Published online Jan 6, 2020. doi: 10.12998/wjcc.v8.i1.11
Peer-review started: October 7, 2019
First decision: November 13, 2019
Revised: November 18, 2019
Accepted: November 30, 2019
Article in press: November 30, 2019
Published online: January 6, 2020
Processing time: 91 Days and 8.6 Hours
As one of the subsets of CD8+ T cells, Tc17 cells have recently been identified and are characterized by the secretion of interleukin (IL)-17, which is related to inflammatory diseases.
To assess the status of Tc17 cells in cervical cancer and investigate the biological function of Tc17 cells in cervical cancer development.
Flow cytometry assay, immunohistochemistry, and immunofluorescence were performed to detect the levels and phenotype of Tc17 cells in blood and tumor samples from patients with cervical cancer. Prior to cell suspension culture, ELISA was carried out to measure the production of IL-6, IL-1β, IL-23, CXCL12, and IL-17 in tumor tissue supernatant and co-cultured supernatant of patients with cervical cancer. In addition, multivariate analysis was performed to identify factors associated with overall survival using the Cox proportional hazards model.
Compared with normal tissues, Tc17 cells specifically accumulated in tumor tissues of cervical cancer patients. Cancer cells produced a greater amount of IL-6, IL-1β, and IL-23, which in turn promoted Tc17 cell polarization. Unlike the traditional cytotoxic CD8+ T cells, Tc17 cells secreted IL-17, which subsequently promoted CXCL12 expression in tumor cells, eventually enhancing the proliferation and migration of tumor cells. Thus, the ratio of tumor-infiltrating Tc17 cells was highly correlated with poor clinical outcome in patients with cervical cancer.
Our data identified the oncogenic role of Tc17 cells in the development of cervical cancer. We propose that the ratio of Tc17 cells may be a useful index in the prognosis of patients with cervical cancer.
Core tip: Inflammation contributes to cancer development. In this study, it was found that cervical cancer-elicited inflammation increased Tc17-polarizing cytokine production, which attenuated cytotoxic CD8+ T cell development. The high level of interleukin-17 production by Tc17 cells led to CXCL12 upregulation and cancer cell migration. Consistent with the oncogenic role of Tc17 cells in cancer development, the ratio of cancer-infiltrating Tc17 cells was highly associated with poor prognosis in patients with cervical cancer. Thus, our data demonstrate that Tc17 cells can be induced in cervical cancers and serve as a meaningful index in the prognosis of patients with cervical cancer.
- Citation: Zhang ZS, Gu Y, Liu BG, Tang H, Hua Y, Wang J. Oncogenic role of Tc17 cells in cervical cancer development. World J Clin Cases 2020; 8(1): 11-19
- URL: https://www.wjgnet.com/2307-8960/full/v8/i1/11.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v8.i1.11
As the fourth most common malignant tumor worldwide, the incidence of new cervical cancer is approximately 130000, accounting for 28% of the total number of cases globally. About 20000 women die of cervical cancer each year[1,2]. Tumor progression has been recognized to be the product of evolving crosstalk between immune cells and tumor cells. By affecting immune cell activation or differentiation, cancer cells escape host immune attack and enhance tumor resistance to immunotherapies[3,4].
As the primary component of tumor-infiltrating lymphocytes, T cells elicit a crucial effector function in cancer eradication. Recent studies showed that T cells that produce interleukin (IL)-17 are detected in human tumors, which have a certain pro-inflammatory effect[3]. On the one hand, Th17 cell polarizing factor may induce differentiation and proliferation of Tc17 cells, despite exhibiting reduced cytotoxic activity in CD8+ T cells, thereby interrupting host immune surveillance[5,6]. Moreover, in a monkey immunodeficiency virus infection model with mammals such as macaques and black-and-white marmosets, Tc17 cells play a pathological role in promoting disease progression[7,8]. However, the role of Tc17 cells in human cervical cancer development remains unclear.
A large number of Tc17 cells were found in black and white monkey tumor tissues, which were induced by the cytokine IL-23[8]. At the same time, other studies have shown that IL-6 may also be crucial for Tc17 cell differentiation[9]. However, it is unclear whether other cytokines can induce Tc17 cell differentiation and how Tc17 cells affect cancer development is still poorly understood.
In this study, we showed that Tc17 cells are highly enriched within cervical cancer tissue. Cervical cancer cells produced a large amount of IL-6, IL-1β, and IL-23, which induced Tc17 cell polarization. Increased levels of IL-17 induced by Tc17 cells led to CXCL12 upregulation in tumor cells, resulting in tumor cell proliferation and migration. Moreover, the percentage of Tc17 cells was associated with tumor progression and clinical outcome in patients with cervical cancer. Our data demonstrate the oncogenic role of Tc17 cells in cancer development and provide a theoretical basis for the clinical treatment of cervical cancer.
Fresh blood, tumor, peritumoral, or matched adjacent tissues (at least 5 cm distant from the tumor site) were obtained from patients with cervical cancer who underwent surgical resection at the Shanghai Seventh People’s Hospital. None of these patients had received chemotherapy or radiotherapy before sampling. Individuals with autoimmune disease, infectious diseases, and multi-primary cancers were excluded. Blood from healthy donors was used for control experiments. The clinical stages of tumors were determined according to the TNM classification system of the International Union Against Cancer.
Fresh tissues were washed 3 times with Hank’s solution containing 1% fetal calf serum (FCS) before being cut into small pieces. The specimens were then placed in RPMI 1640 medium containing 1 mg/mL collagenase IV and 10 mg/mL deoxyribonuclease I and mechanically dissociated using a gentle MACS Dissociator (Miltenyi Biotec, Bergisch Gladbach, Germany). Dissociated cell suspensions were further incubated for 1 h at 37 °C under continuous rotation. The cell suspensions were then filtered through a 70 μm cell strainer (BD Labware, Bedford, MA, United States). Then, part of cells was used for flow cytometry to detect the number of Tc17 cells, and the remaining cells were cultured with IL-17 (0-10 ng/mL), IL-22 (0-10 ng/mL), interferon (IFN)-γ (0-10 ng/mL), IL-17 plus IL-22, or 100% Tc17 cell-polarizing culture for 48 h. The culture supernatants were harvested for ELISA.
Peripheral blood mononuclear cells from cervical cancer patients were isolated by Ficoll density gradient centrifugation. Fresh peripheral blood CD8+ T cells were selected using positive isolation and negative isolation kits, respectively. In a 5-d incubation period, bead-puriWed peripheral CD8+ T cells were co-cultured with autologous blood monocytes at a 2:1 ratio in the presence or absence of recombinant human IL-6 (10 ng/mL), IL-1β (10 ng/mL) and IL-23 (10 ng/mL) in 200 μL RPMI 1640 medium supplemented with 10% FCS. After 5-d incubation, the supernatants were harvested for ELISA and the cells for intracellular cytokine staining.
For detection of intracellular molecules, T lymphocytes were stimulated for 5 h with phorbol myristate acetate (50 ng/mL) plus ionomycin (1 μg/mL) in the presence of GolgiStop (BD Pharmingen, San Diego, CA, United States). Intracellular cytokine staining was performed after fixation and permeabilization using Perm/Wash solution (BD Pharmingen). The lymphocytes were analyzed by multicolor flow cytometry with FACSCanto II (BD Biosciences). Data were analyzed with FlowJo software (TreeStar, Ashland, OR, United States) or FACSDiva software (BD Biosciences).
Paraformaldehyde-fixed and paraffin-embedded samples were cut into 5 μm sections, which were incubated with rabbit anti-IL-17 antibody and stained by horseradish peroxidase anti-rabbit immunoglobulin G followed by diaminobenzidine. The sections were then incubated with mouse anti-CD8 antibody and stained using EnVision G2 System/AP Rabbit/Mouse (Permanent Red) (Dako, Glostrup, Denmark) and subsequently counterstained with hematoxylin. Slides were examined using an Olympus 71 inverted fluorescence microscope.
Immunofluorescence was performed as previously described with minor modifications[10]. In detail, Paraformaldehyde-Waxed cryostat sections of tumor tissues were washed in PBS and blocked for 30 min with 20% rabbit serum in PBS. Sections were incubated with goat anti-human IL-17 antibody (Ab) diluted in 5% rabbit serum. The bound Ab was detected with FITC-conjugated rabbit anti-goat Ab. After washing with PBS, the sections were blocked for 30 min with 20% goat serum in PBS and incubated with mouse anti-human CD8 Ab diluted in 5% goat serum. The bound Ab was detected with TRITC-conjugated goat anti-mouse Ab. After washing with PBS, the slides were examined with an Olympus 71 inverted fluorescence microscope.
Cervical cancer tissues or their matched adjacent normal tissues were homogenized in 1 mL Protein Extraction Reagent (Rockford, IL, United States). Concentrations of IL-6, IL-1β, and IL-23 in the tissue supernatants; concentrations of CXCL12 in the co-culture supernatants or tissue supernatants; and concentrations of IL-17 in the co-culture supernatants were determined using ELISA kits according to the manufacturer’s instructions.
Comparisons between data groups were performed as stated in each figure legend with the use of GraphPad Prism ver.6. A value of P < 0.05 was considered statistically significant. The resulting data were presented as mean ± SE.
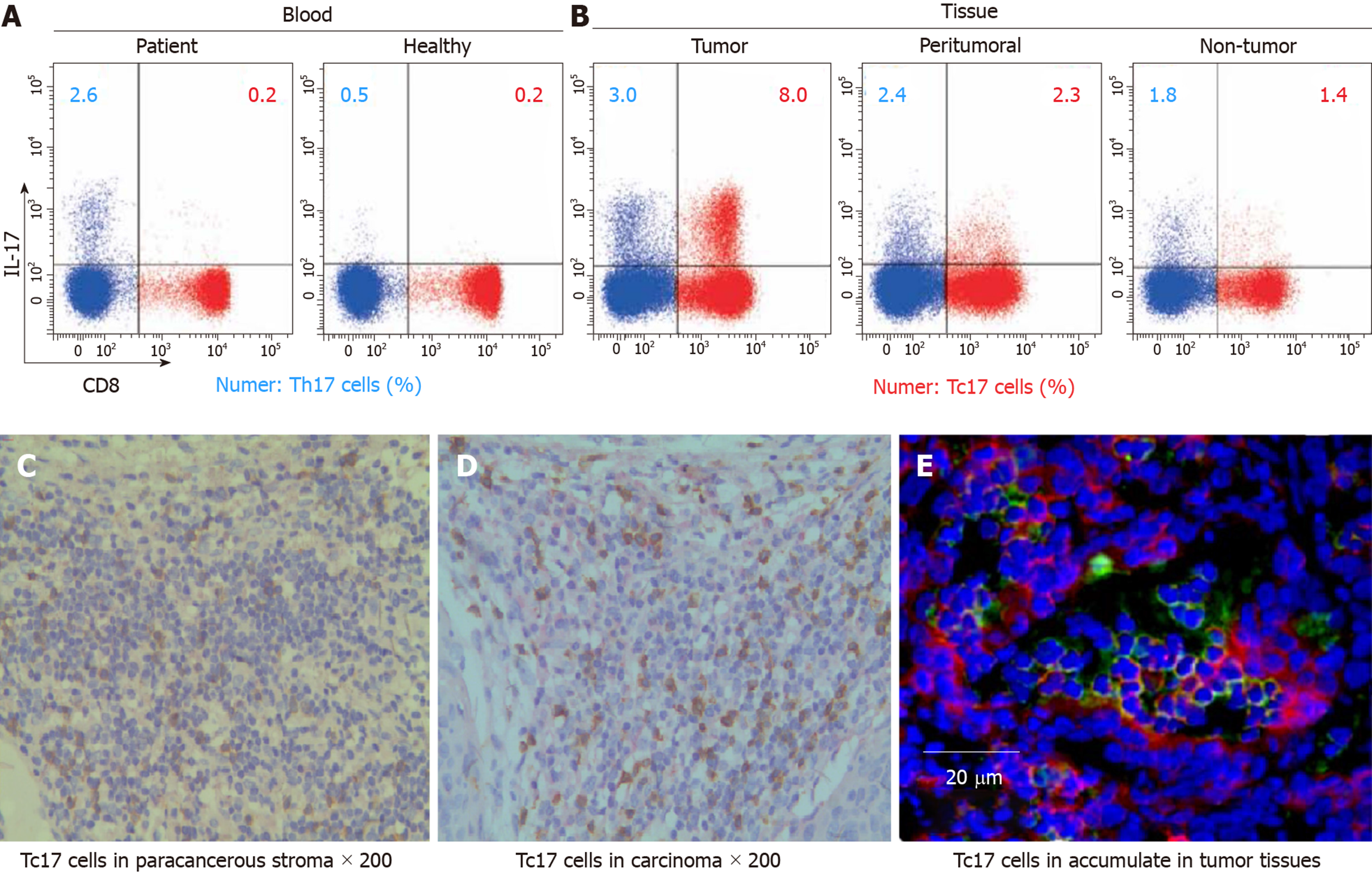
To assess the status of Tc17 cells in human cervical cancer tissues, we isolated immune cells from cancer tissues, matched adjacent normal tissues as well as peripheral blood. Compared with healthy donors, the percentage of Tc17 cells in the peripheral blood from patients with cervical cancers was identical (Figure 1A). Of note, we found that Tc17 cells were selectively induced in tumors compared to their matched adjacent normal tissues (Figure 1A). To further confirm this result, we analyzed the distribution of Tc17 cells in the paracancerous stroma, carcinoma nets, and intra-tumor sites. The results showed that Tc17 cells accumulated in all these sites, especially in carcinoma nets and intra-tumor sites (Figure 1B-D). Thus, these data show that Tc17 cells have an oncogenic role in cervical cancer development.
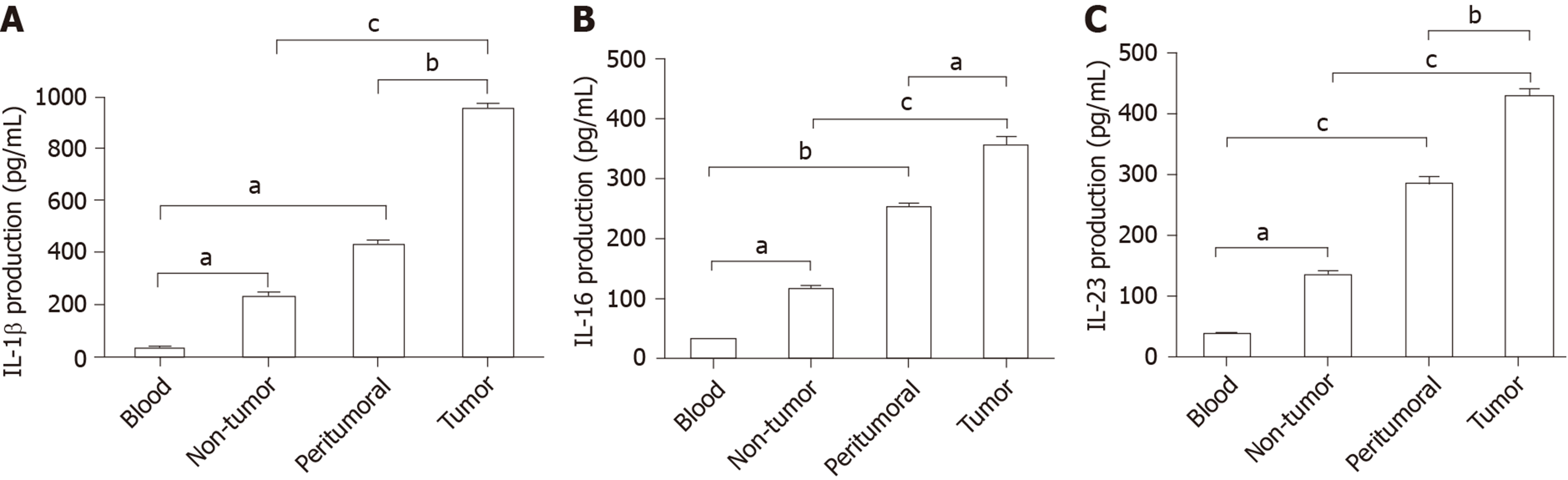
Previous studies revealed that IL-6, IL-1β, and IL-23 are essential for Tc17 cell differentiation[11,12]. To investigate the mechanism of cervical cancer following modulation of Tc17 cell development, we assessed the levels of Tc17-polarizing cytokines in cancer-associated tissues. As expected, the concentrations of IL-6, IL-1β, and IL-23 were significantly increased in peritumoral and intra-tumor tissue relative to their matched normal tissues or peripheral blood (Figure 2A-C). Thus, these data indicate that tumor-derived cytokines play a stimulatory role in the modulation of Tc17 polarization.
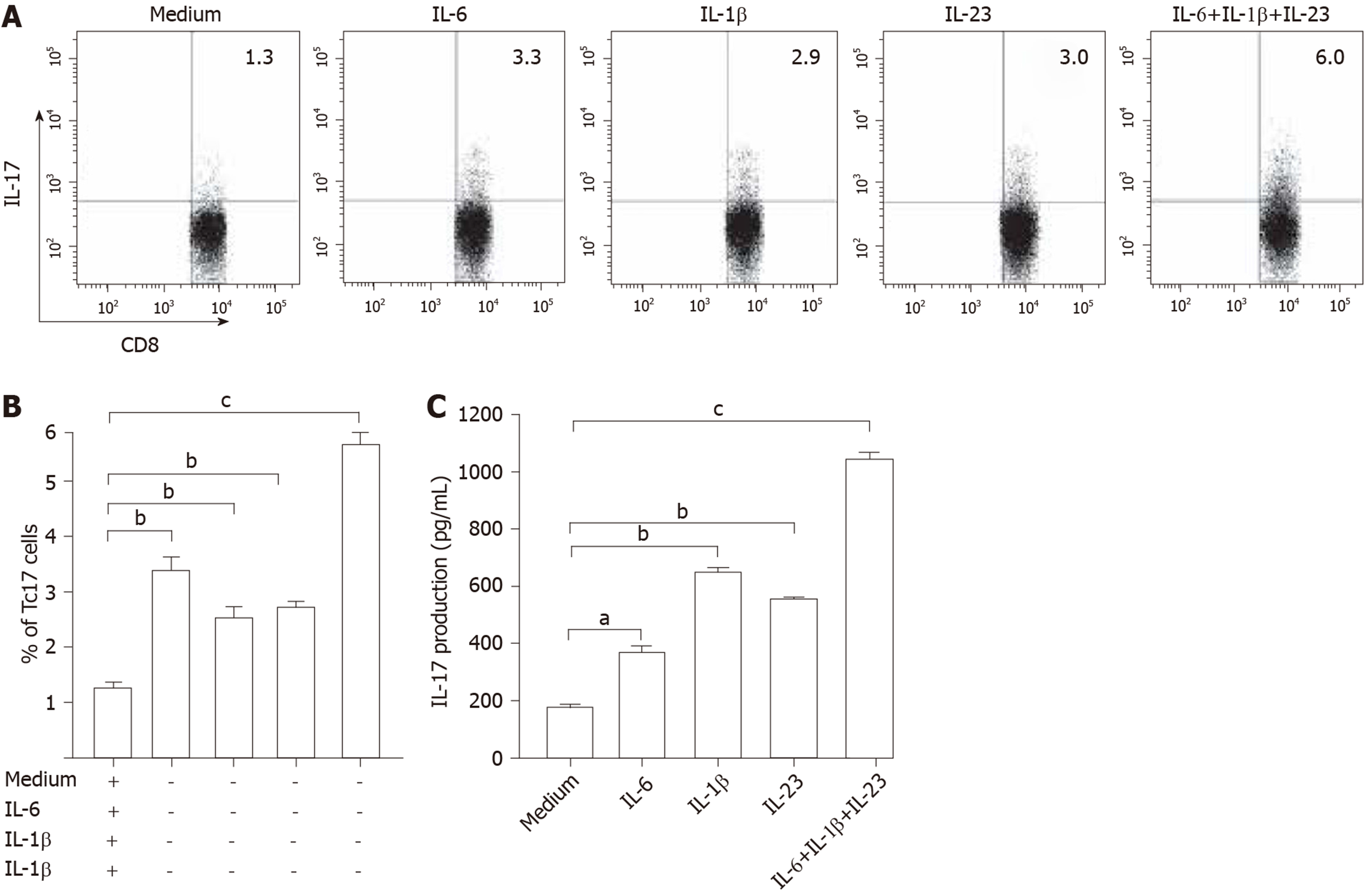
To evaluate the potential role of these cytokines in Tc17 cell differentiation, we isolated peripheral blood CD8+ T cells and autologous blood monocytes of cervical cancer patients. After 5-day co-culture incubation, the supernatants were harvested for ELISA and the cells for intracellular cytokine staining. The results showed that the addition of exogenous IL-6, IL-1β, and IL-23 significantly increased the frequency of Tc17 cells either alone or in combination compared with co-culture without any cytokines added (Figure 3A and B). In addition, the ELISA results showed that IL-17 production was consistent with the percentage of Tc17 cells (Figure 3C). These findings showed that IL-6, IL-1β, and IL-23 act synergistically to induce Tc17 cell polarization in vitro and suggest that a similar process might operate in vivo.
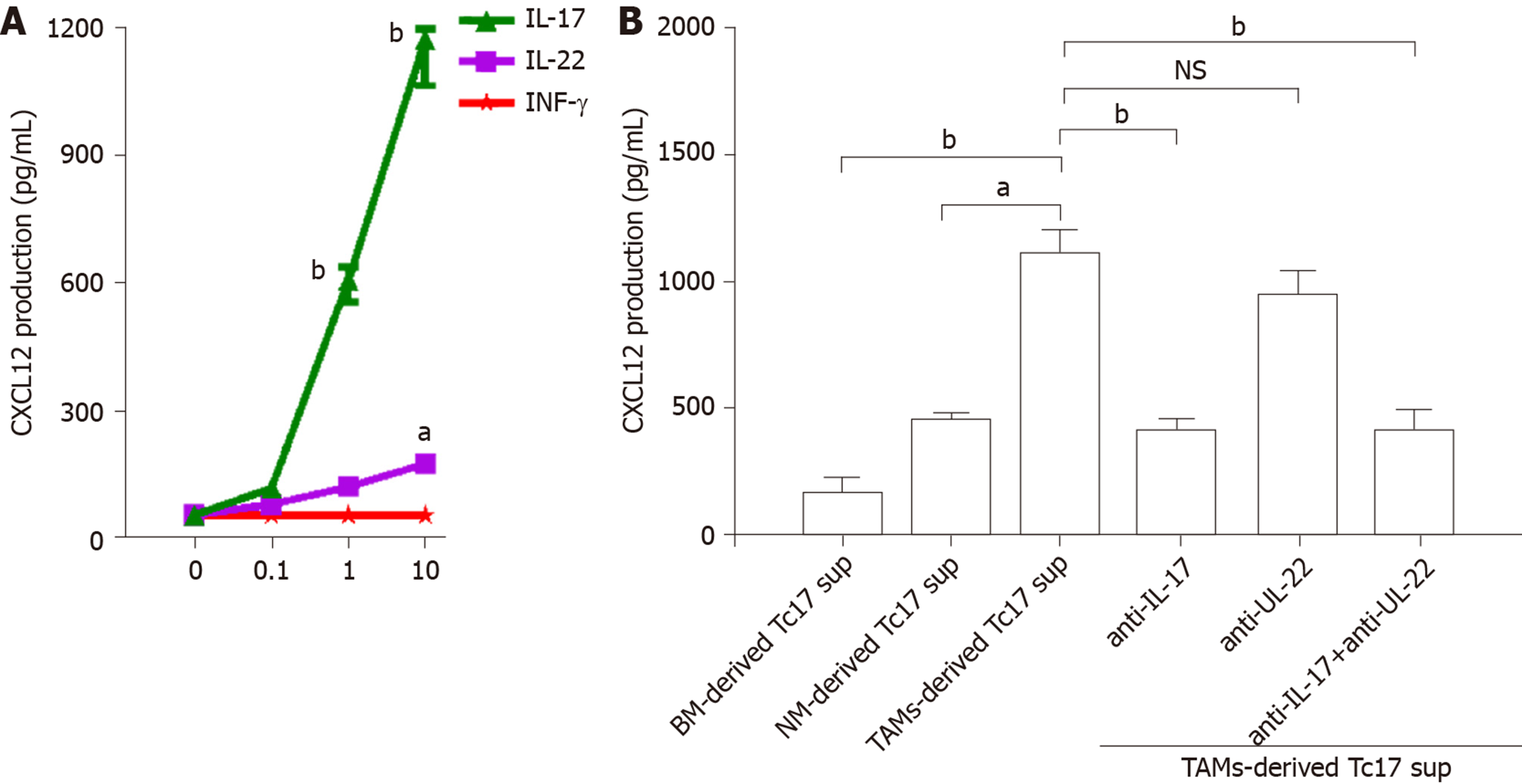
To investigate the biological function of Tc17 cells in cervical cancer development, we isolated primary tumor cells and stimulated them with various concentrations of IL-17, IL-22, and IFN-γ, and the production of CXC12 was detected in the culture supernatants by ELISA. As shown in Figure 4A, CXCL12 expression in cancer cells was increased upon IL-17 stimulation, while IL-22 or IFN-γ exhibited little effects on CXCL12 upregulation in cancer cells (Figure 4A). Moreover, unlike the co-culture supernatants from blood or non-tumor tissue monocytes, TAM-derived Tc17 cell culture supernatants selectively induced CXCL12 production in cancer cells (Figure 4B). The effects of CXCL12 upregulation were attenuated by anti–IL-17 neutralizing antibody, but not anti–IL-22 neutralizing antibody (Figure 4B). These findings suggested that Tc17 cell–derived IL-17 induced chemokine CXCL12 production by tumor cells.
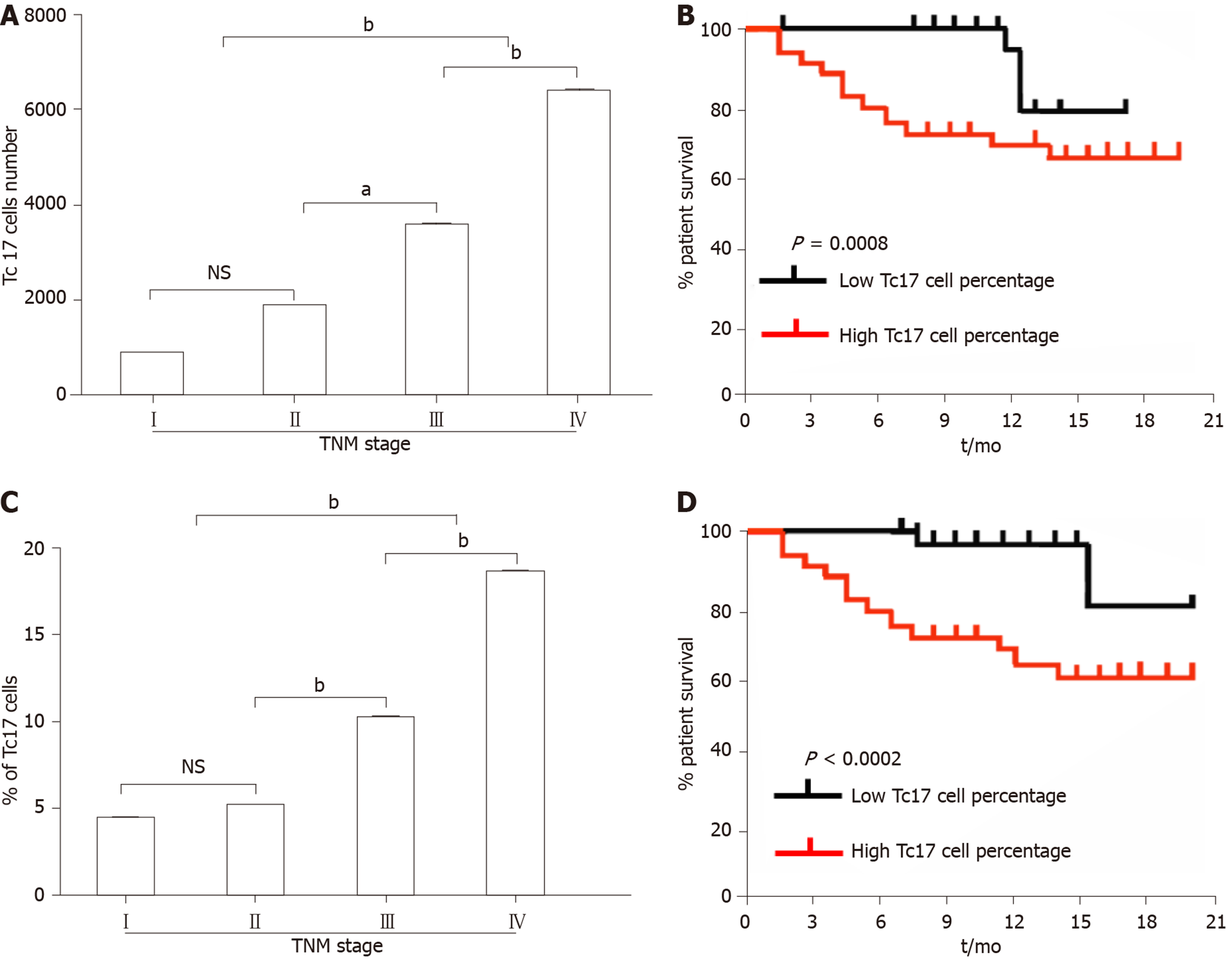
As CXCL12 upregulation drives cancer development, we assessed the relationship between the number of Tc17 cells and the survival rate of patients with cervical cancer. We first analyzed the number of Tc17 cells per million total cells in each tissue from patients in different TNM stages. The results showed that as cancer progressed, the number of Tc17 cells significantly increased in each of the tested tissues (Figure 5A). Conversely, patient survival was significantly reduced (Figure 5A and B). Simultaneously, we analyzed Tc17 cell percentage within the total CD8+ T cells in the same samples, and as cancer progressed, we found that the percentage of Tc17 cells significantly increased in the tested tissues, and was negatively associated with patient survival (Figure 5C and D). These findings suggested that the number of Tc17 cells was related to the survival rate of patients, thereby predicting the clinical outcome of patients with cervical cancer.
Although Tc17 cell-mediated immune regulation has been studied in tumor progression in both mice and black-and-white monkeys[13], the mechanism of Tc17 cells in human cervical cancer has been rarely reported. In this study, we found that Tc17 cells play a stimulatory role in the progression of cervical cancer. Compared with normal tissues, cervical cancer cells produced a greater amount of IL-6, IL-1β, and IL-23A, which are essential for Tc17 cell differentiation[11,12]. Tc17 induction in turn augmented CXCL12 expression in tumor cells via activation of IL-17 signaling. Furthermore, the increased ratio of Tc17 cells in tumors predicted a poor prognosis in patients with cervical cancers. Thus, these data demonstrate that Tc17 cells have an oncogenic role in cervical cancer development.
Recent studies have shown that persistent antigenic stimulation leads to CD8+ T cell exhaustion[14,15]. Blockade of the PD-1/PD-L1 strategy is promising for the treatment of cancer[16]. However, an increasing number of studies have revealed that tumor-elicited inflammation enhances resistance to cancer immunotherapy, which suggests that cancer cells adopt other strategies to escape immune attack. In the present study, we found that Tc17 cells specifically accumulated in cervical cancer tissue. Unlike the necessary role of IL-6 and IFN-γ in stimulation of Tc17 cell expansion in colorectal or liver cancers[14,15], cervical cancer cells secrete abundant Tc17-polarizing cytokines including IL-6, IL-1β, and IL-23, suggesting the enhanced effects on Tc17 cell development is attributed to the cancer microenvironment.
Murine tumor models have shown that Tc17 cells impair immune surveillance and promote de novo carcinogenesis and neovascularization of tumors[16,17]. However, other groups have identified anti-tumor activity of Tc17 cells in mice[18,19]. Therefore, the precise underlying mechanism is still unclear. Our data revealed that increased IL-17 derived from Tc17 cells drives CXCL12 expression in tumor cells, which has been reported to enhance cell proliferation and migration, thereby exacerbating malignant formation. Finally, we assessed the relationship between the number of Tc17 cells and the survival rate of patients with cervical cancer. Similar studies have been conducted in mice and gastric cancer patients[20]. The results showed that the increased ratio of Tc17 in tumors is highly correlated with poor outcome in patients with cervical cancer.
In conclusion, our data demonstrate that Tc17 cells are specifically induced by the cervical cancer microenvironment. Tc17 cells promote tumor development via activation of IL-17 signaling and are associated with the prognosis of patients with cervical cancer. Thus, we propose that Tc17 cells can be used as a useful clinical index in the prognosis of cervical cancer.
The existence of Tc17 cells was recently shown in several types of inflammatory diseases.
The distribution and functions of Tc17 cells in cervical cancer have not been fully elucidated.
To investigate the role of Tc17 cells in the pathogenesis of cervical cancer.
The frequency of Tc17 cells in blood and tumor samples from patients with cervical cancer was determined by flow cytometry. In addition, the levels and phenotype of Tc17 cells in tissue samples from cervical cancer patients were assessed by immunohistochemistry staining.
Tc17 cells specifically accumulate in the tumor tissues of cervical cancer patients. Cervical cancer-elicited inflammation increases Tc17-polarizing cytokine production, which attenuates cytotoxic CD8+ T cell development. High interleukin-17 production by Tc17 cells leads to CXCL12 upregulation and cancer cell migration.
Consistent with the oncogenic role of Tc17 cells in cancer development, the ratio of cancer-infiltrating Tc17 cells is highly associated with poor prognosis in patients with cervical cancer.
This study indicates that Tc17 cells in cervical cancer and their regulatory mechanisms are associated with cancer progression.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): E
P-Reviewer: Ankathil R, Ortiz-Sanchez E, Rangel-Corona R S-Editor: Ma YJ L-Editor: Webster JR E-Editor: Liu JH
| 1. | Chen W, Zheng R, Baade PD, Zhang S, Zeng H, Bray F, Jemal A, Yu XQ, He J. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11444] [Cited by in RCA: 13210] [Article Influence: 1467.8] [Reference Citation Analysis (3)] |
| 2. | Li S, Hu T, Lv W, Zhou H, Li X, Yang R, Jia Y, Huang K, Chen Z, Wang S, Tang F, Zhang Q, Shen J, Zhou J, Xi L, Deng D, Wang H, Wang S, Xie X, Ma D. Changes in prevalence and clinical characteristics of cervical cancer in the People's Republic of China: a study of 10,012 cases from a nationwide working group. Oncologist. 2013;18:1101-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 73] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 3. | Tu JF, Pan HY, Ying XH, Lou J, Ji JS, Zou H. Mast Cells Comprise the Major of Interleukin 17-Producing Cells and Predict a Poor Prognosis in Hepatocellular Carcinoma. Medicine (Baltimore). 2016;95:e3220. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 4. | Yamaguchi Y, Seino Y, Takei H, Kurosumi M, Hayashi S. Detection of estrogen-independent growth-stimulating activity in breast cancer tissues: implication for tumor aggressiveness. Cancer Microenviron. 2014;7:23-31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 5. | Ma J, Shi LL, Deng YK, Wang H, Cao PP, Long XB, Zhang XH, Liu Y, Zeng M, Liu Z. CD8(+) T cells with distinct cytokine-producing features and low cytotoxic activity in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Clin Exp Allergy. 2016;46:1162-1175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 6. | Coughlan L, Lambe T. Measuring Cellular Immunity to Influenza: Methods of Detection, Applications and Challenges. Vaccines (Basel). 2015;3:293-319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Fujita T, Burwitz BJ, Chew GM, Reed JS, Pathak R, Seger E, Clayton KL, Rini JM, Ostrowski MA, Ishii N, Kuroda MJ, Hansen SG, Sacha JB, Ndhlovu LC. Expansion of dysfunctional Tim-3-expressing effector memory CD8+ T cells during simian immunodeficiency virus infection in rhesus macaques. J Immunol. 2014;193:5576-5583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 8. | Nanjappa SG, Hernández-Santos N, Galles K, Wüthrich M, Suresh M, Klein BS. Intrinsic MyD88-Akt1-mTOR Signaling Coordinates Disparate Tc17 and Tc1 Responses during Vaccine Immunity against Fungal Pneumonia. PLoS Pathog. 2015;11:e1005161. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 9. | Mei Z, Zhou L, Zhu Y, Jie K, Fan D, Chen J, Liu X, Jiang L, Jia Q, Li W. Interleukin-22 promotes papillary thyroid cancer cell migration and invasion through microRNA-595/Sox17 axis. Tumour Biol. 2016;37:11753-11762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Li M, Pang Z, Xiao W, Liu X, Zhang Y, Yu D, Yang M, Yang Y, Hu J, Luo K. A transcriptome analysis suggests apoptosis-related signaling pathways in hemocytes of Spodoptera litura after parasitization by Microplitis bicoloratus. PLoS One. 2014;9:e110967. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 11. | Kimura A, Naka T, Kishimoto T. IL-6-dependent and -independent pathways in the development of interleukin 17-producing T helper cells. Proc Natl Acad Sci U S A. 2007;104:12099-12104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 270] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 12. | Nigam P, Kwa S, Velu V, Amara RR. Loss of IL-17-producing CD8 T cells during late chronic stage of pathogenic simian immunodeficiency virus infection. J Immunol. 2011;186:745-753. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 64] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 13. | Alvarez Arias DA, Kim HJ, Zhou P, Holderried TA, Wang X, Dranoff G, Cantor H. Disruption of CD8+ Treg activity results in expansion of T follicular helper cells and enhanced antitumor immunity. Cancer Immunol Res. 2014;2:207-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 36] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 14. | In TSH, Trotman-Grant A, Fahl S, Chen ELY, Zarin P, Moore AJ, Wiest DL, Zúñiga-Pflücker JC, Anderson MK. HEB is required for the specification of fetal IL-17-producing γδ T cells. Nat Commun. 2017;8:2004. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 43] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 15. | Khmaladze Ia, Kelkka T, Guerard S, Wing K, Pizzolla A, Saxena A, Lundqvist K, Holmdahl M, Nandakumar KS, Holmdahl R. Mannan induces ROS-regulated, IL-17A-dependent psoriasis arthritis-like disease in mice. Proc Natl Acad Sci USA. 2014;111:E3669-E3678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 130] [Cited by in RCA: 128] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 16. | Werthmöller N, Frey B, Rückert M, Lotter M, Fietkau R, Gaipl US. Combination of ionising radiation with hyperthermia increases the immunogenic potential of B16-F10 melanoma cells in vitro and in vivo. Int J Hyperthermia. 2016;32:23-30. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 17. | Tosello Boari J, Acosta Rodriguez EV. IL-1β/CD14 pathway induces IL-17 production: Dendritic cells activated with IL-1β set Th17 cells on fire by CD14-mediated mechanisms. Immunol Cell Biol. 2016;94:903-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 18. | Li YX, Zhang L, Simayi D, Zhang N, Tao L, Yang L, Zhao J, Chen YZ, Li F, Zhang WJ. Human papillomavirus infection correlates with inflammatory Stat3 signaling activity and IL-17 level in patients with colorectal cancer. PLoS One. 2015;10:e0118391. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 19. | Li A, Gan Y, Wang R, Liu Y, Ma T, Huang M, Cui X. IL-22 Up-Regulates β-Defensin-2 Expression in Human Alveolar Epithelium via STAT3 but Not NF-κB Signaling Pathway. Inflammation. 2015;38:1191-1200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Shimada K, Uzui H, Ueda T, Lee JD, Kishimoto C. N-Acetylcysteine Ameliorates Experimental Autoimmune Myocarditis in Rats via Nitric Oxide. J Cardiovasc Pharmacol Ther. 2015;20:203-210. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |