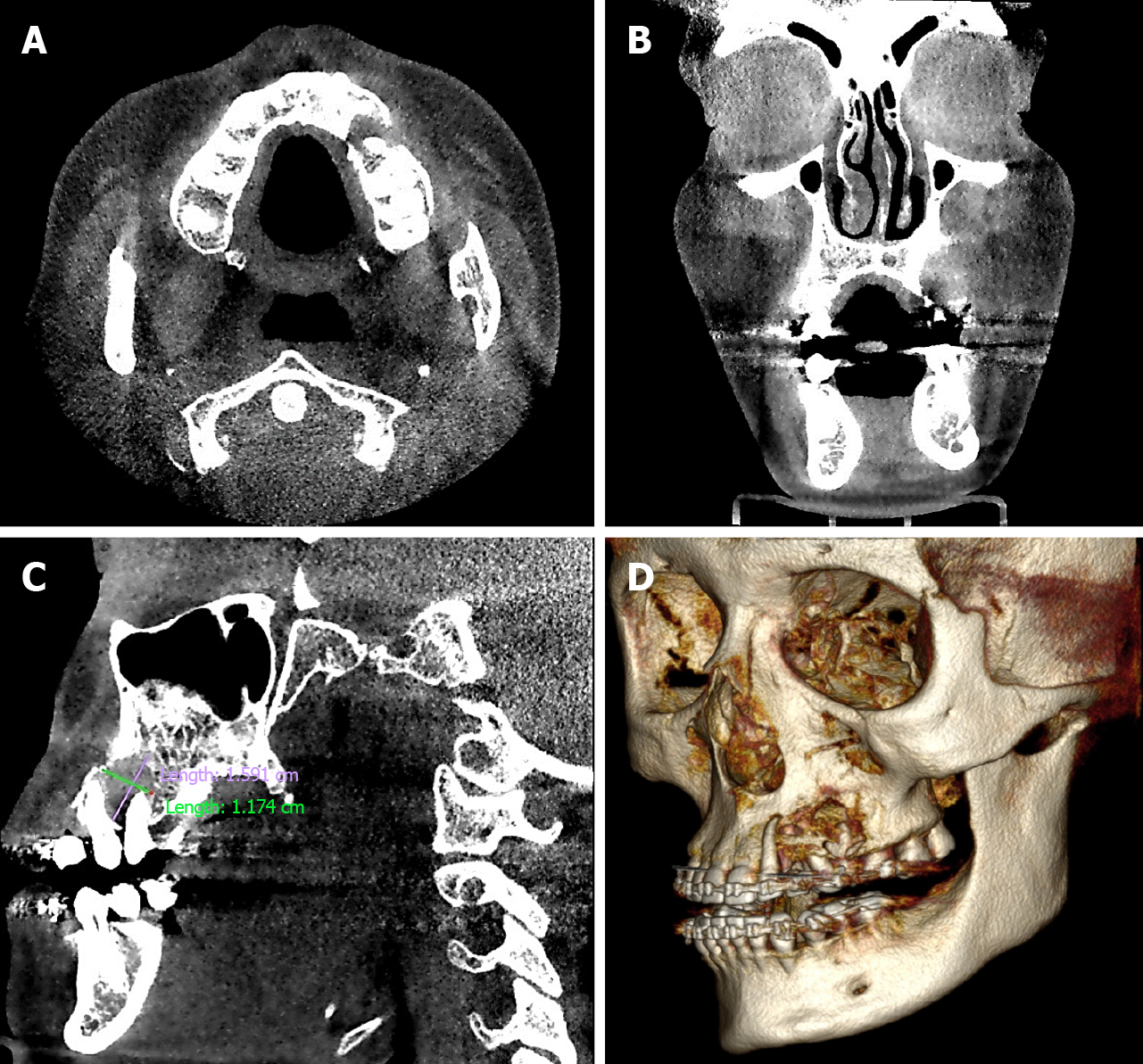
We performed orthopantomography and identified a small radiolucent area at the level of the premolars on the left side (Figure 1B). Noting that the lesion involved bone tissue, computed tomography with 3-dimensional reconstruction was performed (Figure 2), allowing us to determine the extent of the tumor - we observed that the osteolytic lesion affected the vestibular cortex as the palatal bone. Until this point, our differential diagnosis was oriented toward a benign neoplasm (odontogenic myxoma); thus, an excisional biopsy was performed and analyzed by histopathology.
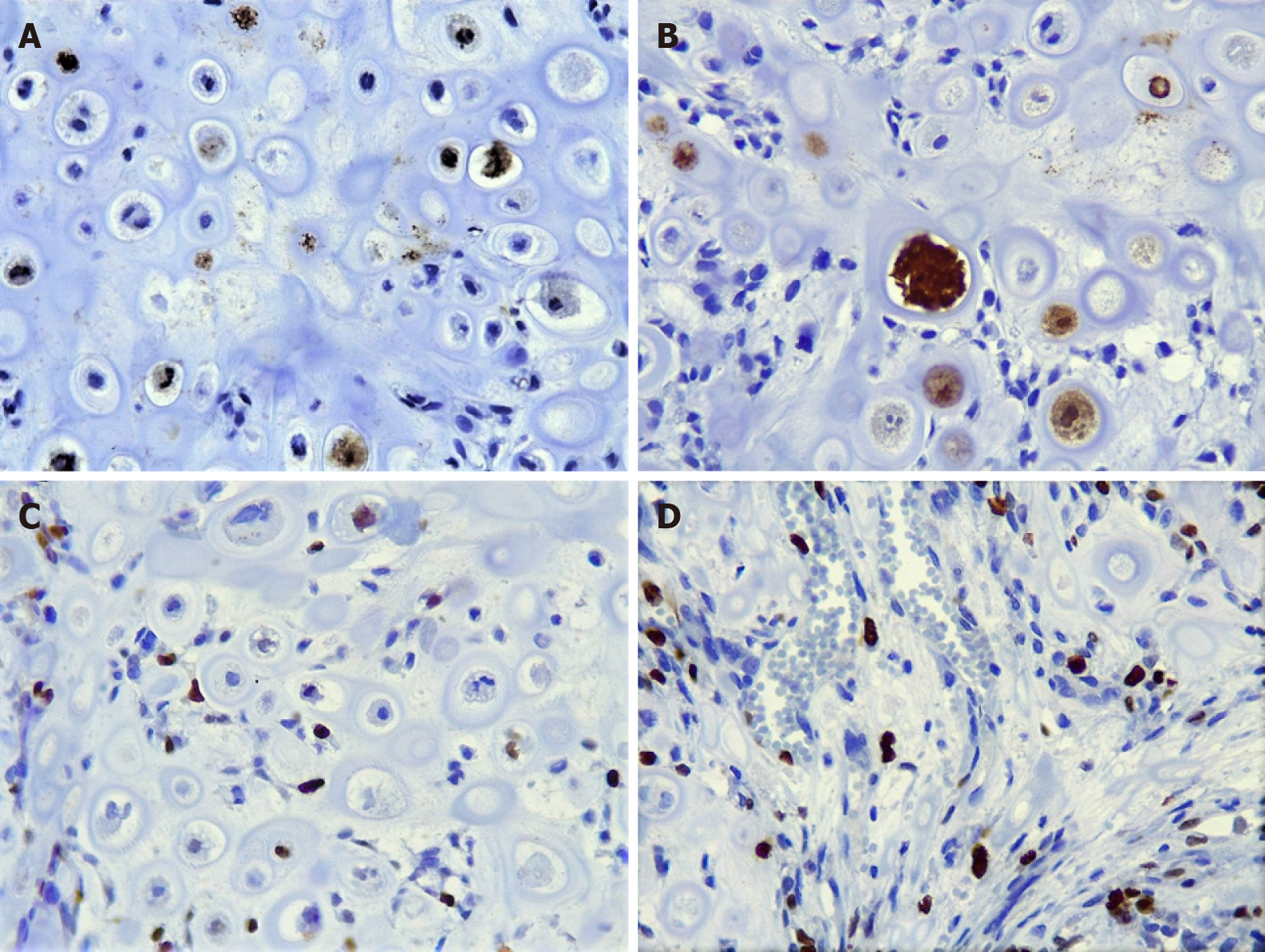
The hematoxylin and eosin (H and E) stain revealed an appearance that was similar to mature hyaline cartilage, hypercellularity, nuclear and cellular pleomorphism, and multinucleated cells, with significant vacuolization. The tumor cells had a circumscribed multilobular pattern, with focal areas of infiltration into the surrounding bone tissue. The focal presence of a component of small ovoid, hyperchromatic, and undifferentiated cells was observed, interspersing the cartilaginous lobes; osteoid matrix formation was absent. To complement the histopathological analysis, we performed immunohistochemistry, staining for S-100 and Ki-67, which showed moderate positivity (50% and 25% respectively), whereas the pan-cytokeratin AE1/AE3 was negative.
These results led us to a definitive diagnosis of grade II chondrosarcoma (Figures 3 and 4).
The patient was referred to the oncology service to rule out the possibility of tumor permanence or metastasis.
Close long-term follow-up.
The relevance of this case report is based on 2 main points: (1) Despite its rarity in the jaws, the diagnosis of chondrosarcoma and other malignancies should be considered, even in lesions with apparent indolent behavior, especially when cortical destruction is observed; and (2) The delay of 9 mo in the diagnosis of this chondrosarcoma highlights the lack of attention to oral pathologies by some clinicians, because the patient complained about the tumor to 2 specialist dentists who did not take any action, until a third specialist performed the biopsy. Therefore, the communication of these clinical cases is paramount.
Chondrosarcoma is a slow-growing neoplasm, characterized by the formation of malignant cartilage that is derived from cartilaginous embryogenic remains. Although several groups have suggested that it affects both sexes equally, others have reported a slight predilection in males, primarily between the second and fifth decades of life, peaking in incidence at age 31 years. Chondrosarcoma rarely affects the jaws (about 224 cases in the literature). Clinically, it usually resembles other less aggressive disorder, delaying the diagnosis[4-6].
Our clinical case is consistent with the age at which chondrosarcoma usually occurs, but this diagnosis was not considered, due its apparently indolent and asymptomatic evolution and lack of pathological changes in the lining mucosa. The patient had routinely attended a dental practice, and it was not until the tumor caused obvious asymmetry that it was referred to a maxillofacial surgeon, affecting the patient’s prognosis. This case is interesting because it shows that that some specialists and general practice dentists do not give due importance to volume increases or do not identify them due to inadequate oral clinical examination, limiting themselves to treating the reason for the consultation and overlooking other situations that need to be identified in a timely manner and Do not compromise as in this case the patient's life.
The radiographic profile of this sarcoma is nonspecific - it can be radiolucent or radiopaque; present with well-circumscribed mixed lesions; or assume a partially delimited, generally unilocular pattern. Due to these heterogeneous characteristics, it is difficult to define the extent of the lesion[2]. Using imaging tools, such as computed tomography, it is possible to identify the expansive, osteolytic, and well-defined tumor mass of the lesion[6], which is why we decided to perform orthopantomography to determine the involvement of the bone tissue. Conversely, computed tomography provided more information, revealing the destruction of bone tissue in greater detail. Based on the clinical and imaging features, the main diagnosis was a benign neoplasm, likely odontogenic, due to the presence of a retained canine. Thus, we decided to perform an excisional biopsy for histopathological analysis to establish a definitive diagnosis.
Chondrosarcoma has several histological subtypes: conventional intramedullary, juxtacortical, clear cell, myxoid, mesenchymal, dedifferentiated, and extraskeletal. The conventional subtype is the most frequent[7,8]; microscopically, it has well-differentiated hyaline cartilage matrix with a lobular appearance and harbors large and irregular lagoons, with binucleation, pleomorphism, and evident nucleoli in the chondroid matrix[9].
The main histopathological differential diagnosis of moderately differentiated chondrosarcoma (grade II) is chondroma, which is even rarer in gnathic bones. The World Health Organization states that any tumor with exclusively chondroblastic differentiation in this region should initially be considered a chondrosarcoma, until proven otherwise[10]. Other differential diagnoses of chondrosarcoma include chondroblastic osteosarcoma, mesenchymal chondrosarcoma, chondroblastoma, and, in the area of the temporomandibular joint, synovial chondromatosis[10,11]. The presence of multiple foci of hyaline cartilage - well differentiated or with atypia - associated with the proliferation of dedifferentiated small cells, invasion of the surrounding bone, cortical destruction, and the absence of osteoid matrix production are important criteria in the diagnosis of this neoplasm[7,10]. This case report represents a conventional chondrosarcoma that might present with difficulties when establishing the diagnosis. However, unlike other cases in the literature, because the sample consisted primarily of hyaline cartilage with marked cellular atypia and the lack of ossification or osteoid material, chondrosarcoma was considered as the diagnosis from the outset.
The histopathological diagnosis can be supported by immunohistochemistry. Cho et al[12] studied several protein biomarkers in 7 cases of chondrosarcomas, reporting that S-100 and D2-40 are expressed intensely and uniformly, whereas AE1/AE3, EMA, GFAP, β-cat, and CEA were negative. Consistent with these findings, we observed nuclear S-100 staining in areas of cartilaginous formation. Although the reaction was intense and useful at the time of the diagnosis, S-100 is a sensitive marker but not specific for chondrosarcoma, because it can be expressed in various types of neoplasms. Thus, with regard to its interpretation, it is extremely important to consider the histomorphological, clinical, and imaging aspects of the lesion.
Evans et al[13] classified this tumor using 3 grades, for which they considered the number of mitoses, cellularity, and size of the nucleus. The 5-year survival rates are 90% for grade I, 81% for grade II, and 43% for grade III tumors[9]. de Souza et al[5] emphasized that cases that are treated with radical surgery have a better prognosis compared with those that undergo conservative surgery. In some cases, radiotherapy or chemotherapy is chosen, but the treatment should be considered tailored according to the clinical and histopathological features of each case.
They also reported a percentage of metastasis of 7.6% and a recurrence rate of 25.4%, which are undoubtedly parameters that can help us understand the behavior of chondrosarcoma[5]. In addition to counting the number of mitoses per field by H and E staining, we included Ki-67 in our immunohistochemical panel, which was moderately positive, leading to a diagnosis of grade II chondrosarcoma, which is extremely important for establishing the treatment.
An early diagnosis will allow surgical procedures to be performed in patients, yielding a good long-term prognosis[3]. In our case, the diagnosis was made using tissue that was acquired by incisional biopsy, after which the patient underwent a second surgical event, in which the tumor was completely removed, with free margins. The specimen was reevaluated, confirming the diagnosis of chondrosarcoma. In conclusion, the identification of the clinical and histopathological characteristics of rare neoplasms in the maxillofacial region, such as chondrosarcomas, allows the pathologist and surgeon to make the appropriate therapeutic decisions, optimizing the patient's prognosis.
It is necessary that both the general practice dentist and specialist perform a systematic and detailed oral clinical examination, in order to avoid delays in the diagnosis and treatment of malignancies, as occurred in the present case, potentially impacting the prognosis and survival of patients. Despite its rarity in the jaws, the diagnosis of chondrosarcoma and other malignancies should be considered even in lesions with an indolent clinical appearance, especially when cortical destruction is observed. Imaging studies and histopathological analyses are mandatory, and immunohistochemistry can be helpful to achieve the correct diagnosis of chondrosarcoma and other rare neoplasms of the gnathic bones.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: D'Orazi V, Yang LY S-Editor: Dou Y L-Editor: A E-Editor: Liu JH
| 1. | Tan HB, Rimmer J. Nasal Chondrosarcoma of the Lower Lateral Cartilage. Medicina (Kaunas). 2019;55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 2. | Hackney FL, Aragon SB, Aufdemorte TB, Holt GR, Van Sickels JE. Chondrosarcoma of the jaws: clinical findings, histopathology, and treatment. Oral Surg Oral Med Oral Pathol. 1991;71:139-143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 35] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Brimioulle M, Bowles PF, Pelser A. Maxillary chondrosarcoma mimicking torus palatinus. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (1)] |
| 4. | Giorgione C, Passali FM, Varakliotis T, Sibilia M, Ottaviani F. Temporo-mandibular joint chondrosarcoma: Case report and review of the literature. Acta Otorhinolaryngol Ital. 2015;35:208-211. [PubMed] |
| 5. | de Souza LL, Pontes FSC, Fonseca FP, da Mata Rezende DS, Vasconcelos VCS, Pontes HAR. Chondrosarcoma of the jaw bones: a review of 224 cases reported to date and an analysis of prognostic factors. Int J Oral Maxillofac Surg. 2019;48:452-460. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 6. | Karadwal A, Chatterjee S. Chondrosarcoma of maxilla. J Oral Maxillofac Pathol. 2018;22:S35-S38. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 7. | Angiero F. Extraskeletal myxoid chondrosarcoma of the left buccal mucosa. Anticancer Res. 2012;32:3345-3350. [PubMed] |
| 8. | Pasta V, Sottile D, Urciuoli P, Del Vecchio L, Custureri F, D'Orazi V. Rare chondrosarcoma of the breast treated with quadrantectomy instead of mastectomy: A case report. Oncol Lett. 2015;9:1116-1120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 9. | Maruya S, Kurotaki H, Fujita S, Sariishi T, Shinkawa H, Yagihashi S. Primary chondrosarcoma arising in the parotid gland. ORL J Otorhinolaryngol Relat Spec. 2001;63:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 12] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | El-Naggar A, Chan JK, Grandis JR, Takata T, Slootweg PJ. WHO Classification of Head and Neck Tumours. 4th ed. Lyon: International Agency for Research on Cancer (IARC), 2017. . |
| 11. | Chen W, DiFrancesco LM. Chondroblastoma: An Update. Arch Pathol Lab Med. 2017;141:867-871. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 77] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 12. | Cho HY, Lee M, Takei H, Dancer J, Ro JY, Zhai QJ. Immunohistochemical comparison of chordoma with chondrosarcoma, myxopapillary ependymoma, and chordoid meningioma. Appl Immunohistochem Mol Morphol. 2009;17:131-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 13. | Evans HL, Ayala AG, Romsdahl MM. Prognostic factors in chondrosarcoma of bone: a clinicopathologic analysis with emphasis on histologic grading. Cancer. 1977;40:818-831. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |