Published online May 6, 2019. doi: 10.12998/wjcc.v7.i9.1053
Peer-review started: January 18, 2019
First decision: January 30, 2019
Revised: March 6, 2019
Accepted: March 26, 2019
Article in press: March 26, 2019
Published online: May 6, 2019
Processing time: 109 Days and 5.7 Hours
There have been few reports about the late effects of disconnected pancreatic duct syndrome (DPDS). Although few reports have described the recurrence interval of pancreatitis, it might be rare for recurrence to occur more than 5 years later. Herein, we describe a case of recurrence in an 81-year-old man after the treatment of walled-off necrosis (WON) with pancreatic transection 7 years ago.
An 81-year-old man visited our hospital with chief complaints of fever and abdominal pain 7 years after the onset of WON due to severe necrotic pancreatitis. His medical history included an abdominal aortic aneurysm (AAA), hypertension, dyslipidemia, and chronic kidney disease. Computed tomography (CT) scan showed that the pancreatic fluid collection (PFC) had spread to the aorta with inflammation surrounding it, and CT findings suggested that bleeding occurred from the vasodilation due to splenic vein occlusion. First, we attempted to perform transpapillary drainage because of venous dilation around the residual stomach and the PFC. However, pancreatic duct drainage failed because of complete main pancreatic duct disruption. Second, we performed endoscopic ultrasound-guided drainage. After transmural drainage, the inflammation improved and stenting for the AAA was performed successfully. The inflammation was resolved, and he has been free from infection for more than 2 years after the procedure.
This case highlights the importance of continued follow-up of patients for recurrence after the treatment of WON with pancreatic transection.
Core tip: There have been a few reports about the late effects of disconnected pancreatic duct syndrome. We describe a case of recurrence in an 81-year-old man after the treatment of walled-off necrosis (WON) with pancreatic transection 7 years ago. Endoscopic transpapillary drainage was attempted first but failed. Thereafter, endoscopic ultrasound-guided drainage was performed. Subsequently, pancreatic inflammation resolved, and abdominal aortic stenting for the aneurysm was performed successfully. The patient has been infection free for more than 2 years post-procedure. This case highlights the importance of continued follow-up of patients for recurrence after the treatment of WON with pancreatic transection.
- Citation: Yamada R, Umeda Y, Shiono Y, Okuse H, Kuroda N, Tsuboi J, Inoue H, Hamada Y, Tanaka K, Horiki N, Takei Y. Management of the late effects of disconnected pancreatic duct syndrome: A case report. World J Clin Cases 2019; 7(9): 1053-1059
- URL: https://www.wjgnet.com/2307-8960/full/v7/i9/1053.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i9.1053
Disconnected pancreatic duct syndrome (DPDS) is characterized by disruption of the main pancreatic duct (MPD), resulting in various upstream pancreatic glands becoming isolated from the MPD downstream[1]. A recent retrospective study reported that DPDS occurred more frequently in patients with walled-off necrosis (WON) than in those with other pancreatic fluid collections (PFCs) (68.3% vs 31.7%)[2]. It was also reported that DPDS due to WON is disadvantageous compared to that due to other PFCs because of the increased need for re-interventions (30% vs 18.5%; P = 0.03), rescue surgery (13.2% vs 4.8%; P = 0.02), and a longer length of admission[2]. However, there have been few reports about the late adverse effects of DPDS. Currently, endoscopic intervention is increasingly considered as a less invasive alternative to surgery for managing DPDS[3]. Thus, endoscopic intervention is considered to be effective for the late effects of DPDS. We describe a case of endoscopic management for the late effects of DPDS with complete duct disruption and pancreatic transection following severe acute pancreatitis.
An 81-year-old man with a medical history of abdominal aortic aneurysm (AAA), hypertension, dyslipidemia, and chronic kidney disease visited our hospital with fever and abdominal pain.
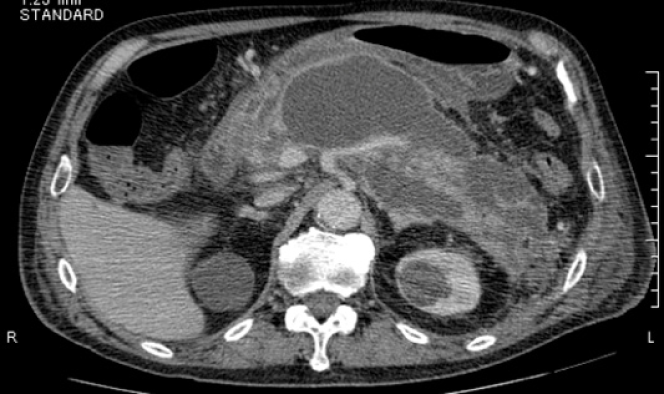
The patient reported having severe acute necrotizing pancreatitis due to a gallstone 7 years prior. He did not drink alcohol. He was referred to our hospital 42 d after onset because of the development of WON in the pancreatic neck region (Figure 1). The patient underwent endoscopic ultrasound (EUS)-guided drainage and necrosectomy eight times. Subsequently, he recovered from WON, his nutritional status improved, and he was discharged from our hospital after 79 d. After discharge, we performed endoscopic choledocholithotomy. Cholecystectomy was not performed because the gallbladder had already shrunk. Although he had a pancreatic fistula due to pancreatic transection following severe pancreatitis, he had been asymptomatic for 6 years. Four years after WON onset, he underwent distal gastrectomy with Roux-en-Y reconstruction for invasive gastric cancer.
On examination, upper abdominal tenderness and gastrointestinal bleeding were noted.
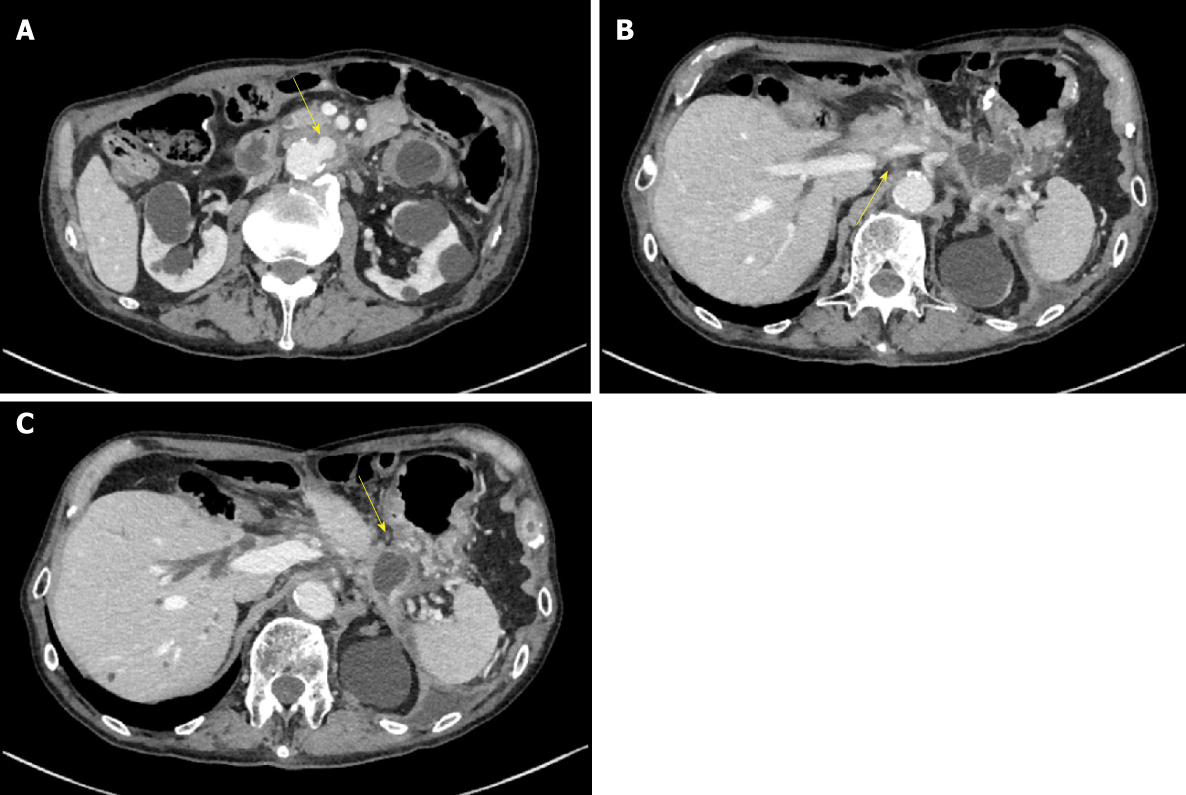
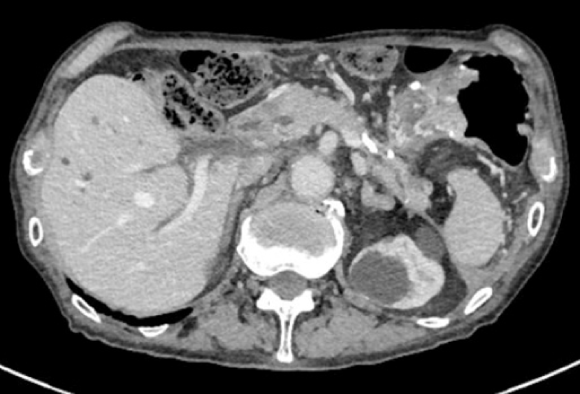
Computed tomography (CT) scan was suggestive of PFC in the pancreatic tail due to pancreatic transection that had spread to the aorta with surrounding inflammation. Inflammation was also found around the aorta and ulcer-like blood flow appeared in the aortic aneurysmal thrombus (Figure 2A). Gastrointestinal bleeding was present, but no active bleeding was observed through the endoscope. The CT scan showed venous dilation mainly around the residual stomach, and it was thought that bleeding occurred from the vasodilator due to splenic vein occlusion (Figure 2B). Because there was a risk of rupture of the AAA owing to the spread of inflammation from the PFC, drainage was deemed necessary.
The final diagnosis of the presented case is late effects of DPDS and infected PFC due to severe acute pancreatitis and WON.
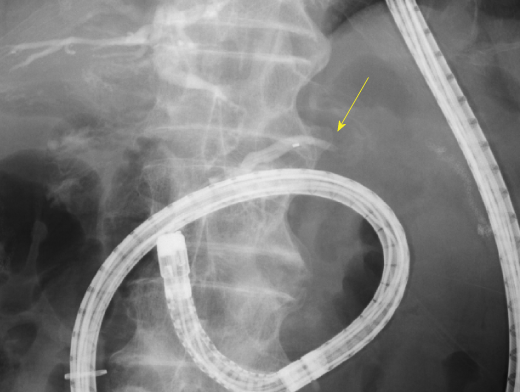
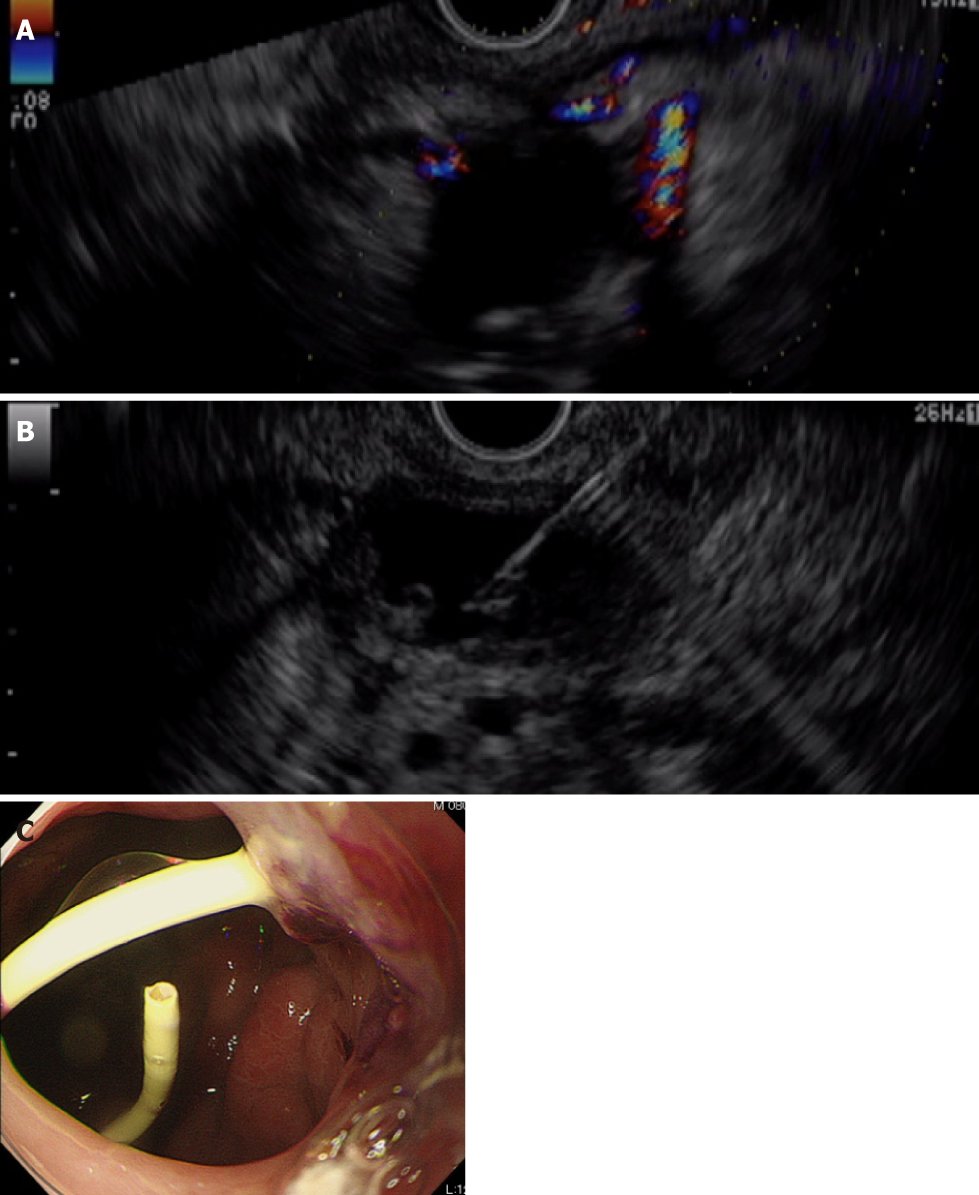
First, we attempted transpapillary drainage via double-balloon endoscopy (DBE) due to difficulty in EUS-guided puncture secondary to venous dilation around the residual stomach and the distribution of the PFC, which was extensive but narrow around the stomach (Figure 2C). DBE retrograde pancreatography showed complete pancreatic duct disruption (Figure 3). Pancreatic duct drainage failed because of complete MPD disruption. Thereafter, we performed EUS-guided drainage. The linear array echo-endoscope showed many vessels surrounding the PFC and stomach. We punctured the PFC using a 19-gauge needle, carefully avoiding the vessels and inserted a double pig catheter (6 French/4 cm) transmurally (Figure 4). After transmural drainage, the inflammation was resolved and stenting for the AAA was performed successfully.
The inflammation further improved after the procedure (Figure 5). The patient has been free from WON infection for more than 2 years after the procedure.
Previously, surgery was the first therapeutic choice for DPDS. Fischer et al[4] retrospectively reviewed operated cases of DPDS; distal pancreatectomy had been performed in whole delayed DPDS cases. They concluded that fluid collection often is not accessible endoscopically and that no long-term data support the patency of the endoscopic approach. However, endoscopic intervention is increasingly considered as a less invasive alternative to surgery in the management of DPDS[3]. Furthermore, guidelines were developed for endoscopic management of acute necrotizing pancreatitis, including the management of DPDS[5].
There are two endoscopic strategies for treating DPDS: Transpapillary placement of the drainage stent and endoscopic transmural drainage of the PFC. The evidence-based multidisciplinary guidelines of the European Society of Gastrointestinal Endoscopy suggested that transpapillary stenting can be considered where partial MPD disruption has occurred[5]. Even though widely practiced in the management of DPDS, transpapillary stenting sometimes results in failure. However, successful transmural drainage does not depend on the presence of communication between the proximal MPD and the disconnected upstream segment. Bang et al[6] reported that the best indication of EUS-guided drainage for DPDS is when WON or PFC is larger than 4 cm at its largest dimension and located within 15 mm of the gastrointestinal lumen. In this case, the PFC distribution was narrow and surrounded the stomach, although it had spread extensively. Thus, we attempted to perform transpapillary drainage first but discontinued the drainage because of complete MPD disruption. Although the width of the PFC was less than 4 cm, transmural drainage was performed safely and successfully.
The present patient showed recurrence of pancreatitis in the form of PFC after a prolonged period of 7 years. Recurrence in the form of a necrotic cavity or pseudocyst has been reported in approximately 10% of patients, even after successful endoscopic treatments. For WON, the recurrence rates were reported to be 9.4% after endoscopic transmural drainage (the single or multiple transluminal gateway technique) in 53 patients[7], 7.8% after combined percutaneous and endoscopic drainage in 103 patients[8], and 10.9% (7%-15%) after endoscopic necrosectomy in a meta-analysis (8 studies, 233 patients)[9]. Although few reports have described the recurrence interval of pancreatitis, Yasuda et al[10] reported that 7.0% of 43 patients showed recurrence within 2-8 months after endoscopic treatment.
Based on the literature, it might be rare for recurrence to occur more than 5 years later. Nevertheless, it is important to continue following patients after the treatment of WON with pancreatic transection because late recurrence due to DPDS may occur.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Antonini F, Cheungpasitporn W, Fusaroli P, Trifan A S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Tann M, Maglinte D, Howard TJ, Sherman S, Fogel E, Madura JA, Lehman GA. Disconnected pancreatic duct syndrome: imaging findings and therapeutic implications in 26 surgically corrected patients. J Comput Assist Tomogr. 2003;27:577-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 69] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Bang JY, Wilcox CM, Navaneethan U, Hasan MK, Peter S, Christein J, Hawes R, Varadarajulu S. Impact of Disconnected Pancreatic Duct Syndrome on the Endoscopic Management of Pancreatic Fluid Collections. Ann Surg. 2018;267:561-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 92] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 3. | Wilechansky RM, Khan AS, Sethi A. Disrupted pancreatic duct treated with a combination of endoscopic cystoduodenostomy and pancreatic duct fistualization through a pseudocyst wall using a lumen-apposing metal stent. Dig Endosc. 2017;29:226-228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 4. | Fischer TD, Gutman DS, Hughes SJ, Trevino JG, Behrns KE. Disconnected pancreatic duct syndrome: disease classification and management strategies. J Am Coll Surg. 2014;219:704-712. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 54] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 5. | Arvanitakis M, Dumonceau JM, Albert J, Badaoui A, Bali MA, Barthet M, Besselink M, Deviere J, Oliveira Ferreira A, Gyökeres T, Hritz I, Hucl T, Milashka M, Papanikolaou IS, Poley JW, Seewald S, Vanbiervliet G, van Lienden K, van Santvoort H, Voermans R, Delhaye M, van Hooft J. Endoscopic management of acute necrotizing pancreatitis: European Society of Gastrointestinal Endoscopy (ESGE) evidence-based multidisciplinary guidelines. Endoscopy. 2018;50:524-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 221] [Cited by in RCA: 287] [Article Influence: 41.0] [Reference Citation Analysis (0)] |
| 6. | Bang JY, Navaneethan U, Hasan MK, Hawes RH, Varadarajulu S. EUS correlates of disconnected pancreatic duct syndrome in walled-off necrosis. Endosc Int Open. 2016;4:E883-E889. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Bang JY, Wilcox CM, Trevino J, Ramesh J, Peter S, Hasan M, Hawes RH, Varadarajulu S. Factors impacting treatment outcomes in the endoscopic management of walled-off pancreatic necrosis. J Gastroenterol Hepatol. 2013;28:1725-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 8. | Ross AS, Irani S, Gan SI, Rocha F, Siegal J, Fotoohi M, Hauptmann E, Robinson D, Crane R, Kozarek R, Gluck M. Dual-modality drainage of infected and symptomatic walled-off pancreatic necrosis: long-term clinical outcomes. Gastrointest Endosc. 2014;79:929-935. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 116] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 9. | Puli SR, Graumlich JF, Pamulaparthy SR, Kalva N. Endoscopic transmural necrosectomy for walled-off pancreatic necrosis: a systematic review and meta-analysis. Can J Gastroenterol Hepatol. 2014;28:50-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 51] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 10. | Yasuda I, Nakashima M, Iwai T, Isayama H, Itoi T, Hisai H, Inoue H, Kato H, Kanno A, Kubota K, Irisawa A, Igarashi H, Okabe Y, Kitano M, Kawakami H, Hayashi T, Mukai T, Sata N, Kida M, Shimosegawa T. Japanese multicenter experience of endoscopic necrosectomy for infected walled-off pancreatic necrosis: The JENIPaN study. Endoscopy. 2013;45:627-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 95] [Article Influence: 7.9] [Reference Citation Analysis (0)] |