Published online Mar 26, 2019. doi: 10.12998/wjcc.v7.i6.785
Peer-review started: November 28, 2018
First decision: January 19, 2019
Revised: February 3, 2019
Accepted: February 26, 2019
Article in press: February 26, 2019
Published online: March 26, 2019
Processing time: 118 Days and 13.8 Hours
Follicular dendritic cell sarcoma (FDCS) is an uncommon type of tumor with low incidence. To date, no standard treatment for the disease has been established. Surgery remains the main treatment. Adjuvant chemotherapy and radiotherapy are optional approaches. Metastatic cases require multidisciplinary collaborative treatments. However, the choice of chemotherapeutic drugs is controversial.
A 66-year-old Chinese woman presented to our hospital complaining of intermittent pain of right upper quadrant. An enhanced computed tomography (CT) scan of the abdomen revealed hepatocellular carcinoma. Subsequently, the patient underwent a radical partial hepatectomy. Primary FDCS of the liver was diagnosed pathologically. Except for regular follow-up examinations, the patient did not receive adjuvant chemotherapy or radiotherapy. However, fluorine-18-fluorodeoxyglucose positron emission tomography/CT (PET/CT) confirmed lymph node metastases in the space of ligamentum hepatogastricum and pancreatic head, as well as the portacaval space. The patient was given systemic chemotherapy with gemcitabine and docetaxel for she was unsuitable for surgery. Satisfactorily, the metastatic lymph nodes were significantly reduced to clinical complete remission after eight cycles of chemotherapy. Then, strengthened radiotherapy was followed when the patient rejected the opportunity of surgery. Eventually, the carcinoma got better control and the patient was free of progression.
This case highlights the importance of making suitable chemotherapy regimens for the rare tumor. The combination of gemcitabine, docetaxel, and consolidated radiotherapy may offer a new promising option for the treatment of metastatic hepatic FDCS in the future.
Core tip: This case report is important because it provides a potential strategy for hepatic follicular dendritic cell sarcoma, which is a rare tumor with no standard treatment to date. The choice of chemotherapeutic drugs is currently controversial. Main systemic chemotherapy drugs focus on those applied on lymphoma and soft tissue sarcoma, but the efficacy is variable. In our case, cytotoxic drugs such as gemcitabine and docetaxel play an important role in antitumor therapy. The role of follow-up radical radiotherapy should not be ignored.
- Citation: Chen HM, Shen YL, Liu M. Primary hepatic follicular dendritic cell sarcoma: A case report. World J Clin Cases 2019; 7(6): 785-791
- URL: https://www.wjgnet.com/2307-8960/full/v7/i6/785.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i6.785
Follicular dendritic cell sarcoma (FDCS) is a rare, indolently growing sarcoma with unclear etiology, originating from follicular dendritic cells, which play an important role in antigen presentation to B cells in lymphoid organs[1]. FDCS mainly occurs in the lymph nodes but can also be present in extranodal sites. The liver is an organ commonly involved in the development of extranodal FDCS. Primary FDCS of the liver constitutes less than 0.1% of primary liver neoplasms[2], and only a small number of single cases, as well as case series, have been reported to date.
Complete surgery with or without adjuvant chemotherapy or radiotherapy is the choice of therapy for the treatment of hepatic FDCS. However, the recurrence of hepatic FDCS and the development of distant metastasis are major causes for therapeutic failure. Systemic chemotherapy with regimens that are applicable to lymphoma and soft tissue sarcoma can bring a certain benefit for the treatment of metastatic FDCS[3,4]. In this paper, we describe a patient with metastatic hepatic FDCS who responded remarkably to a combination of gemcitabine, docetaxel, and radiotherapy treatment.
A 66-year-old female patient had experienced intermittent right epigastric pain for almost 2 mo.
A 66-year-old Chinese female presented to our hospital with intermittent right epigastric pain for 6 mo. An enhanced computed tomography (CT) scan of the abdomen revealed a solid lesion with a size of 2.3 cm × 2.3 cm in the junction of the left lateral lobe of the liver. Contrast-enhanced ultrasound suggested the presence of hepatocellular carcinoma. Subsequently, a radical partial hepatectomy was performed and the operation went smoothly. Except for regular follow-up examinations, the patient did not receive adjuvant chemotherapy or radiotherapy. However, enhanced CT of the abdomen and fluorine-18-fluorodeoxyglucose positron emission tomography/CT (PET/CT) suggested lymph node metastases. She was unsuitable for surgery because of the location and number of metastatic lymph nodes. The patient was admitted to our department for further evaluation and treatment.
She had a 16-year history of chronic hepatitis B virus infection.
Unremarkable.
Vital signs, such as respiration, pulse, temperature, and blood pressure, were within the normal range. There were no other positive signs with the exception of mild tenderness in the right upper abdomen.
The initial laboratory examinations included a complete blood count which showed a normal white blood cell count, haemoglobin, and platelet count. Liver enzymes were elevated: alanine transaminase, 26 IU/L; aspartate transaminase, 23 IU/L; and serum albumin, 27.3 g/L. Quantitative analysis of hepatitis B markers revealed that the level of hepatitis B virus surface antigen was 829.9 IU/mL. She was negative for hepatitis B surface antibody and hepatitis B virus e antigen, but positive for hepatitis B e antibody and hepatitis B core antibody.
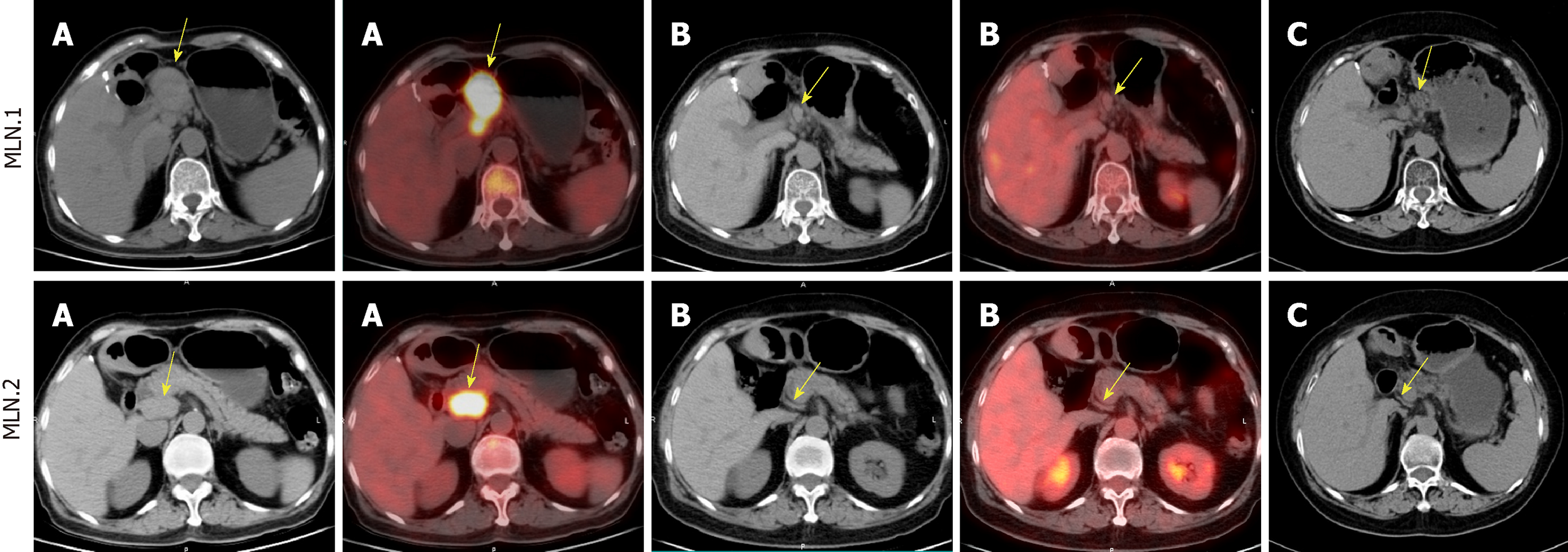
Contrast-enhanced CT of the abdomen revealed enlarged lymph nodes in the portacaval space and the zone of the pancreatic head and ligamentum hepatogas-tricum. PET/CT confirmed metastases according to the history of hepatic FDCS (Figure 1A).
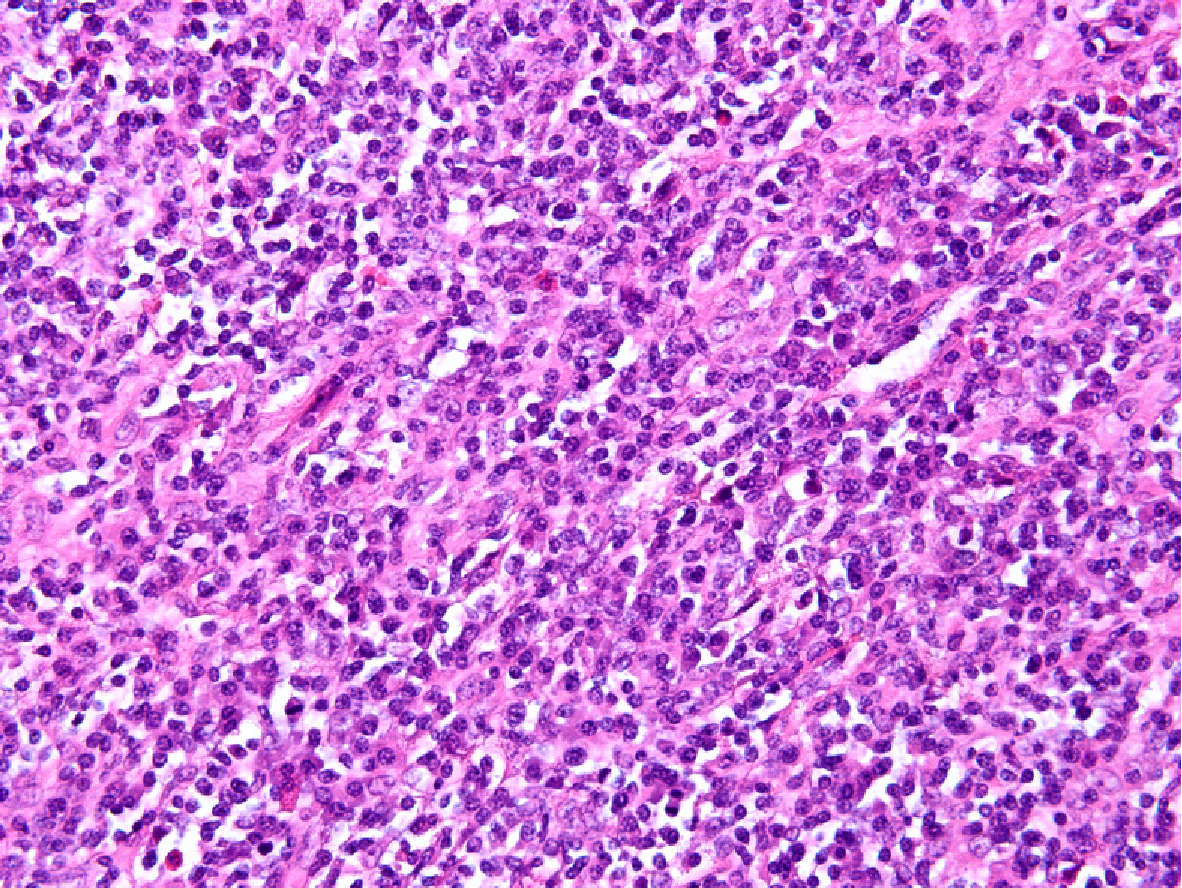
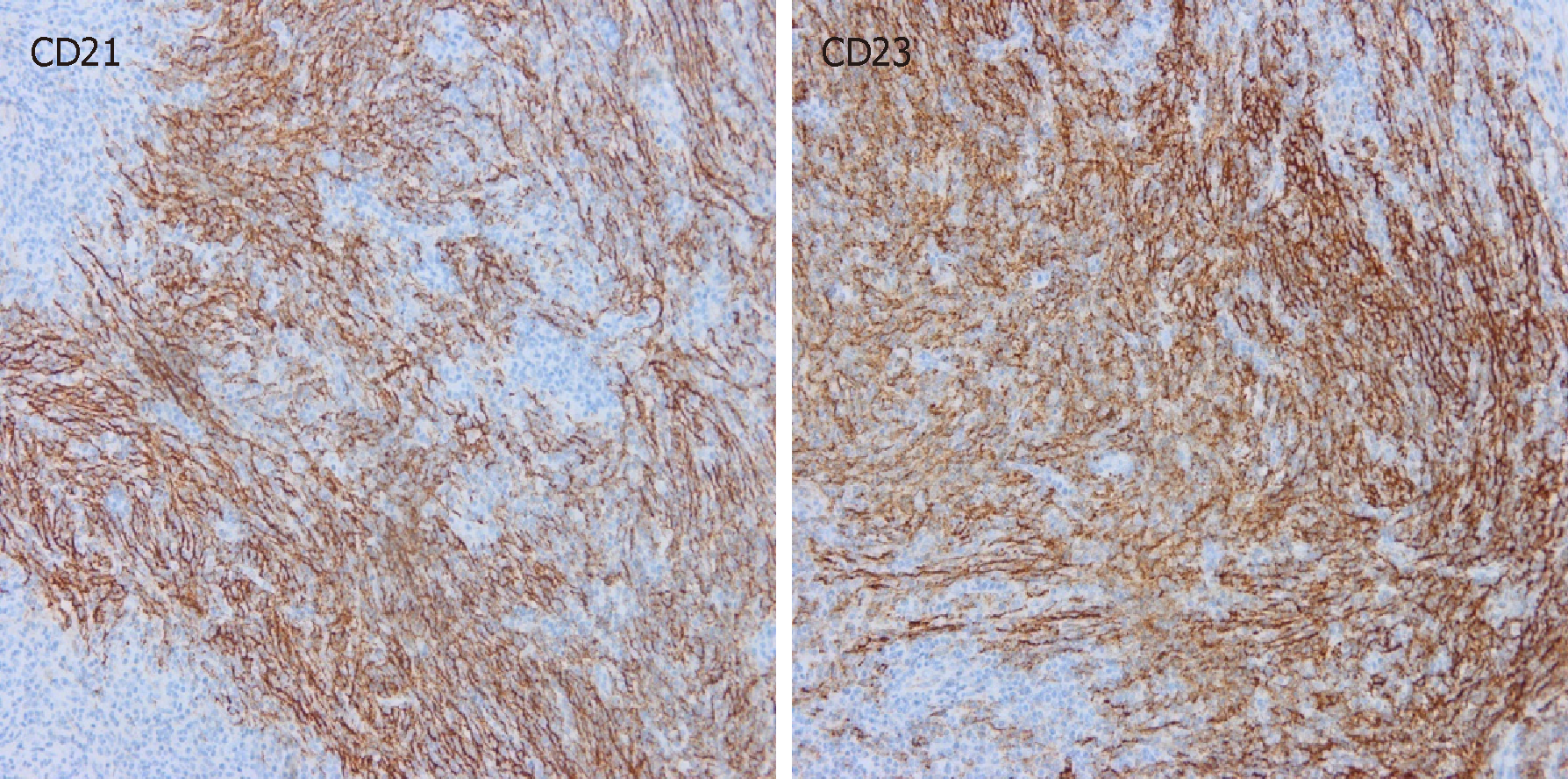
At the microscopic level, the tumor was composed of spindle cells, abundant small lymphocytes and mature plasmocytes (Figure 2). Immunohistochemical staining showed that the spindle cells were positive for CD21 and CD23 (Figure 3), and in situ hybridization revealed that the tumor cells were positive for Epstein-Barr-encoded-RNA and negative for CD34, CD35, and S-100. The positive rate of expression of Ki-67 was 20%. Primary FDCS of the liver was diagnosed pathologically after operation. Considering the history of hepatic FDCS and the images of enlarged lymph nodes, the final diagnosis was hepatic FDCS with celiac lymph node metastases.
At first there were no surgical indications for metastatic lymph nodes. The patient was given systemic chemotherapy with gemcitabine and docetaxel (gemcitabine administered at 1000 mg/m2 intravenously on days 1 and 8, docetaxel administered at 75 mg/m2 intravenously on day 1 every three weeks). Afterwards, the patient was able to undergo resection of its metastatic lymph nodes after chemoherapy, but she refused surgery and chose to receive radiotherapy. A total dose of 50.4 Gy was applied with a conventional fractionation of 1.8 Gy/d.
Images of PET/CT showed that the mass significantly decreased to complete clinical remission after eight cycles of chemotherapy (Figure 1B). And the metastatic lymph nodes were reduced continually one month after radiotherapy (Figure 1C). The patient was free of metastatic progression and in good physical condition at 1 year after chemoradiotherapy.
FDCS, first reported by Monda in 1986[5], is a rare malignant tumor that is derived from the abnormal proliferation and differentiation of follicular dendritic cells in the lymphoid follicles[6]. It has been reported that approximately two-thirds of FDCS occurred in the lymph nodes, mainly involving cervical lymph nodes. The other one third of FDCS were present in extranodal locations, such as the liver, spleen, gastrointestinal tract, lung, and head-and-neck soft tissue[7]. The rate of hepatic FDCS is approximately 13%[8]. There is no obvious age or sex difference in the incidence of FDCS.
FDCSs have an unclear etiology. Approximately 10%-20% of FDCSs are correlated with hyaline vascular Castleman disease, which is considered a cause of FDCS, because the epidermal growth factor receptor can be detected in both of these diseases[6,9]. Epstein-Barr virus (EBV) infection is present in some FDCSs. About 12% of FDCSs can be detected with EBV infection. Furthermore, the incidence rate of hepatic and splenic primary FDCS, especially the inflammatory pseudotumor-like FDCS (IPT-like FDCS), is higher[8,10].
IPT-like FDCS is an exceptionally rare and low-grade malignancy that has not been fully recognized by clinicians and pathologists. By the end of 2016, fewer than 100 cases had been reported, and more than 90% of the cases occurred in the liver or spleen[11]. Hepatic IPT-like FDCS is often differentiated from IPT for similar histomorphology. Detection of EBV and FDC-related immune markers plays an important role in differential diagnosis between two diseases[11,12].
Histopathological examination is the gold standard for the diagnosis of FDCS. Hepatic or splenic FDCS is composed of spindle to ovoid shaped-cells, lymphocytes, and plasmacytes, which are arranged in a cluster, storiform, or spiral pattern[13]. The immunohistochemical markers CD21, CD23, and/or CD35 are generally expressed in FDCS[6]. Rate of Ki-67 positive expression is also an important index of prognosis[14].
There is no guideline or agreement for the treatment of FDCS. Currently, surgery is the primary choice for treatment, and adjuvant chemotherapy or radiotherapy can be used to improve survival in the case of resectable FDCS. A retrospective study indicated that a combination of surgery, chemotherapy, and radiation could lead to a long disease-free survival[15]. The rates of local recurrence and metastasis are approximately 40% to 50% and 10% to 20%, respectively, and they are higher in extranodal FDCS[6]. Systemic chemotherapy, with or without radiotherapy, is usually considered once distant metastasis occurs.
Chemotherapy regimens are mostly used to treat lymphoma and soft tissue sarcoma according to previous case reports. CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone), which is frequently used for lymphoma treatment, has been attempted to treat FDCS. A study reported that a patient with hepatic FDCS was successfully treated with CHOP and surgery; however, the mass did not decrease significantly in size but showed reduced fluorodeoxyglucose avidity and enlarged necrosis in the neoplasm[3]. Another patient with FDCS in the liver and spleen underwent chemotherapy with CHOP and achieved a limited partial response[16]. However, a review reported by Li et al[17] showed that 3 out of 106 patients primarily treated with CHOP chemotherapy did not respond to therapy[17]. In addition, CHOP chemotherapy regimen used in FDCS patients did not achieve an effect similar to that observed in non-Hodgkin’s lymphoma patients. The reason for this situation may be that drugs used in CHOP regimen did not directly break down follicular dendritic cells[18]. Recently, Vermi et al[19] have suggested that follicular dendritic precursors originate from mesenchymal cells rather than lymphatic cells, and that follicular dendritic cells play an important role in the formation of follicular B cells throughout the body during chronic inflammation.
Gemcitabine and taxane are usually used for the treatment of soft tissue sarcoma. A study[4] analyzed 66 FDCS patients, where 10 out of 16 measureable FDCS patients received gemcitabine and taxane; the overall response rate was 80% (complete response, n = 6; partial response, n = 2) with a median duration response of 13.4 mo. Conry et al[18] has reported two cases of FDCS who both achieved a partial response to gemcitabine and docetaxel. The FDCS patient we have reported here also showed a remarkable response with complete clinical response observed by PET/CT after chemotherapy. These findings demonstrate that FDCSs probably originate from mesenchymal tissues.
Radiotherapy plays a key role in multidisciplinary treatment of FDCS. Surgery and adjuvant radiotherapy can improve local control of the disease compared to surgery alone[4,20]. However, for salvage treatment, radiotherapy could not shrink effectively tumor size and the local progression of the disease occurred even after high doses of radiotherapy[4]. It seems that the initial management of FDCS is very important. In the case we have reported here, the tumor metastasized to the abdominal lymph nodes within 34 mo after resection without adjuvant therapy. Chemotherapy and radiotherapy were used to control the disease progression. However, we do not know what role radiotherapy played in achieving complete clinical remission, after administration of chemotherapy composed of gemcitabine and docetaxel.
In conclusion, FDCS has no standard treatment. According to the literature and the remarkable results achieved in our case, it is possible that a combination of gemcitabine, docetaxel and consolidated radiotherapy will offer a new promising option for the treatment of metastatic hepatic FDCS in the future.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Hilmi I, Kai K S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Ohtake H, Yamakawa M. Interdigitating Dendritic Cell Sarcoma and Follicular Dendritic Cell Sarcoma: Histopathological Findings for Differential Diagnosis. J Clin Exp Hematop. 2013;53:179-184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 25] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Patel JA, Piper JB, Wang BG. Follicular dendritic cell sarcoma of the porta hepatis. BMJ Case Rep. 2018;2018:bcr-2017-223232. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Shinagare AB, Ramaiya NH, Jagannathan JP, Hornick JL, Swanson RS. Primary follicular dendritic cell sarcoma of liver treated with cyclophosphamide, doxorubicin, vincristine, and prednisone regimen and surgery. J Clin Oncol. 2011;29:e849-e851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Jain P, Milgrom SA, Patel KP, Nastoupil L, Fayad L, Wang M, Pinnix CC, Dabaja BS, Smith GL, Yu J, Hu S, Bueso Ramos CE, Kanagal-Shamanna R, Medeiros LJ, Oki Y, Fowler N. Characteristics, management, and outcomes of patients with follicular dendritic cell sarcoma. Br J Haematol. 2017;178:403-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 5. | Monda L, Warnke R, Rosai J. A primary lymph node malignancy with features suggestive of dendritic reticulum cell differentiation. A report of 4 cases. Am J Pathol. 1986;122:562-572. [PubMed] |
| 6. | Wu A, Pullarkat S. Follicular Dendritic Cell Sarcoma. Arch Pathol Lab Med. 2016;140:186-190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 7. | Chen T, Gopal P. Follicular Dendritic Cell Sarcoma. Arch Pathol Lab Med. 2017;141:596-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 57] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Cheuk W, Chan JK, Shek TW, Chang JH, Tsou MH, Yuen NW, Ng WF, Chan AC, Prat J. Inflammatory pseudotumor-like follicular dendritic cell tumor: a distinctive low-grade malignant intra-abdominal neoplasm with consistent Epstein-Barr virus association. Am J Surg Pathol. 2001;25:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 219] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 9. | Youens KE, Waugh MS. Extranodal follicular dendritic cell sarcoma. Arch Pathol Lab Med. 2008;132:1683-1687. [PubMed] |
| 10. | Chen TC, Kuo TT, Ng KF. Follicular dendritic cell tumor of the liver: a clinicopathologic and Epstein-Barr virus study of two cases. Mod Pathol. 2001;14:354-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 43] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 11. | Wu YL, Wu F, Yang L, Sun H, Yan XC, Duan GJ. [Clinicopathologic features and prognosis of inflammatory pseudotumor-like follicular dendritic cell sarcomas in liver and spleen: an analysis of seven cases]. Zhonghua Bing Li Xue Za Zhi. 2018;47:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Zhang X, Zhu C, Hu Y, Qin X. Hepatic inflammatory pseudotumour-like follicular dendritic cell tumor: A case report. Mol Clin Oncol. 2017;6:547-549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Martins PN, Reddy S, Martins AB, Facciuto M. Follicular dendritic cell sarcoma of the liver: unusual presentation of a rare tumor and literature review. Hepatobiliary Pancreat Dis Int. 2011;10:443-445. [PubMed] |
| 14. | Yan WX, Yu YX, Zhang P, Liu XK, Li Y. Follicular dendritic cell sarcoma detected in hepatogastric ligament: A case report and review of the literature. World J Clin Cases. 2019;7:116-121. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Soriano AO, Thompson MA, Admirand JH, Fayad LE, Rodriguez AM, Romaguera JE, Hagemeister FB, Pro B. Follicular dendritic cell sarcoma: a report of 14 cases and a review of the literature. Am J Hematol. 2007;82:725-728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 95] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 16. | Fonseca R, Tefferi A, Strickler JG. Follicular dendritic cell sarcoma mimicking diffuse large cell lymphoma: a case report. Am J Hematol. 1997;55:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Li L, Shi YH, Guo ZJ, Qiu T, Guo L, Yang HY, Zhang X, Zhao XM, Su Q. Clinicopathological features and prognosis assessment of extranodal follicular dendritic cell sarcoma. World J Gastroenterol. 2010;16:2504-2519. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 73] [Cited by in RCA: 83] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 18. | Conry RM. Response of follicular dendritic cell sarcoma to gemcitabine and docetaxel: report of two cases and literature review. Clin Sarcoma Res. 2014;4:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 19. | Vermi W, Giurisato E, Lonardi S, Balzarini P, Rossi E, Medicina D, Bosisio D, Sozzani S, Pellegrini W, Doglioni C, Marchetti A, Rossi G, Pileri S, Facchetti F. Ligand-dependent activation of EGFR in follicular dendritic cells sarcoma is sustained by local production of cognate ligands. Clin Cancer Res. 2013;19:5027-5038. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 25] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 20. | Pang J, Mydlarz WK, Gooi Z, Waters KM, Bishop J, Sciubba JJ, Kim YJ, Fakhry C. Follicular dendritic cell sarcoma of the head and neck: Case report, literature review, and pooled analysis of 97 cases. Head Neck. 2016;38 Suppl 1:E2241-E2249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |