Published online Feb 6, 2019. doi: 10.12998/wjcc.v7.i3.382
Peer-review started: September 23, 2018
First decision: November 14, 2018
Revised: December 4, 2018
Accepted: December 21, 2018
Article in press: December 21, 2018
Published online: February 6, 2019
Processing time: 125 Days and 12.7 Hours
Rivaroxaban is a non-vitamin K antagonist oral anticoagulant that does not require coagulation monitoring based on current recommendations. Our goal is to explore whether routine coagulation monitoring should not be required for all patients receiving oral rivaroxaban, what relationship between routine coagulation abnormalities and bleeding, and how to deal with the above clinical situations through our case and review of the literature.
We report a 67-year-old woman with a history of atrial fibrillation who presented to the hospital with worsening dyspnea and cough. Based on electrocardiogram, venous compression ultrasonography, and computed tomography pulmonary angiography, the diagnosis of atrial fibrillation, deep venous thrombosis, and acute pulmonary embolism was confirmed. Her coagulation assays and renal function were normal on admission; she was not underweight, did not have a history of hemorrhagic disease, and her CHA2DS2-VAS, HAS-BLED, and simplified Pulmonary Embolism Severity Index scores were 3, 0, and 0, respectively. Oral rivaroxaban (15 mg twice daily) was administered. The following day, she presented gastrointestinal and gum bleeding, combined with coagulation abnormalities. Following cessation of rivaroxaban, her bleeding stopped and tests improved over the next 2 d. Rivaroxaban was begun again 3 d after recovery. However, she again presented with gastrointestinal and gum bleeding and the abnormal tests, and the therapy was discontinued. At 30-d follow-up after discharge, she presented normal coagulation tests without bleeding.
Although current guidelines recommend that using non-vitamin K antagonist oral anticoagulants including rivaroxaban do not require coagulation monitoring, a small number of patients may develop routine coagulation test changes and bleeding during rivaroxaban therapy, especially in the elderly. Clinicians should pay attention to these patients and further obtain evidence in practice.
Core tip: Guidelines recommend that patients treated with non-vitamin K antagonist oral anticoagulants do not require coagulation monitoring and show that routine coagulation tests generally do not provide an accurate assessment of effects and bleeding for rivaroxaban. However, our case indicates that in real-world situations, a small number of patients may develop changes in prothrombin time, international normalized ratio, and activated partial thromboplastin time with bleeding during rivaroxaban therapy. The results of literature review suggest that routine coagulation assays may be required in special populations including elderly patients, particularly low-weight females or those with renal insufficiency during oral rivaroxaban.
- Citation: Wu HD, Cao HY, Song ZK, Yang S, Tang ML, Liu Y, Qin L. Considerations for routine coagulation monitoring with rivaroxaban: A case report and review of the literature. World J Clin Cases 2019; 7(3): 382-388
- URL: https://www.wjgnet.com/2307-8960/full/v7/i3/382.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i3.382
Rivaroxaban is an Xa factor inhibitor approved for the prevention of stroke in patients with nonvalvular atrial fibrillation (AF)[1] and for the prevention and treatment of venous thromboembolism (VTE)[2]. It has a predictable anticoagulant effect, eliminating the need for routine coagulation monitoring. Compared with vitamin K antagonists (VKAs), it also has a better efficacy/safety ratio, fewer food and drug interactions, a more rapid onset of action, and reduced risk of fatal bleeding. However, many unresolved questions remain about the optimal use of these agents in specific clinical situations involving AF and VTE, whether routine coagulation monitoring should be required for all patients receiving rivaroxaban, and which clinical situations are considered predictive factors associated with coagulation test abnormalities and/or bleeding while prescribing rivaroxaban.
A 67-year-old women presented to the hospital with worsening dyspnea and cough for 4 d.
Her symptoms worsened after mild activities and relieved after rest without taking any medication.
She had a long-term history of AF.
On admission, she was fully conscious, with a blood pressure of 110/70 mmHg, an irregular heart rate of 105 bpm on auscultation and oxygen saturation of 92% on room air. She had mild edema in the lower limbs. Her weight was 58 kg and the remainder of her physical examination was normal.
Laboratory tests revealed normal platelets, hemoglobin, electrolytes, liver function markers, renal function markers (serum creatinine and estimated glomerular filtration rate), cardiac troponin-T, and routine coagulation measurements (prothrombin time [PT], international normalized ratio, and activated partial thromboplastin time [aPTT]). However, her N-terminal pro-brain natriuretic peptide was 5364.00 pg/mL (reference, < 450.00 pg/mL), D-dimer level was 5.65 μg/mL (reference, 0.001–0.50 g/mL), and PaO2 was 60.5 mmHg (reference, 80-100 mmHg). Her electrocardiogram suggested AF, and her transthoracic echocardiogram revealed a left atrial diameter of 51 mm and an ejection fraction of 48%.
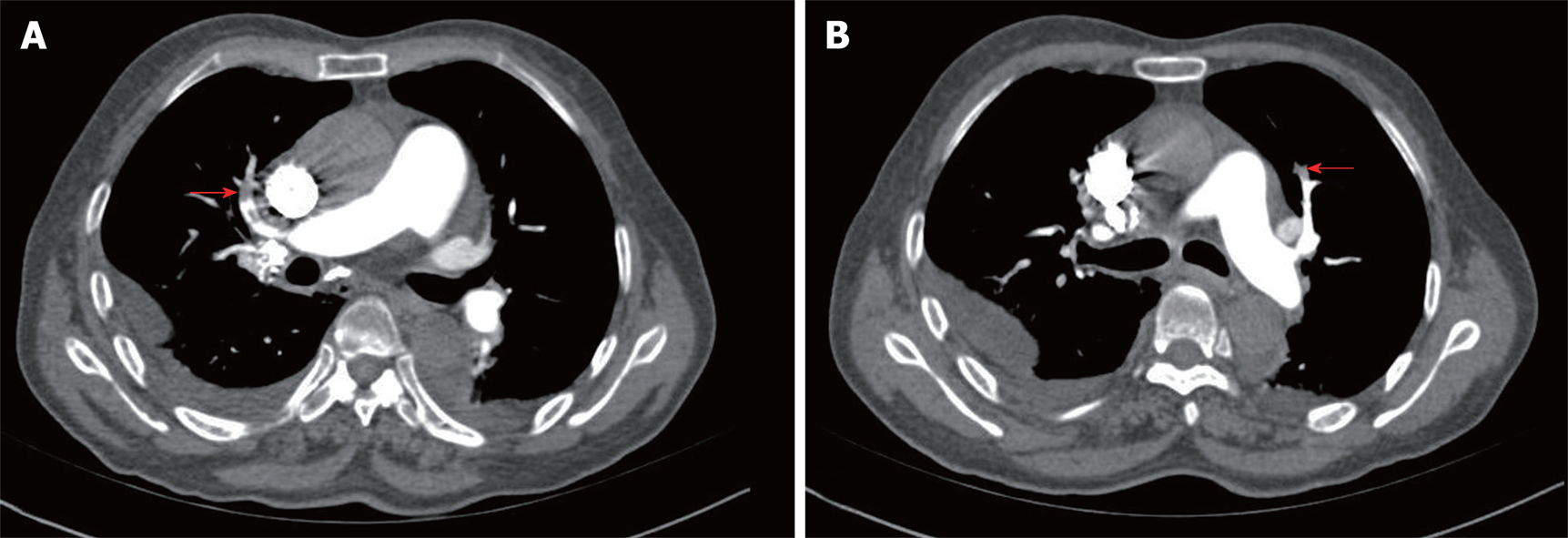
Lower limb venous compression ultrasonography showed a deep vein thromboembolism (DVT) involving bilateral intermuscular veins. Furthermore, computed tomography pulmonary angiography confirmed the presence of an embolus in both upper lobes of the pulmonary artery (Figure 1).
Atrial fibrillation, deep venous thrombosis, and acute pulmonary embolism.
To select an appropriate treatment strategy, the CHA2DS2-VAS, HAS-BLED, and simplified Pulmonary Embolism Severity Index (sPESI) scores were calculated; these values were 3, 0, and 0, respectively. Therefore, anticoagulation therapy with rivaroxaban (15 mg twice daily) was initiated. After 1 d, the patient had maroon stools and gum bleeding with routine coagulation test abnormalities. Rivaroxaban was discontinued, and routine coagulation tests were monitored daily (Table 1). Her maroon stools and gum bleeding disappeared, and routine coagulation tests returned to normal 2 d later. The patient received rivaroxaban again 3 d after coagulation tests returned to normal, and routine coagulation tests were performed 2 and 6 h after rivaroxaban administration (Table 1). Routine coagulation tests illustrated abnormalities 6 h after rivaroxaban (Table 1) and her maroon stools and gum bleeding reappeared. No further rivaroxaban treatment was administered.
| PT (s) | aPTT (s) | INR | |
| Reference range | 11.0-15.0 | 28.0-42.5 | 0.84-1.27 |
| Before treatment | 15.6 | 39.2 | 1.25 |
| Day 2 of drug administration | 64.4 | 99.3 | 7.68 |
| Day 1 after drug stopped | 25.2 | 63.3 | 2.31 |
| Day 2 after drug stopped | 16.1 | 44.6 | 1.3 |
| Day 3 after drug stopped | 17.1 | 45.1 | 1.41 |
| Day 4 after drug stopped | 15.2 | 42.1 | 1.21 |
| Day 5 after drug stopped: drug administration resumed | |||
| 2 h after second drug administration | 18.6 | 55.7 | 1.57 |
| 6 h after second drug administration | 27.7 | 68.8 | 2.61 |
Following cessation of rivaroxaban, her bleeding stopped and coagulation tests returned to normal. At follow-up 30 d after discharge at home, the patient received aspirin instead of an anticoagulant. Routine coagulation tests were normal and bleeding did not recur.
VKAs require regular laboratory monitoring to prevent under-coagulation or over-coagulation. This entails ongoing patient visits to clinics and regular blood collection. Even within the therapeutic range, there is still a significant risk of hemorrhage in patients receiving VKAs[3,4]. In recent years, an improved understanding of coagulation pathways has led to the development of several new parenterally or orally active agents that specifically target single blood coagulation factors.
Rivaroxaban is an orally active, specific, and direct inhibitor of activated factor Xa, with predictable pharmacokinetics and pharmacodynamics across a wide spectrum of patients. Treatment with rivaroxaban does not currently require blood coagulation monitoring[5-7]. It strongly binds plasma proteins (> 90%), peaks in the plasma 2-4 h following oral administration, has a half-life of 5-9 h in healthy young subjects and 11-12 h in elderly subjects, and has a dual mode of elimination (two-thirds are metabolized by the liver and one-third is excreted unaltered by the kidneys)[8]. Treatment with rivaroxaban has fewer considerations for food and drug interactions and greater effectiveness and safety than those of VKAs for prevention of stroke in patients with AF and for treatment of VTE[9]. For the majority of patients, rivaroxaban should be considered the first preferred anticoagulation therapy based on the positive results of large trials and current guidelines[1,10,11]. The ROCKET-AF, EINSTEIN-DVT, and EINSTEIN-PE trials also demonstrated that rivaroxaban could be used to prevent ischemic stroke in patients with AF and as a prophylaxis and treatment for DVT and PE[2,12,13].
In real-world practice, however, changes in routine coagulation tests occur in some patients during rivaroxaban treatment. Our patient in particular had a clear diagnosis of AF, DVT, and PE and an indication for anticoagulant therapy. Although we considered factors that might affect the pharmacokinetics and pharmacodynamics of rivaroxaban in our patient, including her age, weight, renal function, liver function, concomitant medications, comorbidities, routine coagulation tests, and overall frailty, and also assessed and stratified CHA2DS2-VAS, HAS-BLED, and sPESI scores prior to initial prescription, the patient experienced two unexpected changes in routine coagulation assays and bleeding events. Therefore, rivaroxaban had to be discontinued. The patient did not use drugs that affected rivaroxiban metabolism and resulted in rivaroxaban accumulation outside and in the hospital.
First, our review of the literature illustrated that rivaroxaban prolongs PT in a concentration-dependent manner[14]. The impact of rivaroxaban on PT is less sensitive, not specific, varies markedly with different thromboplastin reagents, and is influenced by a variety of other factors, including hepatic impairment and vitamin K deficiency[15]. A normal PT does not rule out clinically relevant concentrations of rivaroxaban and may provide some quantitative information about the risk of bleeding[16]. At present, neither randomized control trials nor clinical trials involve coagulation monitoring for rivaroxaban. Based on previous case reports describing hemorrhaging caused by rivaroxaban, few patients with bleeding who were treated at a normal dosage had prolonged PT[17-19]. Prolongation of both PT and aPTT was reported in only two patients, who had both consumed large oral doses of rivaroxaban (1960 mg and 1400 mg) as a method of suicide[20,21].
Second, aPTT is not suitable for meaningful evaluation of rivaroxaban efficacy due to the nonlinear relationship of aPTT level with rivaroxaban concentration, insufficient sensitivity, and significant variability between reagents. Excessive rivaroxaban may cause PT prolongation, but it has no effect on aPTT. In case reports of bleeding caused by rivaroxaban, none had changes in aPTT[22-24].
Finally, studies have shown that anti-Xa chromogenic assays with rivaroxaban calibrators and controls can accurately measure the anticoagulant effect of rivaroxaban over a wide range of therapeutic levels and plasma concentrations. Low and high plasma levels can be measured with acceptable inter-laboratory precision. In these assays, the absence of anti-Xa activity excludes clinically relevant drug levels[25]. These specific assays accurately quantify plasma levels of the anticoagulant[5,26-28], but are not routinely available at most clinical centers. Excessive plasma concentrations, such as those upon intentional overdose, potentially expose patients to an increased risk of bleeding. Although data on the relationship between plasma levels and clinical outcomes are beginning to emerge, there is no current evidence that routine monitoring or dose titration will improve outcomes, and no studies have investigated whether measuring drug levels and adjusting dosage based on laboratory coagulation parameters reduce the risk for bleeding or thromboembolic complications.
As the use of rivaroxaban increases, clinicians have begun to pay attention to changes in routine coagulation tests and their relationship with bleeding in patients. Some studies suggest that coagulation monitoring may be useful in elderly patients, those who are underweight, or those with renal dysfunction, suspected overdose or bioaccumulation, bleeding, higher HAS-BLED scores, planned invasive procedures and surgery, or those patients with multiple diseases for which multiple drugs are being taken[18,19,29].
The combination of AF and VTE (PE and DVT) is not only common in patients who receive anticoagulation therapy, but is also associated with higher morbidity and mortality[30]. Before initiating anticoagulant therapy to a patient with AF and/or VTE, a clinician should decide whether anticoagulation is indicated by risk/benefit analysis and approved by regulatory authorities and specified guidelines. In addition, patient-related clinical factors and patient preferences should be considered[31].
It is clear from the case described here that differences between individuals exist in real-world situations, especially in elderly adults. Our patient was an elderly woman who had a relatively high risk of routine coagulation changes and bleeding, and she presented rarely both PT and aPTT prolongation at a normal dose of rivaroxaban. However, anti-Xa chromogenic assays were not performed and the quantities and functions of clotting factors were not detected.
Summarily, we suggest that clinicians should now be particularly aware of patients with an increased risk of bleeding and consider monitoring the coagulation status of these individuals during rivaroxaban therapy, because which patients and situations are required to detect routine coagulation is unclear. Conditional centers should also consider using the accurate anti-Xa chromogenic assays to improve detection of drug concentrations and evaluation of bleeding factors.
Although current guidelines recommend that using non-vitamin K antagonist oral anticoagulants including rivaroxaban do not require coagulation monitoring, we found that in clinical practice, a small number of patients may develop routine coagulation changes with bleeding during rivaroxaban therapy. The results of literature review and our case showed that routine coagulation assays may be required in special populations including elderly patients, particularly low-weight females or those with renal insufficiency. Clinicians should pay attention to these patients and further obtain evidence in practice
We thank all participants for their support and participation.
CARE Checklist (2016): The authors have read the CARE Checklist (2016), and the manuscript was prepared and revised according to the CARE Checklist (2016).
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kvolik S, Vaudo G S- Editor: Wang JL L- Editor: Wang TQ E- Editor: Song H
| 1. | Steffel J, Verhamme P, Potpara TS, Albaladejo P, Antz M, Desteghe L, Haeusler KG, Oldgren J, Reinecke H, Roldan-Schilling V, Rowell N, Sinnaeve P, Collins R, Camm AJ, Heidbüchel H; ESC Scientific Document Group. The 2018 European Heart Rhythm Association Practical Guide on the use of non-vitamin K antagonist oral anticoagulants in patients with atrial fibrillation. Eur Heart J. 2018;39:1330-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1188] [Cited by in RCA: 1363] [Article Influence: 227.2] [Reference Citation Analysis (0)] |
| 2. | EINSTEIN Investigators; Bauersachs R, Berkowitz SD, Brenner B, Buller HR, Decousus H, Gallus AS, Lensing AW, Misselwitz F, Prins MH, Raskob GE, Segers A, Verhamme P, Wells P, Agnelli G, Bounameaux H, Cohen A, Davidson BL, Piovella F, Schellong S. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499-2510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2218] [Cited by in RCA: 2258] [Article Influence: 150.5] [Reference Citation Analysis (0)] |
| 3. | Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, Camm AJ, Weitz JI, Lewis BS, Parkhomenko A, Yamashita T, Antman EM. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. 2014;383:955-962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3181] [Cited by in RCA: 3640] [Article Influence: 330.9] [Reference Citation Analysis (0)] |
| 4. | van der Hulle T, Kooiman J, den Exter PL, Dekkers OM, Klok FA, Huisman MV. Effectiveness and safety of novel oral anticoagulants as compared with vitamin K antagonists in the treatment of acute symptomatic venous thromboembolism: a systematic review and meta-analysis. J Thromb Haemost. 2014;12:320-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 355] [Cited by in RCA: 369] [Article Influence: 33.5] [Reference Citation Analysis (0)] |
| 5. | Douxfils J, Mullier F, Loosen C, Chatelain C, Chatelain B, Dogné JM. Assessment of the impact of rivaroxaban on coagulation assays: laboratory recommendations for the monitoring of rivaroxaban and review of the literature. Thromb Res. 2012;130:956-966. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 156] [Cited by in RCA: 176] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 6. | Favaloro EJ, Bonar R, Butler J, Marsden K. Laboratory testing for the new oral anticoagulants: a review of current practice. Pathology. 2013;45:435-437. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 7. | Di Minno A, Spadarella G, Prisco D, Franchini M, Lupoli R, Di Minno MN. Clinical judgment when using coagulation tests during direct oral anticoagulant treatment: a concise review. Semin Thromb Hemost. 2013;39:840-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Kubitza D, Becka M, Wensing G, Voith B, Zuehlsdorf M. Safety, pharmacodynamics, and pharmacokinetics of BAY 59-7939--an oral, direct Factor Xa inhibitor--after multiple dosing in healthy male subjects. Eur J Clin Pharmacol. 2005;61:873-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 485] [Cited by in RCA: 475] [Article Influence: 23.8] [Reference Citation Analysis (0)] |
| 9. | DeWald TA, Becker RC. The pharmacology of novel oral anticoagulants. J Thromb Thrombolysis. 2014;37:217-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Kirchhof P, Benussi S, Kotecha D, Ahlsson A, Atar D, Casadei B, Castella M, Diener HC, Heidbuchel H, Hendriks J, Hindricks G, Manolis AS, Oldgren J, Popescu BA, Schotten U, Van Putte B, Vardas P; ESC Scientific Document Group. 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS. Eur Heart J. 2016;37:2893-2962. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5167] [Cited by in RCA: 4870] [Article Influence: 541.1] [Reference Citation Analysis (0)] |
| 11. | Konstantinides SV, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galiè N, Gibbs JS, Huisman MV, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack C, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Vonk Noordegraaf A, Zamorano JL, Zompatori M; Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). 2014 ESC guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J. 2014;35:3033-3069, 3069a-3069k. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1883] [Cited by in RCA: 1892] [Article Influence: 172.0] [Reference Citation Analysis (0)] |
| 12. | Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, Breithardt G, Halperin JL, Hankey GJ, Piccini JP, Becker RC, Nessel CC, Paolini JF, Berkowitz SD, Fox KA, Califf RM; ROCKET AF Investigators. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. 2011;365:883-891. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6519] [Cited by in RCA: 6884] [Article Influence: 491.7] [Reference Citation Analysis (2)] |
| 13. | EINSTEIN–PE Investigators; Büller HR, Prins MH, Lensin AW, Decousus H, Jacobson BF, Minar E, Chlumsky J, Verhamme P, Wells P, Agnelli G, Cohen A, Berkowitz SD, Bounameaux H, Davidson BL, Misselwitz F, Gallus AS, Raskob GE, Schellong S, Segers A. Oral rivaroxaban for the treatment of symptomatic pulmonary embolism. N Engl J Med. 2012;366:1287-1297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1646] [Cited by in RCA: 1685] [Article Influence: 129.6] [Reference Citation Analysis (0)] |
| 14. | Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al-Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH; ESC Committee for Practice Guidelines. Guidelines for the management of atrial fibrillation: the Task Force for the Management of Atrial Fibrillation of the European Society of Cardiology (ESC). Europace. 2010;12:1360-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 953] [Cited by in RCA: 1028] [Article Influence: 73.4] [Reference Citation Analysis (0)] |
| 15. | Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093-1100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2971] [Cited by in RCA: 3336] [Article Influence: 222.4] [Reference Citation Analysis (0)] |
| 16. | Guo Y, Zhu H, Chen Y, Lip GYH. Comparing Bleeding Risk Assessment Focused on Modifiable Risk Factors Only Versus Validated Bleeding Risk Scores in Atrial Fibrillation. Am J Med. 2018;131:185-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 47] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 17. | Cuker A, Siegal DM, Crowther MA, Garcia DA. Laboratory measurement of the anticoagulant activity of the non-vitamin K oral anticoagulants. J Am Coll Cardiol. 2014;64:1128-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 345] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 18. | Yasaka M, Lip GY. Impact of non-vitamin k antagonist oral anticoagulants on intracranial bleeding in Asian patients with non-valvular atrial fibrillation. Circ J. 2014;78:2367-2372. [PubMed] |
| 19. | Repplinger DJ, Hoffman RS, Nelson LS, Hines EQ, Howland M, Su MK. Lack of significant bleeding despite large acute rivaroxaban overdose confirmed with whole blood concentrations. Clin Toxicol (Phila). 2016;54:647-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 20. | Lane DA, Raichand S, Moore D, Connock M, Fry-Smith A, Fitzmaurice DA; Steering Committee. Combined anticoagulation and antiplatelet therapy for high-risk patients with atrial fibrillation: a systematic review. Health Technol Assess. 2013;17:1-188. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 21. | Linkins LA, Moffat K. Monitoring the anticoagulant effect after a massive rivaroxaban overdose. J Thromb Haemost. 2014;12:1570-1571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 22. | Lehmann T, Hofer KE, Baumann M, Hasler K, Ceschi A, Kupferschmidt H, Rohde G, Korte W. Massive human rivaroxaban overdose. Thromb Haemost. 2014;112:834-836. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 23. | Lindhoff-Last E, Samama MM, Ortel TL, Weitz JI, Spiro TE. Assays for measuring rivaroxaban: their suitability and limitations. Ther Drug Monit. 2010;32:673-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 120] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 24. | Helin TA, Pakkanen A, Lassila R, Joutsi-Korhonen L. Laboratory assessment of novel oral anticoagulants: method suitability and variability between coagulation laboratories. Clin Chem. 2013;59:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 25. | Molenaar PJ, Dinkelaar J, Leyte A. Measuring Rivaroxaban in a clinical laboratory setting, using common coagulation assays, Xa inhibition and thrombin generation. Clin Chem Lab Med. 2012;50:1799-1807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 38] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Lindhoff-Last E, Ansell J, Spiro T, Samama MM. Laboratory testing of rivaroxaban in routine clinical practice: when, how, and which assays. Ann Med. 2013;45:423-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 46] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 27. | Hillarp A, Baghaei F, Fagerberg Blixter I, Gustafsson KM, Stigendal L, Sten-Linder M, Strandberg K, Lindahl TL. Effects of the oral, direct factor Xa inhibitor rivaroxaban on commonly used coagulation assays. J Thromb Haemost. 2011;9:133-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 222] [Cited by in RCA: 218] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 28. | Gómez-Outes A, Suárez-Gea ML, Lecumberri R, Terleira-Fernández AI, Vargas-Castrillón E. Direct-acting oral anticoagulants: pharmacology, indications, management, and future perspectives. Eur J Haematol. 2015;95:389-404. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 59] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 29. | Spiller HA, Mowry JB, Aleguas A, Griffith JR, Goetz R, Ryan ML, Bangh S, Klein-Schwartz W, Schaeffer S, Casavant MJ. An Observational Study of the Factor Xa Inhibitors Rivaroxaban and Apixaban as Reported to Eight Poison Centers. Ann Emerg Med. 2016;67:189-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | De Giorgi A, Fabbian F, Molino C, Misurati E, Tiseo R, Parisi C, Boari B, Manfredini R. Pulmonary embolism and internal jugular vein thrombosis as evocative clues of Lemierre's syndrome: A case report and review of the literature. World J Clin Cases. 2017;5:112-118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 10] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 31. | Heidbuchel H, Berti D, Campos M, Desteghe L, Freixo AP, Nunes AR, Roldán V, Toschi V, Lassila R. Implementation of non-vitamin K antagonist oral anticoagulants in daily practice: the need for comprehensive education for professionals and patients. Thromb J. 2015;13:22. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |