Published online Mar 26, 2019. doi: 10.12998/wjcc.v7.i6.798
Peer-review started: December 10, 2018
First decision: January 5, 2019
Revised: February 11, 2019
Accepted: February 18, 2019
Article in press: February 18, 2019
Published online: March 26, 2019
Processing time: 107 Days and 13.1 Hours
The rate of positive resection margins (R1) in patients with low rectal cancer is substantial. Recommended remedies such as extended resection or chemoradiotherapy have their own serious drawbacks. It has been reported that photodynamic therapy (PDT) as a remedial treatment for esophageal cancer. Colorectal cancer and esophageal cancer has many similarities, however, PDT as a salvage therapy for rectal cancer is rare.
Here, we describe a 56-year-old man who was admitted to the hospital due to a 6-mo history of hemafecia, which had been aggravated for 1 mo. Colonoscopy revealed a 3 × 4 cm ulcerated mass in the rectum 4 cm from the anus. Preoperative pathological examination showed villous adenoma, moderate-to-high-grade dysplasia, good differentiation, and invasion of the mucosal muscle. The patient had R1 after ultra-low anterior resection, but he refused extended resection and experienced severe liver function impairment after 3 cycles of chemotherapy. Ultimately, the patient underwent PDT to remove R1. After five years of follow-up, there was no liver function impairment, recurrence, metastasis, sexual dysfunction, or abnormal defecation function.
This is the first case worldwide in which R1 of rectal cancer were successfully treated by PDT.
Core tip: The rate of R1 in patients with low rectal cancer is substantial, especially when sphincter preservation is desired. Many people refuse to remove the anal sphincter. Thus, further extended resection cannot be performed. In addition, postoperative chemoradiotherapy, which is the second choice to compensate for R1, has disappointing outcomes and numerous drawbacks. Because of many advantages and few side effects of photodynamic therapy (PDT), this approach is increasing in frequency. This case report describes PDT as a salvage therapy for R1 in a patient with low rectal cancer after ultra-low anterior resection; sphincter preservation was achieved, and no recurrence was observed after 5 years.
- Citation: Zhang SQ, Liu KJ, Yao HL, Lei SL, Lei ZD, Yi WJ, Xiong L, Zhao H. Photodynamic therapy as salvage therapy for residual microscopic cancer after ultra-low anterior resection: A case report. World J Clin Cases 2019; 7(6): 798-804
- URL: https://www.wjgnet.com/2307-8960/full/v7/i6/798.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i6.798
Low rectal cancer refers to rectal cancer within 5 cm of the dentate line. Currently, total mesorectal excision (TME) is the first approach for rectal cancer. However, sphincter preservation in patients with low rectal cancer is controversial. Some people consider abdominoperineal resection (APR) to be the standard, whereas others see the benefit of sphincter preservation[1]. The latter approach is currently trending. The ultimate goal of surgery is to obtain a good prognosis. Many studies have confirmed that a positive resection margin (R1) is a significant factor affecting the prognosis of patients with rectal cancer. Unfortunately, although advances in surgical techniques and multimodality therapy have reduced the rate of R1 in patients with low rectal cancer, this outcome still occurs in a large proportion of patients[2]. Patients with R1 need extended resection, which often means that the anus cannot be retained. This outcome is unacceptable for many patients and their families. Alternatively, as a remedy and alternative, postoperative chemoradiotherapy (CRT) may be performed, but the effects and side effects of this approach are questionable. In recent years, photodynamic therapy (PDT) has become an emerging treatment because of its unique advantages and its potential for further development. This article reports the first case of PDT as a salvage treatment for microscopic residual cancer after ultra-low anterior resection (ULAR) for low rectal cancer. This report aims to explore remedial treatment of a positive microscopic distal margin and the potential value of PDT in a patient with low rectal cancer.
A 56-year-old male patient without hepatitis was admitted to the hospital on November 29, 2012, due to a 6-mo history of hemafecia, which had been aggravated for 1 mo.
The patient had good health and no relevant personal or family history.
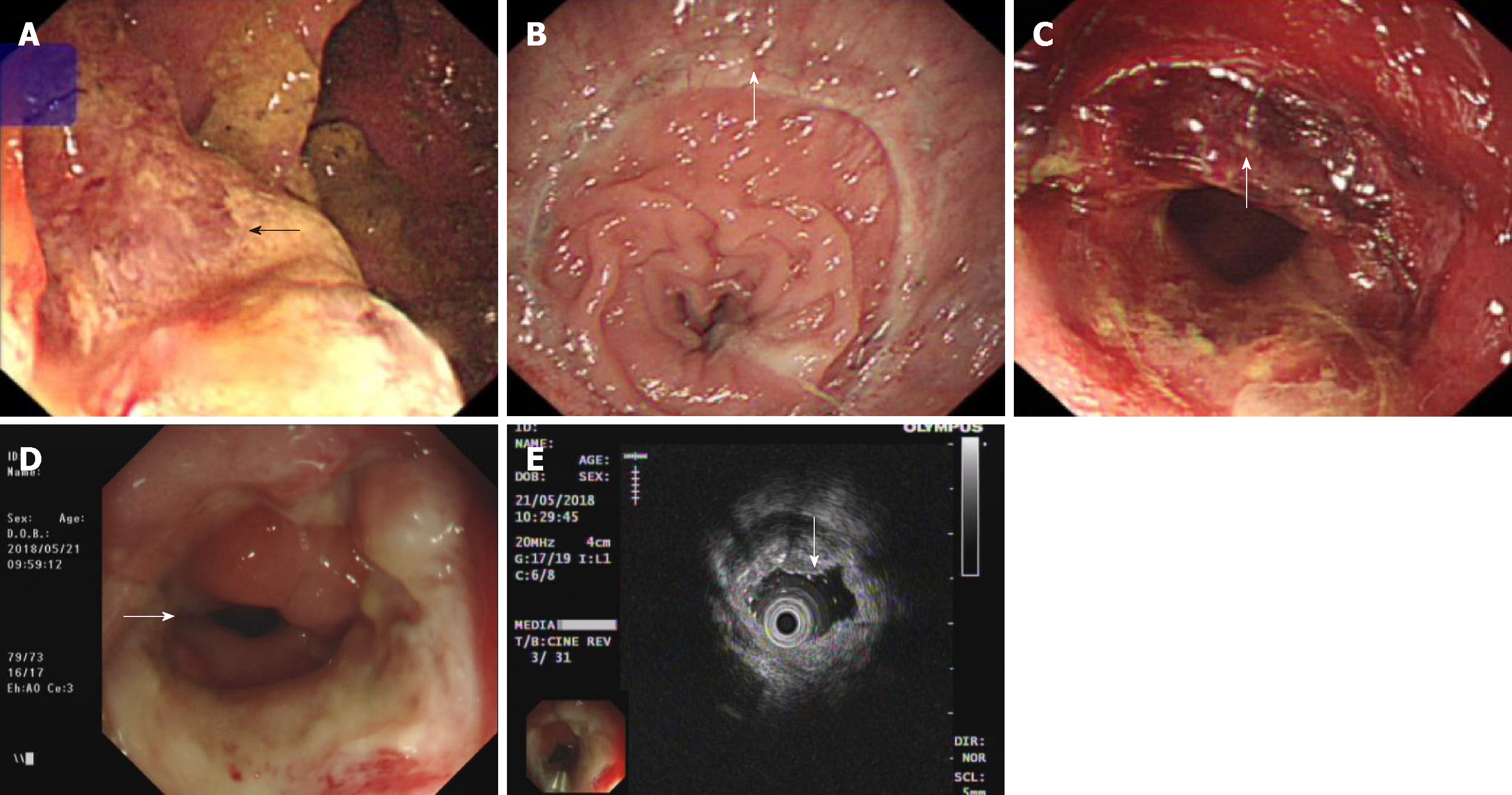
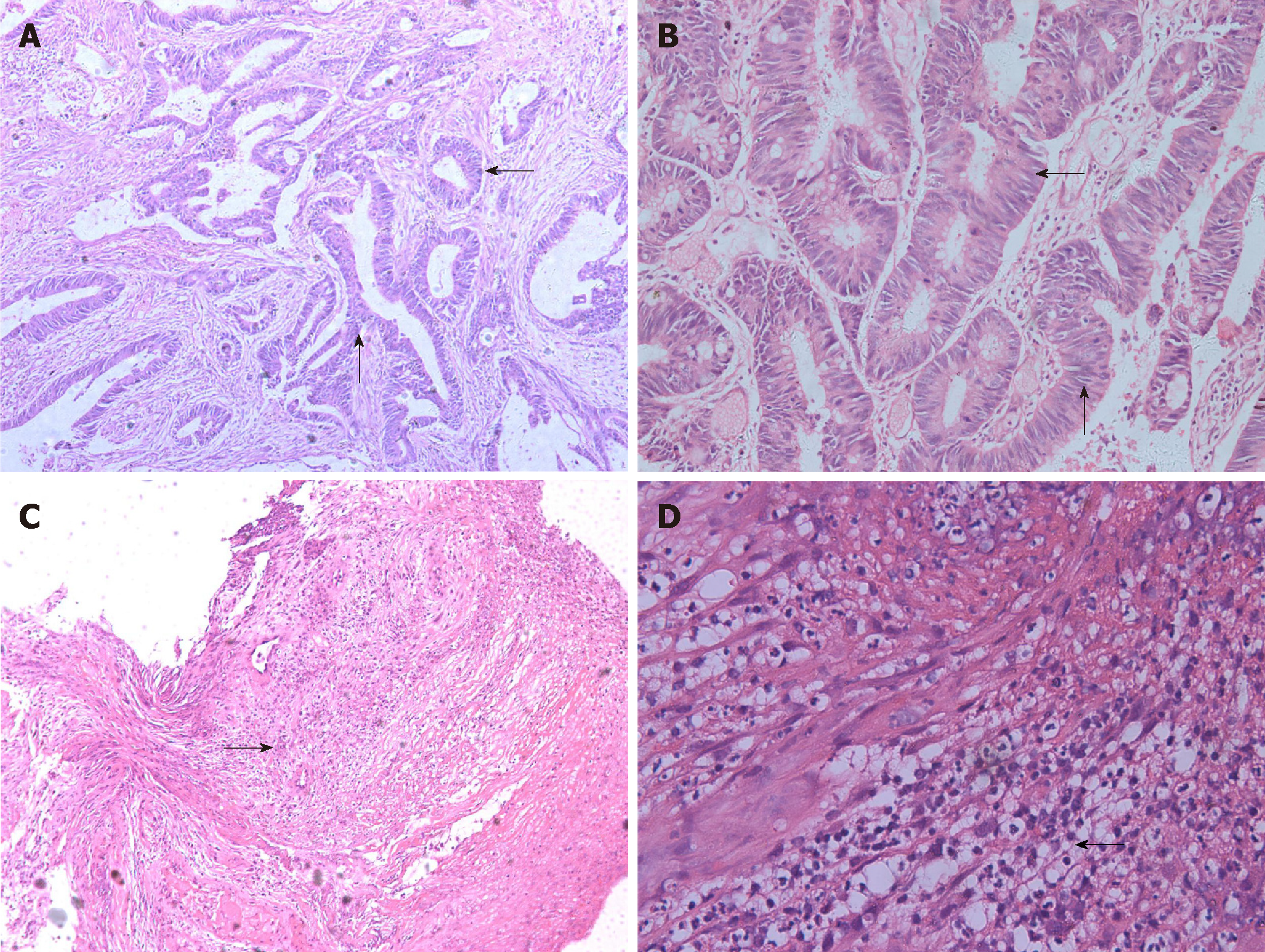
A digital rectal examination could touch half of the annular mass in the back wall of the rectum 4-5 cm away from the anal sphincter. The boundary of the mass was still clear, the range of motion of the mass was normal, and the intestinal canal was narrow, but a finger could be passed through. After the examination, scarlet coloured blood was present on the gloves. Additionally, no abnormalities were found on routine blood tests, routine urine tests, a urinary sediment examination, routine faecal tests, an occult blood test, blood biochemistry, and C12. Chest X-ray and abdominal B ultrasound also showed no abnormalities. However, colonoscopy revealed a 3 × 4 cm ulcerated mass in the rectum 4 cm from the anus (Figure 1A). Preoperative pathological examination showed villous adenoma, moderate-to-high-grade dysplasia, good differentiation, and invasion of the mucosal muscle. On December 5, 2012, the patient underwent TME and ileostomy by laparoscopy. After surgery, there was a rectal mucosal inflammation change without residual tumour on colonoscopy (Figure 1B). Postoperative histopathologic examination revealed that the tumour was a moderately differentiated adenocarcinoma and a partial mucinous adenocarcinoma; the TNM stage was T2N0M0, with no cancer in the incision margin and no metastasis to the lymph nodes. Unfortunately, cancer was detected on the distal surgical margin (Figure 2A and B). We suggested to the patient’s family that the patient should undergo an extended resection, which would require removal of the anus. However, the family rejected the proposal and asked for postoperative chemotherapy. Therefore, FOLFOX6 was administered on December 26, 2012. The patient recovered well and did not show obvious chemotherapeutic toxicity. Subsequently, FOLFOX6 was administered twice, and the patient’s liver function was not significantly abnormal. Until March 29, 2013, the patient received chemotherapy for the fourth time; however, the patient complained of abdominal pain, and the physical examination showed deep tenderness in the right upper abdomen, with no rebound pain, liver area percussion pain, and a positive result for Murphy’s syndrome. At the same time, the patient’s skin and sclera showed yellowing.
Laboratory tests showed that routine blood tests were normal. The neutrophil ratio was 76.50%, and the remaining results are as follows: alanine aminotransferase (ALT) 104.8 U/L, aspartate aminotransferase (AST) 232.7 U/L, total bilirubin (TBIL) 25.8 µmol/L, direct bilirubin (DBIL) 17.3 µmol/L, total bile acid (TBA) 218.2 µmol/L, and alkaline phosphatase (ALP) 220.2 U/L. There was no abnormality on abdominal B ultrasound. On March 30, 2013, the following liver function results were obtained: ALT 249.6 U/L, AST 212.8 U/L, TBIL 86.8 µmol/L, DBIL 63.7 µmol/L, TBA 387.5 µmol/L, r-glutamyl transferase 512.6 U/L, and ALP 309.5 U/L. Abdominal ultrasound revealed that the inner diameter of the common bile duct was approximately 9 mm. Then, a consultation was requested from a gastroenterologist, who considered the patient to have drug-induced hepatitis and recommended liver-protective treatment. Therefore, we stopped the chemotherapy programme. After two wk of treatment, the symptoms of skin and scleral yellowing gradually faded, and the results showed that the liver function had gradually improved but remained abnormal. Unfortunately, on April 20, 2013, the patient again complained of pain at the umbilicus, and the sclera was once again stained. Liver function tests showed ALT 158.1 U/L, AST 136.1 U/L, TBIL 55.9 µmol/L, DBIL 42.8 µmol/L, TBA 329.0 µmol/L, and ALP 265.6 U/L. The patient’s immune system index and complete hepatitis set showed no abnormalities.
Additionally, no obvious abnormalities were observed on magnetic resonance imaging of the liver and bile duct system. We continued to treat the patient with liver care. After two weeks, the jaundice gradually subsided, but liver function indicators were still abnormal. Considering the comparative advantage of the severe side effects of radiotherapy and chemotherapy and the relative advantages of PDT, we obtained consent from the patient and his family. On May 6, 2013, we implemented PDT for the patient. In the first 48 h of PDT, intravenous porphyrin was administered at a dose of 2 mg/kg. We used a 4-cm-long fibre to transmit a laser with a wavelength of 630 nm covering the proximal (1 cm) and distal ends of the anastomosis (3 cm). The power intensity was 100 mw/cm2, the irradiation time was 20 min, and the interval was 10 min. As expected, no side effects were observed throughout the PDT process. Approximately 3 wk after PDT, a circular piece of mucosal tissue fell off the anus (Figure 1C). The results of the liver function review were normal until discharge. Subsequently, the patient underwent repeated colonoscopy, and no recurrence was found. At that time, intestinal canal stenosis appeared, which recovered after expansion treatment of the anal rectum. The patient also showed no yellowing of the skin or sclera, and liver function was normal. Five years after the operation, the patient was generally in good condition, with no liver function impairment, sexual dysfunction, or abnormal defecation function. At that time, no recurrence was found on endoscopic ultrasonography (Figure 1D and E) or pathological examination (Figure 2C and D).
Rectal cancer (T2N0M0 with microscopic residual cancer).
The patient underwent TME operation. Pathological examination revealed a positive distal surgical margin. Chemotherapy was interrupted at three cycles of FOLFOX6 due to liver function damage. Thereafter, the patient received PDT irradiation to anastomosis area by colonoscopy.
No recurrence, metastasis, liver function impairment, or sexual dysfunction was observed and defecation function was normal. Mild stenosis around anastomosis area was found by colonoscopy without impact on daily life.
Colorectal cancer ranks among the top three cancers in both men and women worldwide[3]. Similarly, colorectal cancer ranks high in China[4]. Among colorectal cancers, the most unique form is low rectal cancer. Low rectal cancer refers to rectal cancer within 5 cm of the dentate line. Because the tumour is close to the anus and the anatomy of the pelvic cavity is complex, it is difficult to operate on such cancers. On the one hand, it is necessary to completely remove the tumour; on the other hand, it is necessary to retain the anus and the pelvic structure as much as possible. At present, the principle approach for radical surgery for rectal cancer is TME for radical resection, according to need, combined with adjuvant therapy, such as chemotherapy, radiation therapy (RT), and targeted therapy[4,5]. However, sphincter preservation for patients with low rectal cancer is controversial. Some support APR as a standard approach, while others support sphincter preservation. According to statistics, among patients with low rectal cancer who undergo TME surgery, there is a substantial risk of R1. A study from the Yonsei University College of Medicine, Republic of Korea reported a large series of TME patients (876 subjects), of whom 55 had R1 (5.5%), with 48 patients having a circumferential R1 and 7 patients having a distal R1[6]. In a large retrospective Dutch study, the rate of R1 was nearly 19%[7]. In the CLASICC trial of laparoscopic vs open surgery, the authors reported an R1 rate of 13%[8]. In addition, a positive distal margin is an important factor affecting prognosis. Kim et al[6] found that recurrence of anastomotic site is strongly related to R1 by multivariate analysis. A study from the Netherlands (Dutch-Swedish short-course RT trial) also supported these conclusions. In this RT trial, R1 was an independent prognostic factor not only for local relapse (LR) but also for metastases. When the total number of patients who developed metastases was considered, the difference was significant: 37.6% in patients with R1 vs 12.7% in patients with negative resection margins (R0) (P < 0.0001)[7]. Therefore, to avoid positive distal resection margins as far as possible, the operative distal resection margin should be at least 2 cm. This requirement may be relatively easy for patients with colon cancer or high rectal cancer. However, for patients with low rectal cancer, especially ultralow rectal cancer, this recommendation is hard to achieve without disregarding the patient’s anal sphincter and pelvic nerve function. Admittedly, new adjuvant therapy, as well as laparoscopy and robotics, have been considered for such patients, but the power of these techniques is limited. As a result, how to compensate for R1 is still a question worth exploring. Patients with intraoperative incipient positive margins should be recommended to undergo prolonged or extended resection. Extended resection surgery can remove positive margins, thus reducing the recurrence rate at the anastomosis and distant metastasis. However, reoperation carries a substantial risk and increases financial and physical burdens. More importantly, for most patients with low rectal cancer, prolonged or extended resection surgery means that the anal sphincter cannot be preserved, the pelvic nerve structure is impaired, and the patient experiences micturition and defecation and sexual disorders. This outcome has a substantial impact on the future quality of life of patients and their families, and many people cannot accept this result. In addition to surgery, the second option is adjuvant CRT. The European Society for Medical Oncology first added CRT to its guidelines in 2013[9]. Studies have shown that preoperative CRT can reduce the risk of locoregional recurrence[10], and this approach can reduce tumour size and the pathological stage of rectal carcinoma and increase the tumour resection rate and the anus retention rate[9-12]. However, the effect of postoperative CRT on the compensation of positive distal margins is not as good as expected, and there are numerous drawbacks. A retrospective study showed that in the neoadjuvant CRT group, only 14.3% of R1 patients developed LR. However, in the adjuvant CRT cohort, 50% of R1 patients showed LR after 2 years (P < 0.01)[13,14]. In a retrospective study from the Yonsei University College of Medicine, 13% of patients with R1 who were treated with adjuvant CRT developed LR[13]. In addition, in an experiment, 56 of 120 patients in the surgery-only group with R1 received postoperative radiotherapy, but the results showed that radiotherapy was of little significance[15]. Consequently, adjuvant therapy has a limited effect on the compensation of postoperative R1. However, CRT has many shortcomings[4]. First, there is resistance to chemotherapy drugs. Studies have found that almost all CRC patients develop resistance to chemotherapy[16]. Second, chemotherapy drugs produce treatment toxicity, including mild reactions such as nausea, vomiting, and hair loss and serious reactions such as liver and kidney failure. When cancer cells become resistant to one chemotherapy drug, they become resistant to others. In contrast, the main advantage of targeted therapy is that it reduces the toxicity of drugs through targeted action, but it still cannot avoid the problem of drug resistance[17]. For radiotherapy, there is no drug resistance, however, not only is the treatment ineffective, but many side effects occur[5]. Unfortunately, drug resistance and the toxic side effects of CRT in rectal cancer remain difficult problems. In particular, patients with liver and kidney dysfunction before surgery may even experience liver and kidney failure. In addition, long-term, multi-course treatment is a substantial challenge for patients and their families. Thus, in addition to continued optimization of surgery and CRT, we are also exploring whether there are new ways to compensate R1. In recent years, PDT has become an emerging treatment modality[4]. PDT as a mode of treatment differs from both conventional chemotherapy and radiotherapy. PDT is composed of photosensitizer, light, and reactive oxygen species (ROS). First, the photosensitizer accumulates in cancer cells, and then induces ROS formation via stimulation by light irradiation. Since photosensitizers are only locally deposited and activated in cancer cells, leading to tumour cell death through ROS effects, PDT has a targeted effect and does little harm to normal cells[16]. The greatest advantage of PDT, in addition to its high selectivity to tumours, is the lack of cross-resistance. A large amount of preclinical evidence shows that PDT can not only overcome drug resistance, but even make drug-resistant cells sensitive again[16,18]. Additionally, PDT has other advantages over traditional therapy. Treatment side effects, such as phototoxic and photoallergic reactions, are caused only by excessive amounts of photosensitizers[5]. On the other hand, PDT may cause inflammation and edema in the treated area, therefore, intestinal stenosis may be founded. However, there have been no reports on liver and kidney toxicity. Moreover, with the improvement of photosensitizers, the side effects of PDT are constantly being overcome. Therefore, PDT is gradually expanding its clinical applications with unique advantages and minor side effects. The tumour in the ULAR patient presented in this article had already invaded the mucosal muscle, with no lymph node or distant metastasis (T2N0M0) and no neoadjuvant therapy. Unfortunately, the microscopic distal margin was positive, and we suggested that the resection should be extended; this approach would mean that the anus would not be retained, and a fistula would be performed, which was strongly rejected by the patient’s family. Therefore, FOLFOX6 was used for chemotherapy after surgery. After three cycles of chemotherapy, the patient developed severe liver function impairment, and no obvious improvement was found after treatment for liver function impairment; thus, continued treatment was not possible. Therefore, after obtaining consent from the patient and the patient’s family, PDT was performed. No side effects were observed throughout the PDT process. After treatment, residual cancer was not detected, and the patient’s liver function improved gradually. After 8 mo of follow-up, no residual tumour recurrence was observed; intestinal canal stenosis was detected at that time but recovered after expansion treatment of the anal rectum. We tracked the patient's postoperative condition until 5 years after surgery. He lived as a normal person, without any discomfort. Endoscopic ultrasonography and pathological examination also confirmed that there was no recurrence at the anastomosis. This case provides a new approach for patients with positive microscopic distal margins, especially patients who have already experienced CRT side effects; PDT may be a better choice for these patients. We can infer that PDT has the same effect on positive circumference resection margins. Whether PDT can become a new treatment to compensate for R1 and whether the results of PDT in compensating for R1 are better than those of CRT require further investigation. In addition, whether the patient's intestinal stenosis was due to chemotherapy or PDT requires further study.
Photodynamic removal of positive distal margins is a safe and effective salvage treatment for R1 in patients with low rectal cancer. Further clinical trials should be conducted to compare therapeutic value between PDT and CRT, upon which postoperative stenosis should be investigated also to clarify the affection of PDT.
We thank Dr. Wen-Di Xu, Guang Liu, and Zhe-Xin Huang for help with the preparation and revision of the manuscript.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Friedel D, Vaudo G S-Editor: Yan JP L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Rullier E, Denost Q, Vendrely V, Rullier A, Laurent C. Low rectal cancer: Classification and standardization of surgery. Dis Colon Rectum. 2013;56:560-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 158] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 2. | Rickles AS, Dietz DW, Chang GJ, Wexner SD, Berho ME, Remzi FH, Greene FL, Fleshman JW, Abbas MA, Peters W, Noyes K, Monson JR, Fleming FJ; Consortium for Optimizing the Treatment of Rectal Cancer (OSTRiCh). High Rate of Positive Circumferential Resection Margins Following Rectal Cancer Surgery: A Call to Action. Ann Surg. 2015;262:891-898. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 3. | Kawczyk-Krupka A, Bugaj AM, Latos W, Zaremba K, Wawrzyniec K, Kucharzewski M, Sieroń A. Photodynamic therapy in colorectal cancer treatment--The state of the art in preclinical research. Photodiagnosis Photodyn Ther. 2016;13:158-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 4. | Ouyang G, Xiong L, Liu Z, Lam B, Bui B, Ma L, Chen X, Zhou P, Wang K, Zhang Z, Huang H, Miao X, Chen W, Wen Y. Inhibition of autophagy potentiates the apoptosis-inducing effects of photodynamic therapy on human colon cancer cells. Photodiagnosis Photodyn Ther. 2018;21:396-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Hodgkinson N, Kruger CA, Abrahamse H. Targeted photodynamic therapy as potential treatment modality for the eradication of colon cancer and colon cancer stem cells. Tumour Biol. 2017;39:1010428317734691. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 66] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Kim YW, Kim NK, Min BS, Huh H, Kim JS, Kim JY, Sohn SK, Cho CH. Factors associated with anastomotic recurrence after total mesorectal excision in rectal cancer patients. J Surg Oncol. 2009;99:58-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 7. | Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O'Callaghan C, Myint AS, Bessell E, Thompson LC, Parmar M, Stephens RJ, Sebag-Montefiore D; MRC CR07/NCIC-CTG CO16 Trial Investigators; NCRI Colorectal Cancer Study Group. Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: A prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial. Lancet. 2009;373:821-828. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 833] [Cited by in RCA: 745] [Article Influence: 46.6] [Reference Citation Analysis (0)] |
| 8. | Guillou PJ, Quirke P, Thorpe H, Walker J, Jayne DG, Smith AM, Heath RM, Brown JM; MRC CLASICC trial group. Short-term endpoints of conventional versus laparoscopic-assisted surgery in patients with colorectal cancer (MRC CLASICC trial): Multicentre, randomised controlled trial. Lancet. 2005;365:1718-1726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2360] [Cited by in RCA: 2290] [Article Influence: 114.5] [Reference Citation Analysis (0)] |
| 9. | Li Y, Wang J, Ma X, Tan L, Yan Y, Xue C, Hui B, Liu R, Ma H, Ren J. A Review of Neoadjuvant Chemoradiotherapy for Locally Advanced Rectal Cancer. Int J Biol Sci. 2016;12:1022-1031. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 187] [Cited by in RCA: 142] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 10. | Glimelius B. Neo-adjuvant radiotherapy in rectal cancer. World J Gastroenterol. 2013;19:8489-8501. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 44] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 11. | Watanabe T, Muro K, Ajioka Y, Hashiguchi Y, Ito Y, Saito Y, Hamaguchi T, Ishida H, Ishiguro M, Ishihara S, Kanemitsu Y, Kawano H, Kinugasa Y, Kokudo N, Murofushi K, Nakajima T, Oka S, Sakai Y, Tsuji A, Uehara K, Ueno H, Yamazaki K, Yoshida M, Yoshino T, Boku N, Fujimori T, Itabashi M, Koinuma N, Morita T, Nishimura G, Sakata Y, Shimada Y, Takahashi K, Tanaka S, Tsuruta O, Yamaguchi T, Yamaguchi N, Tanaka T, Kotake K, Sugihara K; Japanese Society for Cancer of the Colon and Rectum. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23:1-34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 576] [Cited by in RCA: 603] [Article Influence: 86.1] [Reference Citation Analysis (0)] |
| 12. | Wen B, Zhang L, Wang C, Huang R, Peng H, Zhang T, Dong J, Xiao W, Zeng Z, Liu M, Gao Y. Prognostic significance of clinical and pathological stages on locally advanced rectal carcinoma after neoadjuvant chemoradiotherapy. Radiat Oncol. 2015;10:124. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Fekete Z, Muntean A, Irimie A, Hica S, Resiga L, Todor N, Nagy V. What is the significance of a microscopically positive resection margin in the curative-intent treatment of rectal adenocarcinoma? A retrospective study. J BUON. 2013;18:989-995. [PubMed] |
| 14. | Shin JW, Amar AH, Kim SH, Kwak JM, Baek SJ, Cho JS, Kim J. Complete mesocolic excision with D3 lymph node dissection in laparoscopic colectomy for stages II and III colon cancer: Long-term oncologic outcomes in 168 patients. Tech Coloproctol. 2014;18:795-803. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Marijnen CA, Nagtegaal ID, Kapiteijn E, Kranenbarg EK, Noordijk EM, van Krieken JH, van de Velde CJ, Leer JW; Cooperative investigators of the Dutch Colerectal Cancer Group. Radiotherapy does not compensate for positive resection margins in rectal cancer patients: Report of a multicenter randomized trial. Int J Radiat Oncol Biol Phys. 2003;55:1311-1320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 190] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 16. | Kukcinaviciute E, Sasnauskiene A, Dabkeviciene D, Kirveliene V, Jonusiene V. Effect of mTHPC-mediated photodynamic therapy on 5-fluorouracil resistant human colorectal cancer cells. Photochem Photobiol Sci. 2017;16:1063-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 17. | Panczyk M. Pharmacogenetics research on chemotherapy resistance in colorectal cancer over the last 20 years. World J Gastroenterol. 2014;20:9775-9827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 98] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 18. | Spring BQ, Rizvi I, Xu N, Hasan T. The role of photodynamic therapy in overcoming cancer drug resistance. Photochem Photobiol Sci. 2015;14:1476-1491. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |