Published online Mar 26, 2019. doi: 10.12998/wjcc.v7.i6.717
Peer-review started: November 16, 2018
First decision: December 29, 2018
Revised: January 23, 2019
Accepted: February 26, 2019
Article in press: February 26, 2019
Published online: March 26, 2019
Processing time: 131 Days and 11.2 Hours
Transduodenal ampullectomy (TDA) is not in wide clinical use due to its low radical effect and a high recurrence rate of tumors. However, TDA is still an effective treatment method; it has great clinical value in cases of duodenal benign tumors, precancerous lesions, and benign and malignant borderline tumors, and can avoid the risks associated with pancreaticoduodenectomy with larger resection range and greater thoroughness than endoscopic papillectomy.
To investigate the surgical method choice and the coincidence rate of pathological diagnoses in TDA for ampullary neoplasms.
Ten patients with ampullary neoplasms underwent TDA based on the fact that their endoscopic biopsy results suggested benign lesions, and the endoscopic ultrasound (EUS)-assessed tumors were resectable. All cases underwent duodenal ampullary lesion endoscopic biopsy, intraoperative frozen-section pathological examination, and postoperative pathological examination.
This study included seven patients with benign tumors and three with malignant tumors (1 pTis, 2 pT1), according to the postoperative pathology results. The coincidence rate of the postoperative pathology results with the intraoperative frozen-section biopsy results was 100% (10/10), and the coincidence rate with the endoscopic biopsy results was 70% (7/10) based on pathological characteristics. The endoscopic biopsy false-negative rate was 30% (3/10). All patients were followed for 6 to 70 mo without tumor recurrence or metastasis.
The coincidence rate of postoperative pathology results, intraoperative frozen-section pathology results, and endoscopic biopsy results is the restraining factor of TDA clinical application. Endoscopic biopsy results and EUS have importance relevance to surgical planning. Intraoperative frozen-section pathology results have a significant influence on the choice of surgical procedure.
Core tip: Transduodenal ampullectomy (TDA) is still an effective treatment method and is of great clinical value in cases of duodenal benign tumors, precancerous lesions, and benign and malignant borderline tumors. To investigate the surgical method choices and the coincidence rate of pathological diagnoses in TDA, we retrospectively analyzed ten patients with ampullary neoplasms who underwent TDA based on the fact that their endoscopic biopsy results suggested benign lesions and the endoscopic ultrasound-assessed tumors were resectable. The coincidence rate of postoperative pathology results, intraoperative frozen-section pathology results, and endoscopic biopsy results is the restraining factor of TDA clinical application.
- Citation: Liu F, Cheng JL, Cui J, Xu ZZ, Fu Z, Liu J, Tian H. Surgical method choice and coincidence rate of pathological diagnoses in transduodenal ampullectomy: A retrospective case series study and review of the literature. World J Clin Cases 2019; 7(6): 717-726
- URL: https://www.wjgnet.com/2307-8960/full/v7/i6/717.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i6.717
Ampullary neoplasms refer to benign and malignant tumors located around the ampulla of Vater, including duodenal papillary tumors, tumors of the ampulla of Vater, and tumors of the terminal portion of the common bile duct, but usually excluding pancreatic tumors. The most common ampullary neoplasm is duodenal papillary tumor. Among ampullary neoplasms, benign tumors are rare[1]; the most common benign lesions are villous adenomas and tubular villous adenomas[2]. Other rare tumors include hamartoma, adenomyoma, carcinoid, gastrointestinal stromal tumor, neuroendocrine tumor, and mixed neuroendocrine tumor[3-9]. In addition, ectopic pancreas can also mimic a tumor of the ampulla of Vater[10].
At present, the treatments for ampullary tumors mainly include pan-creaticoduodenectomy (PD), transduodenal ampullectomy (TDA) or local resection (LR), and endoscopic papillectomy (EP). Ampullectomy was first introduced by Halsted in 1899, and pancreatoduodenectomy was introduced in 1935 by Whipple. In recent years, due to the development of minimally invasive technique, EP has also been used to treat duodenal papilla tumors and is especially recommended for the treatment of benign papillary lesions[11,12]. Endoscopic ultrasound (EUS) is also used to guide the selection of tumor resection methods[13].
TDA has not gained wide clinical use due to its low radical effect and a high recurrence rate of tumors[14]. However, TDA is still an effective treatment method with great clinical value in cases of benign duodenal tumors, precancerous lesions, and benign and malignant borderline tumors; it can avoid the risk associated with PD and offers larger resection range and greater thoroughness than EP. The safety of TDA in patients with benign periampullary tumors is similar to that of EP[15].
TDA can be considered as a treatment choice between EP and radical PD[16] in cases in which preoperative imaging examination indicates no evidence of abdominal lymph node, liver, or lung metastasis and in which there is evidence of a normal digestive tract tumor index. The pathology results for ampullary lesions can be obtained through endoscopic biopsy. However, due to limitations in acquiring tissue for biopsy, the biopsy results may be false-negative. During the process of TDA, intraoperative frozen-section pathological examination is needed to further determine the pathological characteristics and the status of resection margins. The coincidence rate of postoperative pathology results, intraoperative frozen-section pathology results, and endoscopic biopsy results is the factor restraining its clinical application. Endoscopic biopsy results and intraoperative frozen-section pathology results have a significant influence on surgical planning.
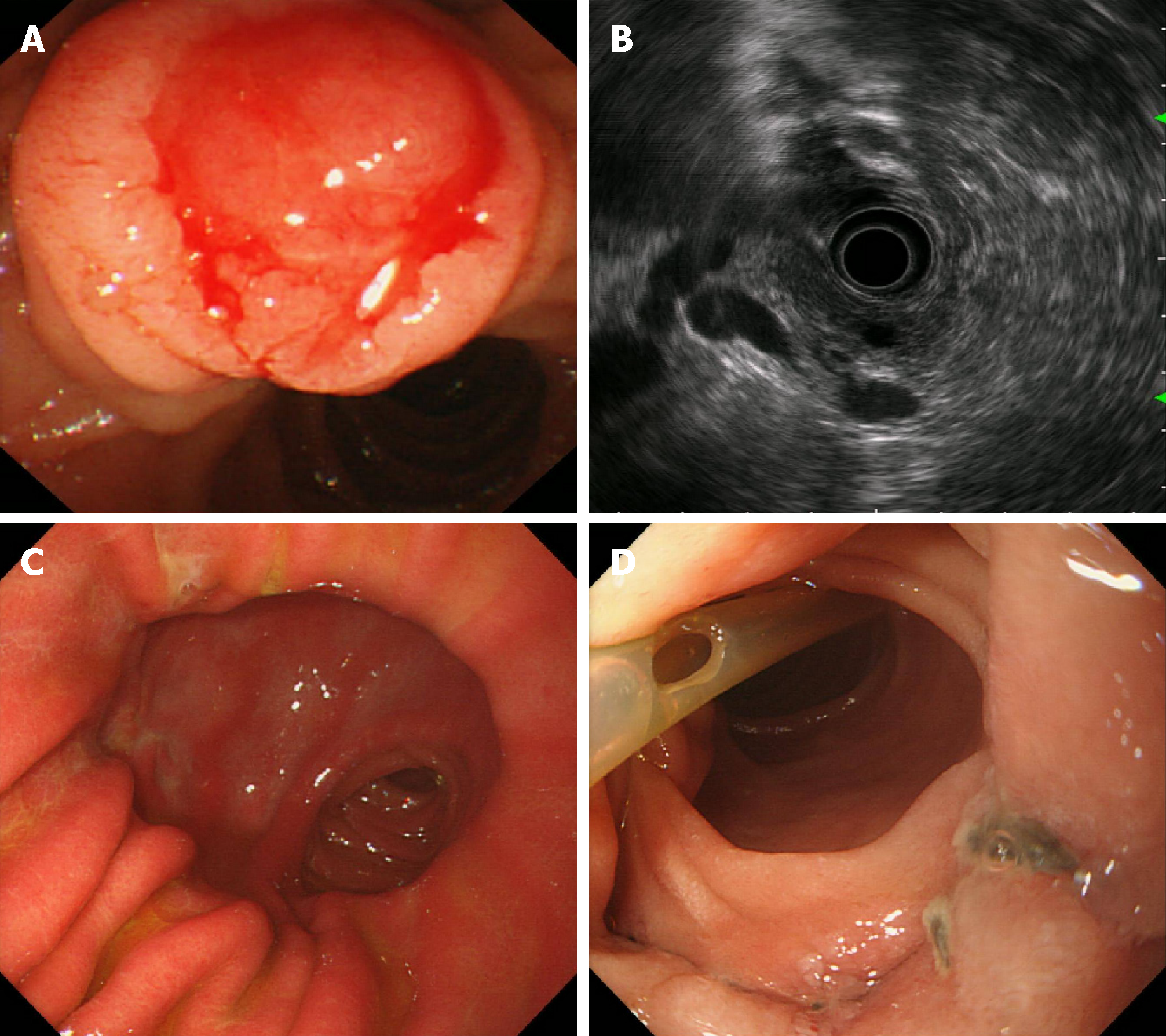
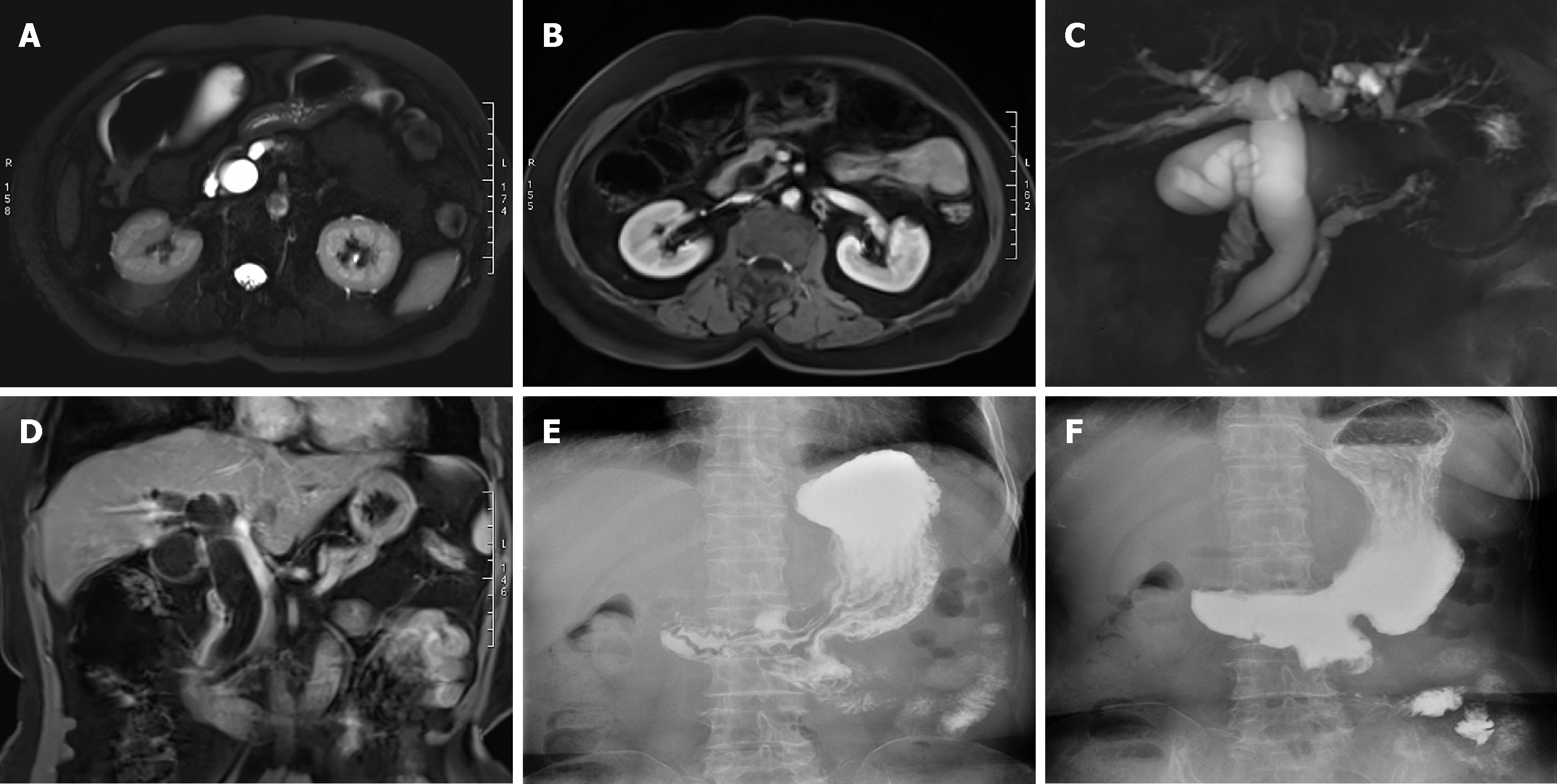
From December 2011 to October 2017, ten patients (four men and six women) with an average age of 66.2 years (range, 51-77 years) underwent TDA for ampullary neoplasms at our hospital. Endoscopic biopsy results showed benign lesions (Table 1). Main clinical symptoms were jaundice and abdominal pain. All cases underwent a duodenal papilla biopsy (Figure 1A and B) and an intraoperative frozen-section pathological examination. All patients underwent EUS, computed tomography (CT), or magnetic resonance imaging (MRI) prior to surgery (Figure 2A-D) as well as preoperative examination, which indicated that there was no hepatic or pulmonary metastasis or abdominal lymph node enlargement. This study was approved by the ethics committee of our hospital, and all patients provided informed consent to par-ticipation in the study. This study was a single-center nonconsecutive retrospective case series study, and the work was reported in accordance with the PROCESS criteria[17].
| Case | Sex | Age (y) | Tumor size (cm) | Resection margin | Biopsy pathology | Frozen-section pathology | Postoperative pathology | Tumor recurrence | Survival time (m) | Outcome | Complication |
| 1 | M | 56 | 1.5×1.5 | R0 | 3 | 1 | 1 | No | 70 | Alive | No |
| 2 | F | 60 | 2.0×1.0 | R0 | 1 | 2 | 2 (pTis) | No | 41 | Alive | No |
| 3 | M | 77 | 1.5×1.5 | R0 | 4 | 1 | 1 | No | 32 | Alive | No |
| 4 | F | 69 | 1.5×1.5 | R0 | 4 | 2 | 2 (pT1) | No | 29 | Alive | No |
| 5 | F | 59 | 2.0×1.3 | R0 | 1 | 5 | 5 | No | 18 | Alive | No |
| 6 | F | 67 | 2.4×2.2 | R0 | 1 | 1 | 1 | No | 13 | Alive | No |
| 7 | F | 64 | 1.5×1.2 | R0 | 3 | 2 | 2 (pT1) | No | 13 | Alive | No |
| 8 | M | 57 | 2.5×1.5 | R0 | 1 | 1 | 1 | No | 11 | Alive | No |
| 9 | M | 51 | 1.5×1.5 | R0 | 6 | 6 | 6 | No | 7 | Alive | No |
| 10 | F | 62 | 1.0×1.0 | R0 | 1 | 6 | 6 | No | 6 | Alive | Yes (bleeding) |
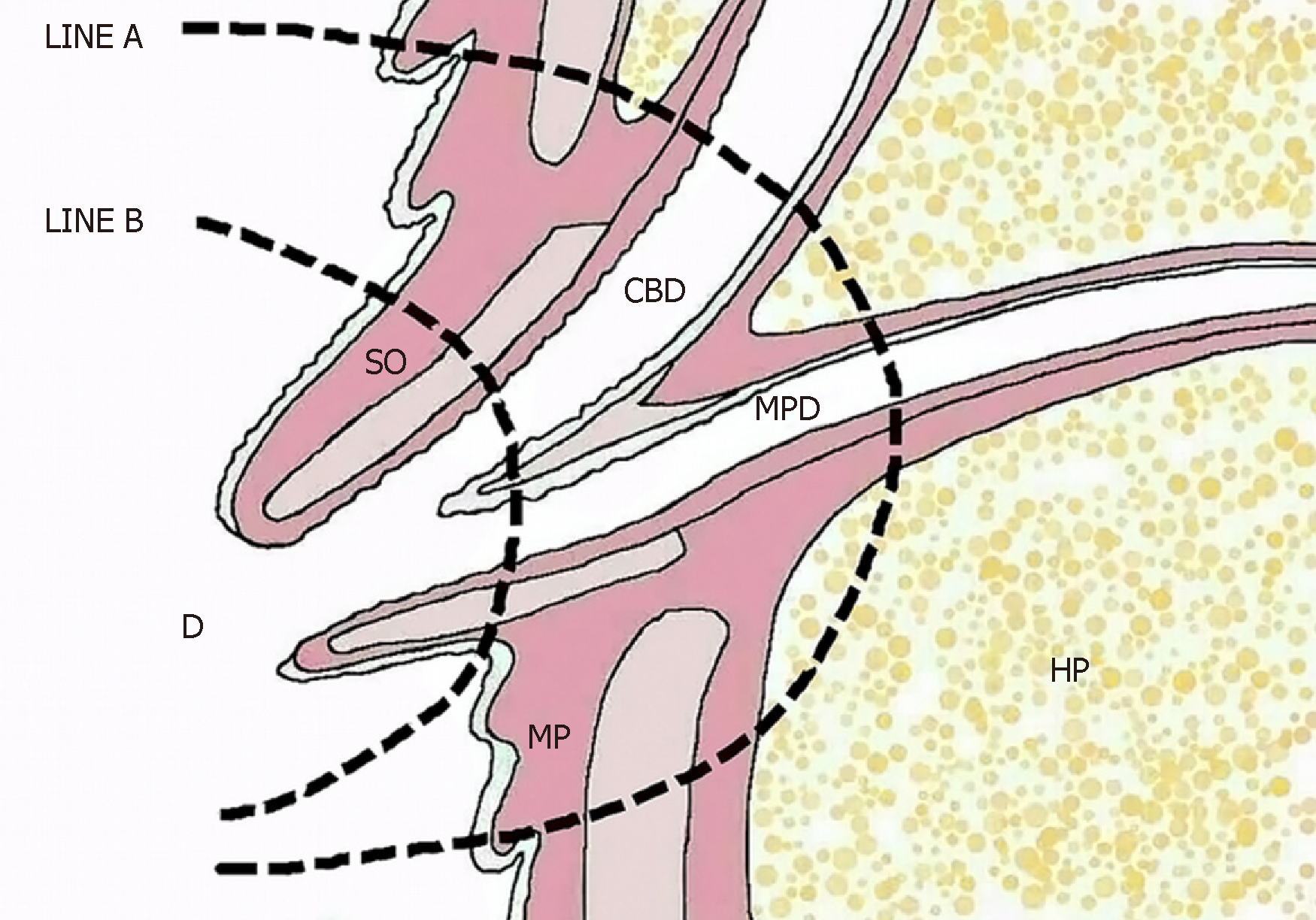
Surgical scope: With regard to surgical scale, TDA is more extensive than EP (Figure 3) and causes less surgical trauma than PD, involving a wide range of duodenal papilla regional excision covering the papilla, the terminal portion of the common bile duct, the ampulla of Vater, the end of the main pancreatic duct, and the related posterior wall of the duodenum (including the mucosa and the muscularis mucosa). Following the excision, the common bile duct end and the end of the main pancreatic duct were reconstructed. Intraoperative frozen-section pathological examination is required to confirm the R0 resection margin of the tumor.
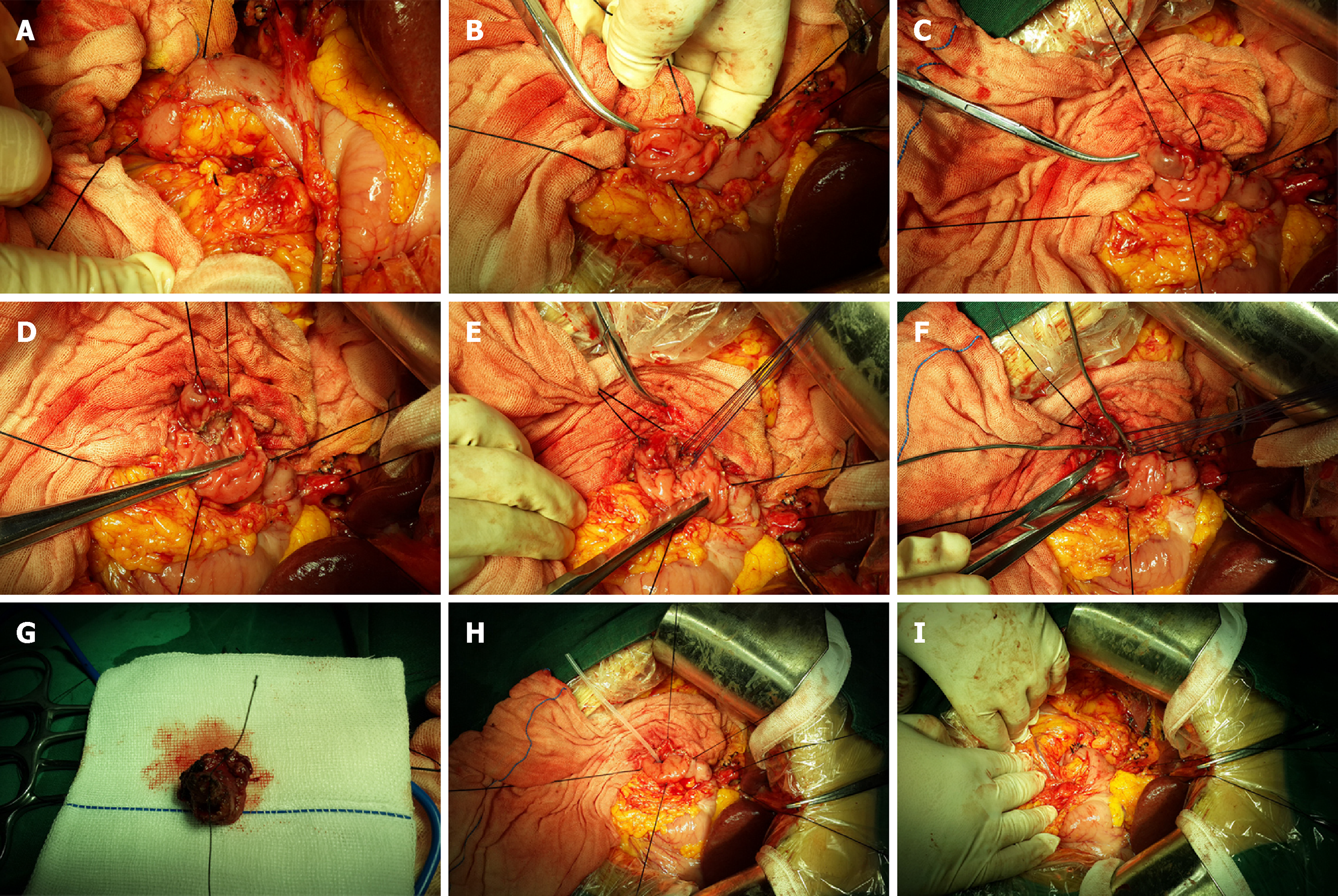
Surgical procedure: A Kocher incision was made to fully dissociate the lateral peritoneum of the duodenal descending part, exposing the duodenal descending part and the transverse part to make it possible to accurately detect the lesion (Figure 4A). This was also important in reducing the suturing tension of the duodenal incision. The surgeon held the duodenal descending portion and the pancreas head with the left hand, determined the location of the duodenal papilla, gently touched the tumor with the thumb and forefinger, evaluated tumor size, mobility, texture, and the extent of invasion by the lesion, and explored the degree of fixation of the tumor to determine whether it was appropriate to perform TDA. If the tumor has infiltrated the duodenal muscular layer or even other tissue such as the pancreas and appears stiff and fixed, it is not suitable for local tumor excision. In addition, if lymph node metastasis is found through operative exploration and confirmed by intraoperative frozen-section pathology, a more appropriate surgical method such as radical PD surgery should be conducted instead to permit lymph node dissection.
During the operation, surgical resection of the duodenal papilla, the sphincter of Oddi, and the gallbladder should be routinely performed to avoid gallbladder infection and dysfunction. The front wall of the common bile duct was incised, and the probe was inserted into the distal end. A lengthwise incision was then made in the anterior wall of the descending section of the duodenum to reveal the duodenal papilla and the periampullary lesion (Figure 4B).
The duodenal papilla was exposed and lifted up using a transfixion suture (Figure 4C). Maintaining an appropriate distance around the base of the periampullary tumor, the duodenal mucosa and the mucosal muscularis were incised to the distal normal bile duct area, with good hemostasis performed (Figure 4D). The full layers of the distal bile duct were incised circlewise; if no tumor tissue was visible, the surgeon continued to incise part of the pancreas and to cut off the main pancreatic duct (Figure 4E). For implantation and reconstruction of the duodenal wall with the common bile duct and the main pancreatic duct, we sutured the common bile duct end and anastomosed the main pancreatic duct end with the duodenal wall using interrupted sutures. Reliable hemostasis was performed, leaving no residual cavity. Excision and suturing were performed simultaneously. The bile duct and the pancreatic duct passed through the biliary probe after replantation (Figure 4F). Periampullary tumor resection was completed (Figure 4G). Confirmation of the absence of tumor residual tissue in the R0 resection margin depended on intraoperative frozen-section pathological examination.
The common bile duct and the main pancreatic duct share a common wall, and a silicone supporting tube was inserted within and fixed to the main pancreatic duct (Figure 4H). We sutured the duodenal intestinal wall with single-layer absorbable longitudinal sutures (Figure 4I). In the meantime, a T drainage tube was placed inside the common bile duct for decompression. Absorbable interrupted sutures were used to close the anterior wall. Gastrojejunostomy reduced the pressure on the duodenum, benefitting anastomotic healing.
Surgical skills: The longitudinal incision of the duodenal wall was carefully protected to prevent edema. A single layer of absorbable sutures was used to close the incision. The intestinal wall tissue and the orthotopic suture were incised to reduce the tension caused by slitting and suturing. The common bile duct and the main pancreatic duct were respectively anastomosed with the duodenal posterior wall, leaving no residual cavity. The main pancreatic duct was placed inside a supporting tube to reduce the occurrence of pancreatic leakage. Gastrojejunostomy (Billroth II type) was performed to reduce duodenal compression. A T drainage tube was placed inside the common bile duct for decompression. Together with gastrointestinal decompression, this reduced the occurrence of duodenal leakage. In this group of patients, no duodenal leakage, obstruction, or delayed gastric emptying occurred after the longitudinal incision and suturing of the duodenal wall.
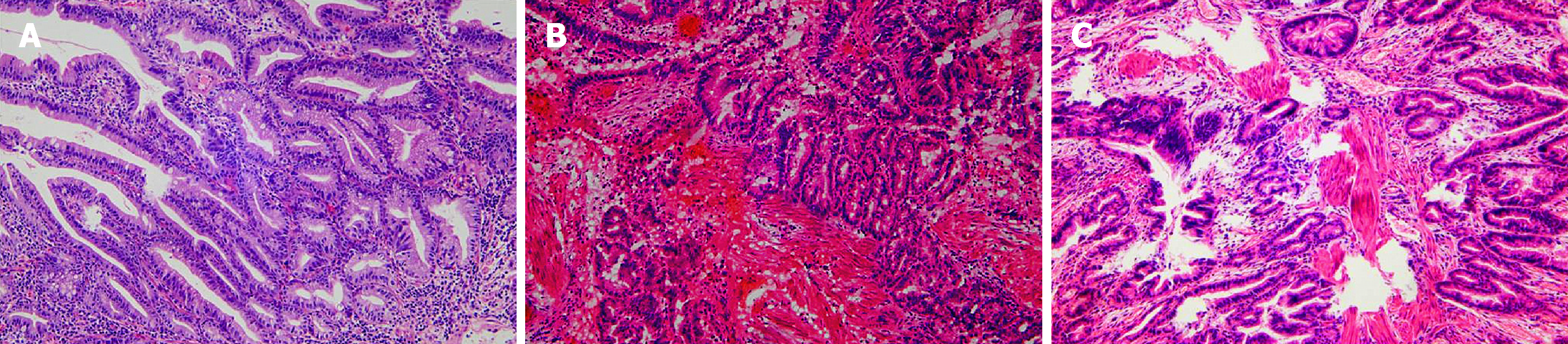
The postoperative pathology results showed that there were seven patients with benign tumors (tubulovillous adenoma; dysplasia; adenomatous hyperplasia; serrated adenoma; intraepithelial neoplasia) and three patients with malignant tumors (adenocarcinoma, 1 pTis, 2 pT1, Figure 5A-C). In one case, there was a history of breast cancer. Specific information on the ten patients who participated in the study is shown in Table 1.
The endoscopic biopsy false-negative rate was 30% (3/10). The coincidence rate of the postoperative pathology results with the intraoperative frozen-section biopsy results was 100% (10/10), and the coincidence rate of the endoscopic biopsy results with the pathological characteristics was 70% (7/10). All patients recovered well without bile leakage, pancreatic leakage, intestinal fistula, duodenal obstruction, peptic ulcer, or delayed gastric emptying (Figure 1C and 2E). One patient experienced gastrointestinal hemorrhage due to ampullary anastomotic stoma errhysis and recovered after conservative treatment. Endoscopic reexamination showed no anastomotic tumor relapse but showed that in some cases, the pancreatic duct supporting tube fell off (Figure 1D). No fever, cholangitis, or liver abscess caused by bile duct reflux was observed (Figure 2F).
For patients with TDA, endoscopic surveillance is very important. After 1-3 mo, regular endoscopic surveillance and radiography of the upper digestive tract were performed to observe the ampullary anastomotic stoma to confirm that no neoplasm recurrence appeared. All patients were followed for 6 to 70 mo without tumor recurrence or metastasis.
With improvements in surgical technology and the quality of sutures, the classic Halsted surgical methods should receive corresponding improvement. In this case series study, the endoscopic biopsy false-negative rate was 30% (3/10). The coincidence rate of the postoperative pathology results with the intraoperative frozen-section biopsy results was 100% (10/10), and the coincidence of the postoperative pathology results with the endoscopic biopsy results was 70% (7/10).
Among gastrointestinal tumors, the morbidity incidence of benign and malignant ampullary tumors in the abdomen is approximately 5%[18]. Ampullary carcinoma constitutes only 0.2% of gastrointestinal tumors and 7% of total cases of periampullary carcinoma, and it is classified into two histological subtypes: gastrointestinal and pancreaticobiliary. Each subtype has a different etiology and distinct clinical features[19]. The progression from ampullary adenoma to cancer is similar to the malignant changes that occur in colonic polyps. Therefore, preventive resection is necessary. Ampullary adenoma is considered a precancerous lesion[1].
For choledochopancreatic junction on the ampulla of Vater, Y type accounted for 47% of the observed cases, and V type accounted for 50%. Openings in the duodenal papilla[20], which is the anatomical basis for TDA of ampullary tumors, also achieve a radical cancer curative effect. Ampullary tumors cause dilation of the bile duct and of the main pancreatic duct, making it possible for bile-pancreas duct formation to occur. The formation and embedding of the bile-pancreas duct into the duodenal wall actually involves the anastomosis of the lower end of the bile-pancreas duct with the posterior wall of the duodenum.
Ampullary tumors grow slowly along the duodenal wall or the bile duct in a noninvasive manner. Gastrointestinal adenocarcinoma is the most common malignant epithelial neoplasm, followed by pancreaticobiliary carcinoma[21]. Most malignant tumors are early carcinomas that present less invasion of the membranae serosa layer, a higher degree of differentiation, and a lower degree of malignancy. Jaundice is an early clinical symptom, and these tumors can easily be detected by endoscopic examination and imaging.
There is some controversy concerning the methods used to treat ampullary neoplasm, mainly concerning the thoroughness of TDA. Because of the biological characteristics of ampullary neoplasm, Sharp et al[22] considered that TDA is applicable to benign tumors and high-risk patients with local malignancies. Preoperative evaluation by EUS of tumors of the ampulla of Vater and Tis-stage tumors could also involve endoscopic resection[23]. In addition, with respect to the size of benign tumors and the stage of malignant tumors, the study of Lee et al[24] indicated that TDA is only applicable to Tis-stage malignant tumors and is not suitable for T1-stage malignant tumors. Demetriades et al[25] thought that local excision was safe, producing satisfactory long-term survival rates in patients with benign lesions < 2 cm, pT1, and well-differentiated ampullary tumors without nodal involvement. According to the study results of Feng et al[26], LR instead of PD is considered to be suitable for patients with malignant tumors less than 2 cm in diameter, I/II, or pT1/T2 N0 because of its low risk and low associated mortality. Tumor-node-metastasis (TNM), pT, and pN stages still serve as independent predictors of survival rate.
The study results of Beger et al[27] indicated that LR is suitable for ampullary adenoma or low-grade malignancy involving pTis and pT1 N0M0, G1, or G2. The latest research demonstrated that LR is applicable to duodenal carcinoma and T1a-stage ampullary carcinoma according to the depth of tumor invasion[28]. Lai et al[29] found that ampullectomy is associated with shorter hospital stays and lower incidence of complications than PD.
In cases involving benign neoplasms, precancerous lesions, local malignant neoplasms, early highly differentiated adenocarcinoma, and Tis- and T1-stage lesions, TDA could achieve the radical treatment aim in cases in which a benign tumor is less than 2.5 cm, a malignant tumor is less than 2.0 cm by EUS, the resection margin is R0, and there is no abdominal lymph node metastasis or remote organ metastasis. The selection of surgical method mainly depends on the results of preoperative endoscopic biopsy and EUS. Intraoperative frozen-section pathology is necessary, and if it is inconsistent with endoscopic biopsy, the surgical method should be chosen according to the TNM classification. TDA can be selected for N0M0, pTis- and pT1-stage low-grade malignancies and early adenocarcinoma.
In regard to this disease, EUS and endoscopic biopsy, in addition to CT and MRI, are reasonable methods for determination of the nature of the tumor and for guiding surgical planning. However, it may also be that preoperative endoscopic biopsy and intraoperative frozen-section pathology of TDA are not consistent with postoperative pathology; this is related to the fact that less tissue or less deep tissue is sampled by endoscopic biopsy and also to the coincidence rate between frozen-section pathology and postoperative pathology.
There are several advantages of this surgical procedure. First, the operation involved less surgical trauma, shorter surgery time and hospital stay, and less risk of perioperative death. Second, there was no pancreatic leakage, biliary stricture, or refluxing cholangitis when the bile-pancreas duct was anastomosed with the posterior wall of the duodenum and a support tube was placed in the pancreatic duct; thus, the patients had high quality of life after surgery. Finally, there was no duodenal leakage, duodenal obstruction, or delayed stomach emptying after the longitudinal incision and suturing of the duodenal wall. The disadvantages of this method are: (1) the surgery did not include lymph node dissection for malignant tumors; and (2) the intraoperative frozen-section pathology had a certain false-negative rate and therefore did not ensure that a complete R0 tumor resection margin was achieved.
A high recurrence rate was not observed because most patients who received TDA suffered benign tumors and low-grade malignant tumors. Due to the lack of observation of a large number of cases, no comparison with survival rate or survival time after PD was made. For cases involving malignant tumors, there is a possibility of a high recurrence rate and low survival rate compared to PD.
EUS and endoscopic biopsy are necessary for ampullary tumors and can determine whether TDA should be selected. Considering the limitations of endoscopic biopsy, intraoperative frozen-section pathology is required to further clarify the pathological characteristics of the lesion and the state of the tumor resection margin. This method is suitable for patients with benign tumors, local or low-grade malignancies, R0 tumor resection margins and without lymphatic metastasis or other visceral metastatic lesions. The surgeon should make a surgical plan after carefully studying all of the patient’s examination results. This operation requires surgeons with particular surgical experience and operative skills. The surgeon should determine to choose TDA or classical PD according to the range of the lesion and the degree of infiltration by the tumor based on endoscopic and intraoperative observation combined with preoperative and intraoperative pathologic results. Regular postoperative endoscopic follow-up is required. Further research on survival rate and rate of recovery compared with radical PD surgery and based on more cases should be considered.
In conclusion, the coincidence rate of postoperative pathology results, intraoperative frozen-section pathology results, and endoscopic biopsy results is the restraining factor of TDA clinical application. Preoperative endoscopic biopsy pathology results and EUS provide an importance reference for surgical planning. Intraoperative frozen-section pathology results have a significant influence on the surgical procedure. Single-layer longitudinal slitting and suturing of the duodenal wall are safe.
Transduodenal ampullectomy (TDA) is not in wide clinical use due to its low radical effect and a high recurrence rate of tumors. However, TDA is still an effective treatment method that has great clinical value in cases of duodenal benign tumors, precancerous lesions, and benign and malignant borderline tumors; it can avoid the risks associated with pancreaticoduodenectomy (PD) and offers a larger resection range and greater thoroughness than endoscopic papillectomy (EP).
The investigation of surgical method choice and the coincidence rate of pathological diagnoses in TDA can improve our understanding of TDA. TDA is still an effective treatment method and is of great clinical value in cases of duodenal benign tumors, precancerous lesions, and benign and malignant borderline tumors.
The main objective of this study was to investigate surgical method choice and the coincidence rate of pathological diagnoses in TDA.
Ten patients with ampullary neoplasm underwent TDA based on the fact that the endoscopic biopsy results suggested benign lesions and the endoscopic ultrasound (EUS)-assessed tumors were resectable. All cases underwent duodenal ampullary lesion endoscopic biopsy, intraoperative frozen-section pathology, and postoperative pathological examination.
There were seven patients with benign tumors and three with malignant tumors (1 pTis, 2 pT1) according to the postoperative pathology results. The coincidence rate of the postoperative pathology results with the intraoperative frozen-section biopsy results was 100% (10/10), and the coincidence rate with the endoscopic biopsy results based on pathological characteristics was 70% (7/10). The false-negative rate of endoscopic biopsy results was 30% (3/10). All patients were followed for 6 to 70 months without tumor recurrence or metastasis.
The coincidence rate of postoperative pathology results, intraoperative frozen-section pathology results, and endoscopic biopsy results is the restraining factor of TDA clinical application. Endoscopic biopsy results and EUS are an importance reference for surgical planning. Intraoperative frozen-section pathology results have a significant influence on the surgical procedure.
Due to the small sample size and the retrospective nature of the study, it is difficult to draw reliable conclusions. However, TDA is still an effective treatment method that has great clinical value in cases involving duodenal benign tumors, precancerous lesions, and benign and malignant borderline tumors. Based on the coincidence rate of postoperative pathology results, intraoperative frozen-section pathology results, and endoscopic biopsy results, TDA can be considered as a treatment choice between EP and radical PD.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Fujino Y, Vaudo G S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Park SH, Kim YI, Park YH, Kim SW, Kim KW, Kim YT, Kim WH. Clinicopathologic correlation of p53 protein overexpression in adenoma and carcinoma of the ampulla of Vater. World J Surg. 2000;24:54-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 2. | Treitschke F, Beger HG. Local resection of benign periampullary tumors. Ann Oncol. 1999;10 Suppl 4:212-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 3. | Allgaier HP, Schwacha H, Kleinschmidt M, Thimme R, Schöffel U, Blum HE. Ampullary hamartoma: A rare cause of biliary obstruction. Digestion. 1999;60:497-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Kwon TH, Park DH, Shim KY, Cho HD, Park JH, Lee SH, Chung IK, Kim HS, Park SH, Kim SJ. Ampullary adenomyoma presenting as acute recurrent pancreatitis. World J Gastroenterol. 2007;13:2892-2894. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 5. | Sun JH, Chao M, Zhang SZ, Zhang GQ, Li B, Wu JJ. Coexistence of small cell neuroendocrine carcinoma and villous adenoma in the ampulla of Vater. World J Gastroenterol. 2008;14:4709-4712. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Ghidirim G, Rojnoveanu G, Mişin I, Cernîi A, Gurghiş R. [Carcinoid of the minor duodenal papilla associated with multiple jejunal leiomyomas in type 1 neurofibromatosis]. Chirurgia (Bucur). 2009;104:491-494. [PubMed] |
| 7. | Leung K, Worni M, Galeotti J, Blazer D. A Novel Approach: Local Resection for Ampullary GIST-Case Report and Review of Literature. J Gastrointest Cancer. 2017;48:376-378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 8. | Fukasawa H, Tounou S, Nabetani M, Michida T. Endoscopic Resection of Ampullary Neuroendocrine Tumor. Intern Med. 2017;56:499-503. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Deschamps L, Dokmak S, Guedj N, Ruszniewski P, Sauvanet A, Couvelard A. Mixed endocrine somatostatinoma of the ampulla of vater associated with a neurofibromatosis type 1: a case report and review of the literature. JOP. 2010;11:64-68. [PubMed] |
| 10. | Hsu SD, Chan DC, Hsieh HF, Chen TW, Yu JC, Chou SJ. Ectopic pancreas presenting as ampulla of Vater tumor. Am J Surg. 2008;195:498-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | Will U, Müller AK, Fueldner F, Wanzar I, Meyer F. Endoscopic papillectomy: data of a prospective observational study. World J Gastroenterol. 2013;19:4316-4324. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 28] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Ceppa EP, Burbridge RA, Rialon KL, Omotosho PA, Emick D, Jowell PS, Branch MS, Pappas TN. Endoscopic versus surgical ampullectomy: an algorithm to treat disease of the ampulla of Vater. Ann Surg. 2013;257:315-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 82] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 13. | Ito K, Fujita N, Noda Y, Kobayashi G, Horaguchi J. Diagnosis of ampullary cancer. Dig Surg. 2010;27:115-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Martin JA, Haber GB. Ampullary adenoma: clinical manifestations, diagnosis, and treatment. Gastrointest Endosc Clin N Am. 2003;13:649-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 58] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 15. | Kim AL, Choi YI. Safety of duodenal ampullectomy for benign periampullary tumors. Ann Hepatobiliary Pancreat Surg. 2017;21:146-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 13] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Kim J, Choi SH, Choi DW, Heo JS, Jang KT. Role of transduodenal ampullectomy for tumors of the ampulla of Vater. J Korean Surg Soc. 2011;81:250-256. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 17. | Agha RA, Fowler AJ, Rajmohan S, Barai I, Orgill DP; PROCESS Group. Preferred reporting of case series in surgery; the PROCESS guidelines. Int J Surg. 2016;36:319-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 302] [Cited by in RCA: 334] [Article Influence: 37.1] [Reference Citation Analysis (0)] |
| 18. | Scarpa A, Capelli P, Zamboni G, Oda T, Mukai K, Bonetti F, Martignoni G, Iacono C, Serio G, Hirohashi S. Neoplasia of the ampulla of Vater. Ki-ras and p53 mutations. Am J Pathol. 1993;142:1163-1172. [PubMed] |
| 19. | Perysinakis I, Margaris I, Kouraklis G. Ampullary cancer--a separate clinical entity? Histopathology. 2014;64:759-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 30] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Funabiki T, Matsubara T, Miyakawa S, Ishihara S. Pancreaticobiliary maljunction and carcinogenesis to biliary and pancreatic malignancy. Langenbecks Arch Surg. 2009;394:159-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 127] [Cited by in RCA: 110] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 21. | Gassler N, Knüchel R. [Tumors of Vater's ampulla]. Pathologe. 2012;33:17-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 22. | Sharp KW, Brandes JL. Local resection of tumors of the ampulla of Vater. Am Surg. 1990;56:214-217. [PubMed] |
| 23. | Okano N, Igarashi Y, Hara S, Takuma K, Kamata I, Kishimoto Y, Mimura T, Ito K, Sumino Y. Endosonographic preoperative evaluation for tumors of the ampulla of vater using endoscopic ultrasonography and intraductal ultrasonography. Clin Endosc. 2014;47:174-177. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Lee H, Park JY, Kwon W, Heo JS, Choi DW, Choi SH. Transduodenal Ampullectomy for the Treatment of Early-Stage Ampulla of Vater Cancer. World J Surg. 2016;40:967-973. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 29] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 25. | Demetriades H, Zacharakis E, Kirou I, Pramateftakis MG, Sapidis N, Kanellos I, Betsis D. Local excision as a treatment for tumors of ampulla of Vater. World J Surg Oncol. 2006;4:14. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 26. | Feng J, Zhou X, Mao W. Prognostic analysis of carcinoma of the ampulla of Vater: pancreaticoduodenectomy versus local resection. Hippokratia. 2012;16:23-28. [PubMed] |
| 27. | Beger HG, Treitschke F, Gansauge F, Harada N, Hiki N, Mattfeldt T. Tumor of the ampulla of Vater: experience with local or radical resection in 171 consecutively treated patients. Arch Surg. 1999;134:526-532. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 231] [Cited by in RCA: 241] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 28. | Kohga A, Yamamoto Y, Sano S, Sugiura T, Okamura Y, Ito T, Ashida R, Ishiwatari H, Matsubayashi H, Sasaki K, Uesaka K. Surgical Strategy for T1 Duodenal or Ampullary Carcinoma According to the Depth of Tumor Invasion. Anticancer Res. 2017;37:5277-5283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 29. | Lai JH, Shyr YM, Wang SE. Ampullectomy versus pancreaticoduodenectomy for ampullary tumors. J Chin Med Assoc. 2015;78:339-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |