Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4398
Peer-review started: September 27, 2019
First decision: November 19, 2019
Revised: November 22, 2019
Accepted: November 26, 2019
Published online: December 26, 2019
Processing time: 89 Days and 4.2 Hours
Gastrointestinal stromal tumors (GISTs) associated with neurofibromatosis are uncommon compared to their gastrointestinal counterparts. Patients with neurofibromatosis type 1 (NF-1) have an increased risk of developing gastrointestinal tumors, including rare types such as GIST.
A 60-year-old male Chinese patient was diagnosed with NF-1 10 years ago and presented with upper abdominal discomfort and black stools. Endoscopic ultrasonography and an enhanced abdominal computed tomography scan revealed a mass located 4 cm from the muscular layer of the descending duodenum. A 59-year-old Chinese woman who was diagnosed with NF-1 25 years ago presented with sudden unconsciousness and black stools. Multiple masses in the duodenum were noted by echogastroscopy and an enhanced abdominal computed tomography scan. Both patients presented with cutaneous neurofibromas. The histologic examination of tumors from both patients revealed spindle cells and low mitotic activity. Immunohistochemically, the tumor cells showed strong positivity for KIT (CD117), DOG-1, CD34, and Dehydrogenase Complex Subunit B, and negativity for SMA, desmin, S-100, and β-catenin. None of the six tumors from two patients had KIT exon 9, 11, 13, or 17 or platelet-derived growth factor receptor α exon 12 or 18 mutation, which is a typical finding for sporadic GISTs. None of the six tumors from the two patients had a BRAFV600E mutation. The patients were alive and well during the follow-up period (range: 0.6-5 yr).
There have been only a few previous reports of GISTs associated with NF-1. Although GISTs associated with NF-1 have morphologic and immunohistochemical similarities with GISTs, the pathogenesis, incidence, genetic background, and prognosis are not completely known. A medical history of NF-1 in a patient who has gastrointestinal bleeding or anemia and an intra-abdominal mass with nonspecific computed tomography features may help in diagnosing GIST by virtue of the well-known association of these two entities. Molecular genetic studies of cases indicated that GISTs in NF-1 patients have a different pathogenesis than sporadic GISTs.
Core tip: Gastrointestinal stromal tumors (GISTs) associated with neurofibromatosis are uncommon compared to their gastrointestinal counterparts. Here we reported two cases of KIT and platelet-derived growth factor receptor α wild-type GISTs with neurofibromatosis type 1. Although GISTs with neurofibromatosis type 1 have morphologic and immunohistochemical similarities with common GISTs, the pathogenesis, incidence, genetic background, and prognosis are not completely known. A medical history of neurofibromatosis type 1 in a patient who has gastrointestinal bleeding or anemia and an intra-abdominal mass with nonspecific computed tomography features may help in diagnosing GIST by virtue of the well-known association of these two entities.
- Citation: Kou YW, Zhang Y, Fu YP, Wang Z. KIT and platelet-derived growth factor receptor α wild-type gastrointestinal stromal tumor associated with neurofibromatosis type 1: Two case reports. World J Clin Cases 2019; 7(24): 4398-4406
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4398.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4398
Gastrointestinal stromal tumors (GISTs) are mesenchymal neoplasms characterized by activating mutations in the related receptor tyrosine kinases [KIT and platelet-derived growth factor receptor α (PDGFRA)][1,2].
GISTs occur throughout the gastrointestinal tract. GISTs are twice as common in the stomach as the jejunum and ileum. GISTs are relatively rare in the duodenum, rectum, and elsewhere. GISTs usually occur in older adults (median age: 55–65 yr) and most cases are sporadic[2]. KIT-positive GISTs have been documented in connection with the neurofibromatosis type 1 (NF-1) tumor syndrome, and most gastrointestinal tract mesenchymal tumors in patients with NF-1 tumors previously interpreted as other tumor types were likely GISTs. The pathogenesis of GISTs with NF-1 is unclear. Data derived from a clinicopathologic and molecular genetics study of NF-1 patients with GISTs are scant[3,4].
In this study, we analyzed two cases of GISTs in NF-1 patients and reviewed the literature to develop a more complete understanding of the clinicopathologic profile, prognosis, and KIT, PDGFRA, dehydrogenase complex subunit B (SDHB), and BRAF mutation status.
Case 1: A 60-year-old man was admitted to our hospital due to upper abdominal discomfort and black stools for 10 d.
Case 2: A 59-year-old woman was admitted to our hospital due to gastrointestinal bleeding for 1 wk.
Case 1: Ten days prior to admission, the patient presented with upper abdominal discomfort and black stool.
Case 2: One week prior to admission, the patient presented with sudden unconsciousness when she ascended a flight of stairs. The patient subsequently had black stool. She was evaluated at a local hospital and underwent gastroscopy, which showed superficial gastritis and multiple duodenal protuberant lesions. Therefore, she was transferred to our hospital for further treatment.
Case 1: He had a 6-mo history of hypertension. The blood pressure was well-controlled with nifedipine sustained-release tablets. Resection of a cervical fibroma was performed 5 years ago.
Case 2: The patient did not have any history of hypertension, type 2 diabetes mellitus, or chronic gastric ulcer. The patient had no history of hepatitis or tuberculosis.
Case 1: The patient was diagnosed with NF-1 10 years ago. All of his children (two males and one female) had neurofibromatosis, but no GISTs were demonstrated. The patient had no other significant past history or family history.
Case 2: The patient had neurofibromatosis and was diagnosed 25 years ago. Her brother and two children (one male and one female) had neurofibromatosis, but no GISTs were reported in her family. The patient had no other significant past history or family history.
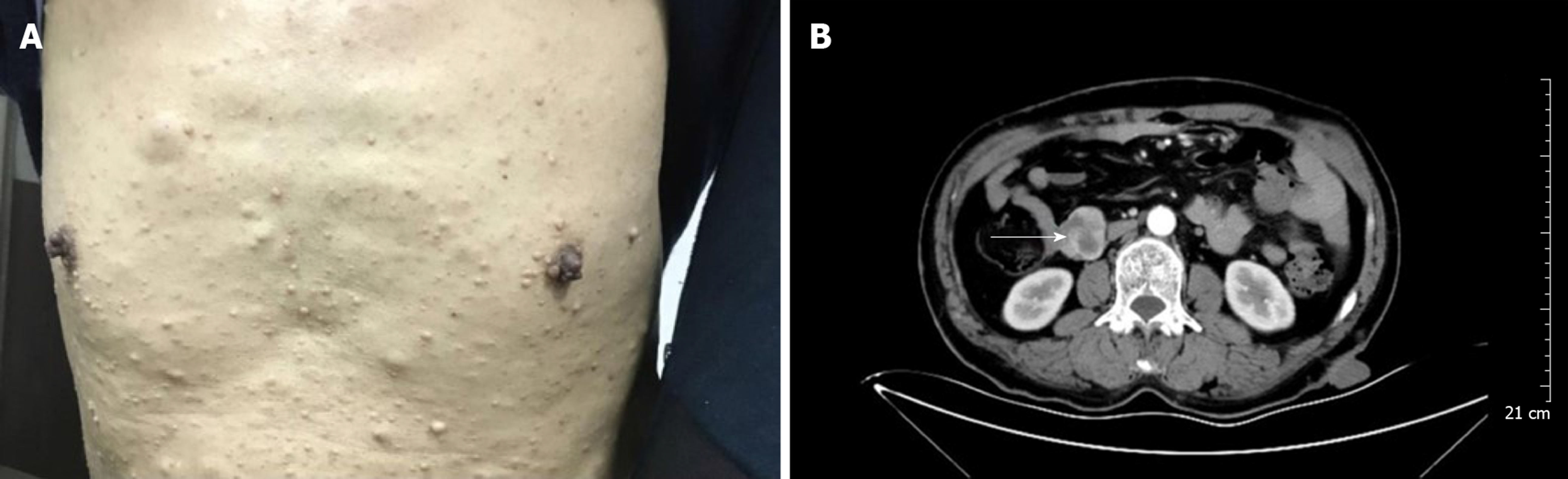
Case 1: The patient's blood pressure and blood glucose were normal. His body temperature was normal 6 d before the operation. The physical examination did not reveal any abdominal masses, and the abdomen was non-tender with no rebound tenderness. There were numerous café-au-lait patches and multiple cutaneous neurofibromas involving the upper limbs and abdomen (Figure 1A). There were no abnormalities in the electrocardiogram, chest x-ray, or preoperative echocardiogram.
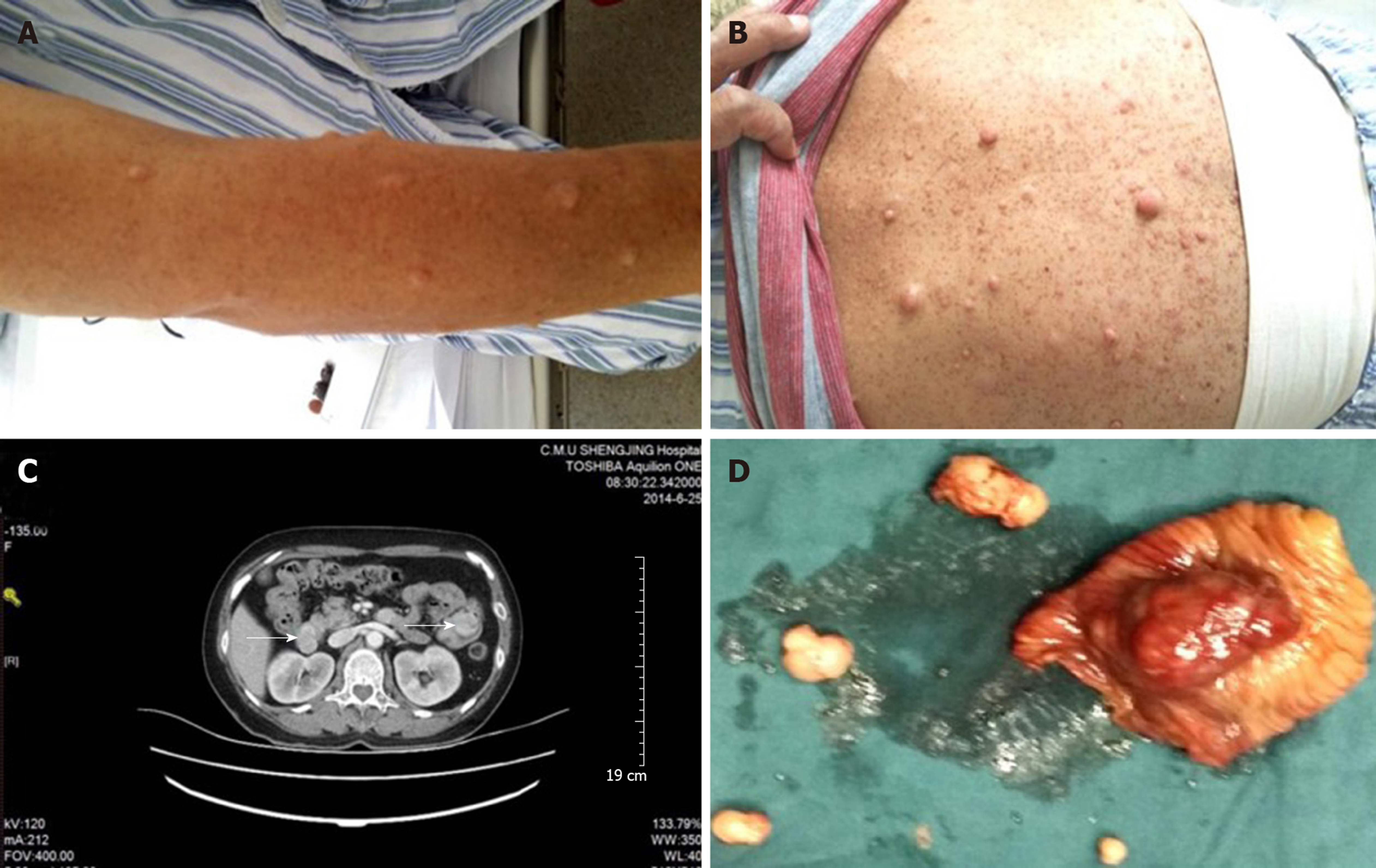
Case 2: The patient's blood pressure and blood glucose were normal. Her body temperature was normal 6 d before the operation. The physical examination did not reveal any abdominal masses, and the abdomen was non-tender with no rebound tenderness. There were numerous café-au-lait patches and multiple cutaneous neurofibromas on the upper limbs and abdomen (Figure 2A and B). There were no abnormalities in the electrocardiogram, chest x-ray, or preoperative echocardiogram.
Case 1: Except for average hemoglobin and red blood cell level under normal value, other laboratory examinations were normal.
Case 2: Except for average hemoglobin and red blood cell level under normal value, other laboratory examinations were normal.
Case 1: Endoscopic ultrasonography revealed hypoechoic protuberant lesions of the proper muscular layer of the descending duodenum. An enhanced abdominal computed tomography (CT) scan showed a mass at the junction of the descending and horizontal duodenum that was highly suggestive of a stromal tumor (Figure 1B). The enhanced CT scan also showed subcutaneous nodules of the abdominal wall that were slightly hypodense and had no apparent enhancement.
Intra-operatively, a large (4.0 cm × 3.0 cm × 3.0 cm) basal mass, hard and growing outside the intestinal wall, was noted at the junction of the descending and horizontal duodenum. After completely dissociating the mass, a local duodenectomy, distal subtotal gastrectomy, and anterior gastrointestinal anastomosis were performed. Two subcutaneous tumors were removed at the same time. The operation was successful and the recovery was uneventful.
Case 2: A contrast-enhanced CT scan was performed and revealed multiple tumors in the initial segment of the duodenum and jejunum, suggesting a diagnosis of GISTs (Figure 2C). On the basis of the radiologic findings, the tumors were surgically excised during the operation (Figure 2D). One mass in the duodenum was located in the anterior wall of the bulbar junction with the following characteristics: 2 cm × 2 cm in size; exogenous type; and completely encapsulated. A larger mass in the small intestine was located approximately 40 cm away from the Treitz ligament (4 cm × 4 cm × 4 cm in size), accounted for 80% of the intestinal lumen, and was brown-tan in color with a hemorrhagic appearance. The cut section had the appearance of fish flesh. The other two masses (1.5 cm × 1 cm in size) were located approximately 15 cm away from the Treitz ligament. The duodenal neoplasms were resected, the duodenum was repaired, and a distal gastrectomy, Roux-Y anastomosis before the gastrointestinal-jejunal colon, partial intestinal resection, and excision of the intestinal wall tumors were performed. The operation was performed without complication. The postoperative recovery was uneventful.
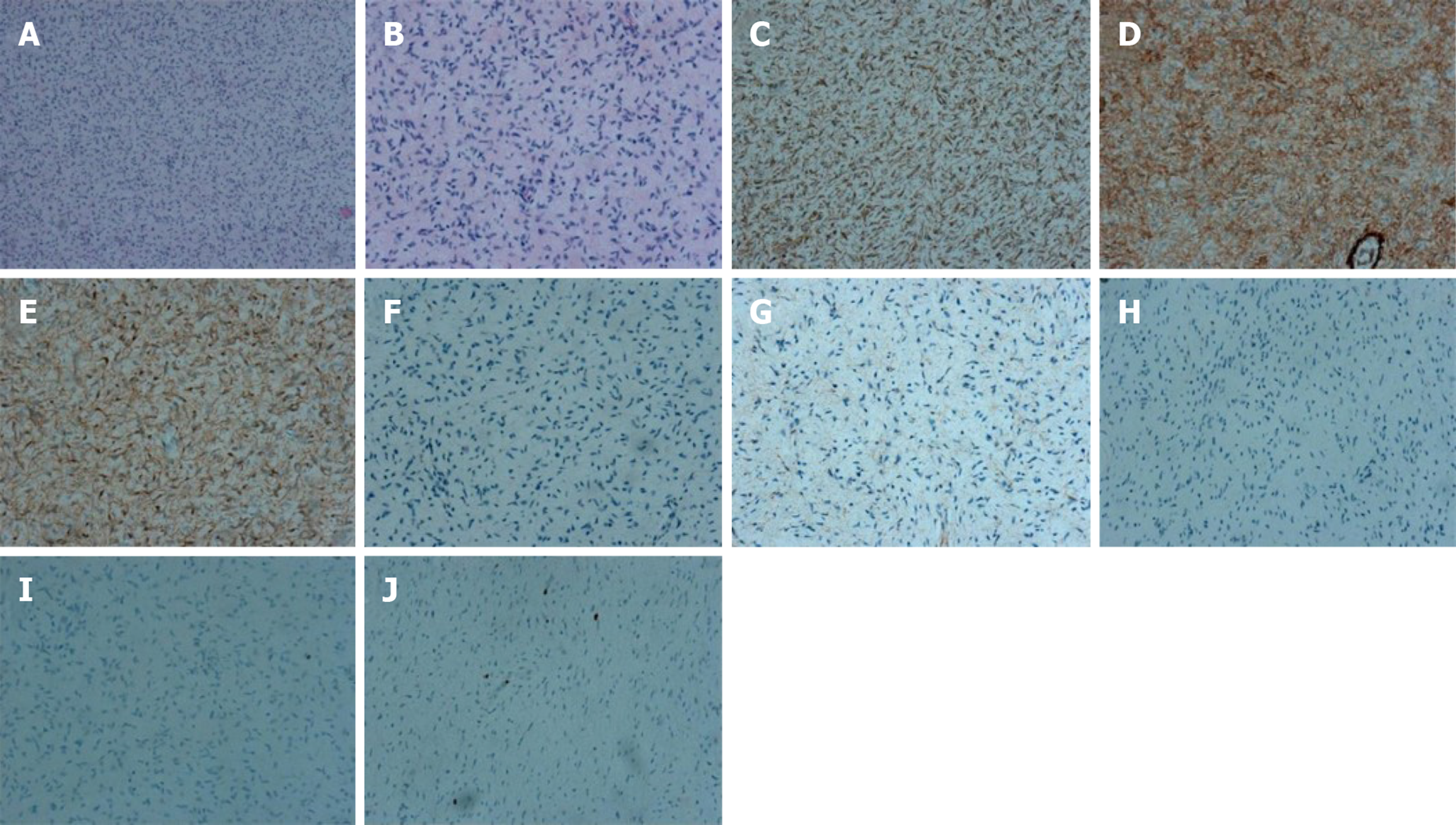
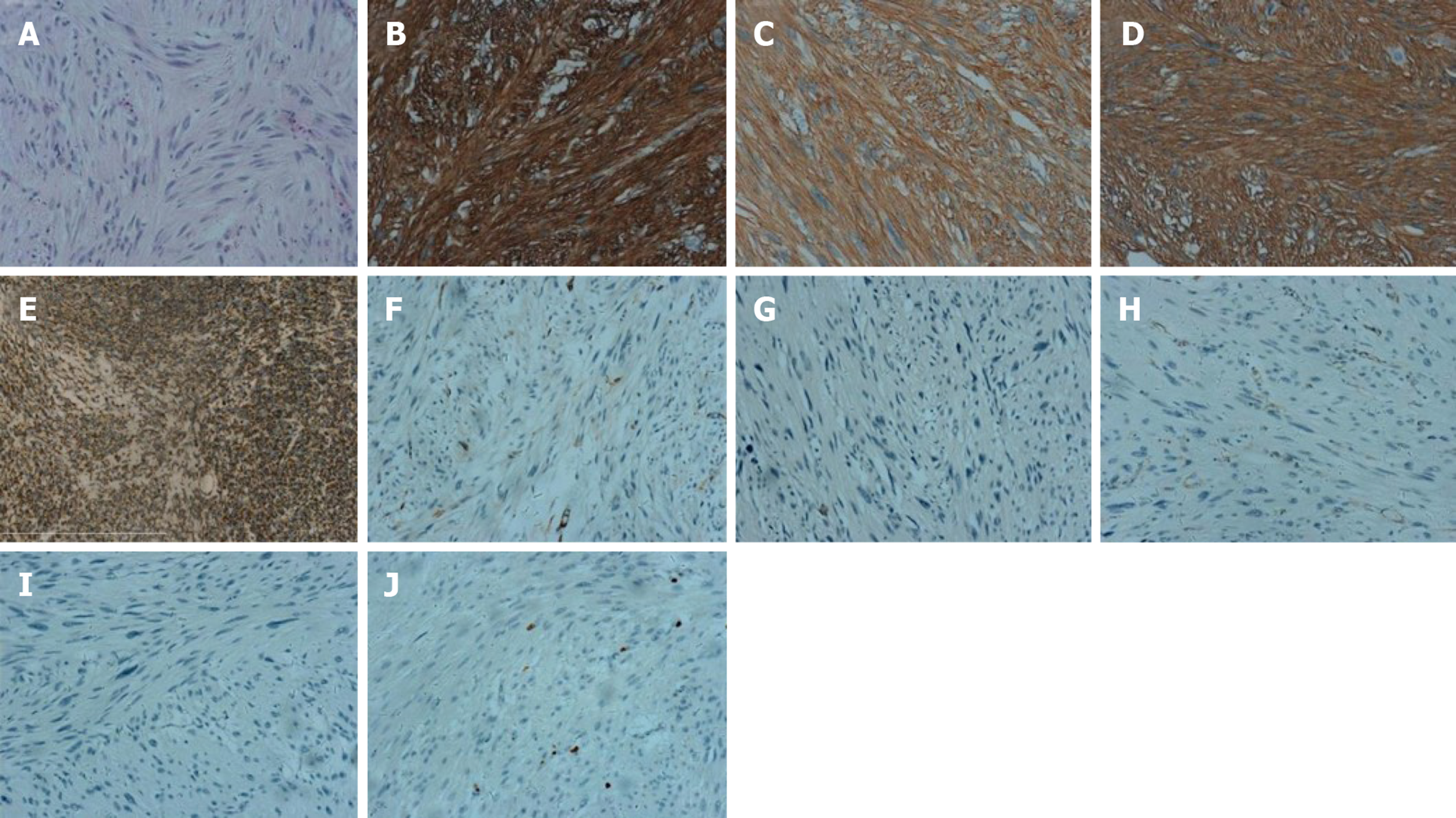
Case 1: Pathology diagnosis and molecular analysis were performed after the operation. Histologically, multiple nodules from skin were typically composed of relatively uniform long spindle cells. Immunohistochemical staining revealed the following: CD34 (+); VIM (+); S-100 (+); CK (-); EMA (-); SMA (-); desmin (-) and Ki67 (2%) (Figure 3).
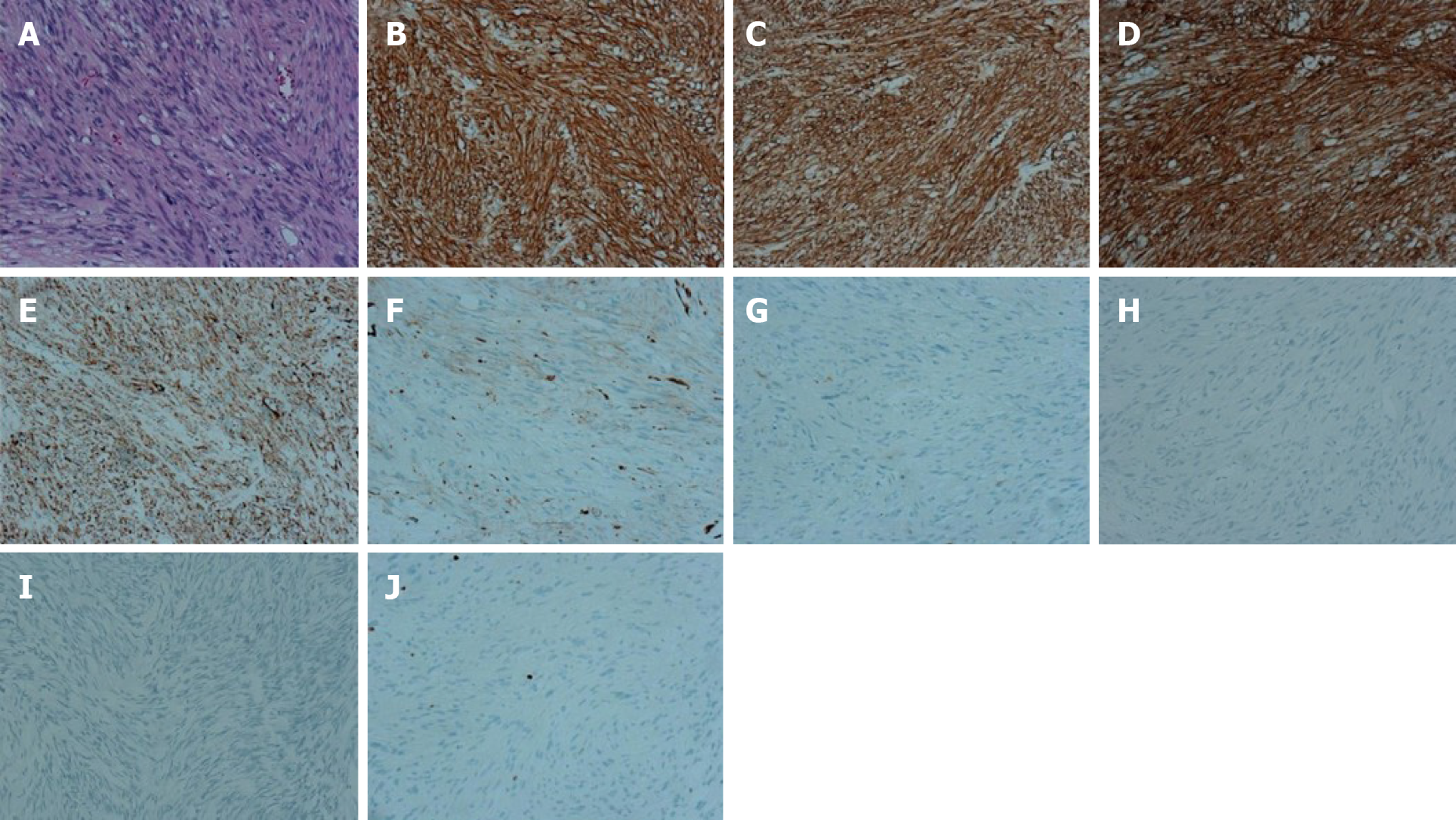
Histologically, multiple tumors from the duodenum were typically composed of relatively uniform spindle-shaped cells. Immunohistochemical staining of the nodule from duodenum revealed: CD117 (+), DOG-1 (+), CD34 (+), SDHB (+), SMA (-), S-100 (-), desmin (-), β-catenin (-), and Ki67 (2%) (Figure 4).
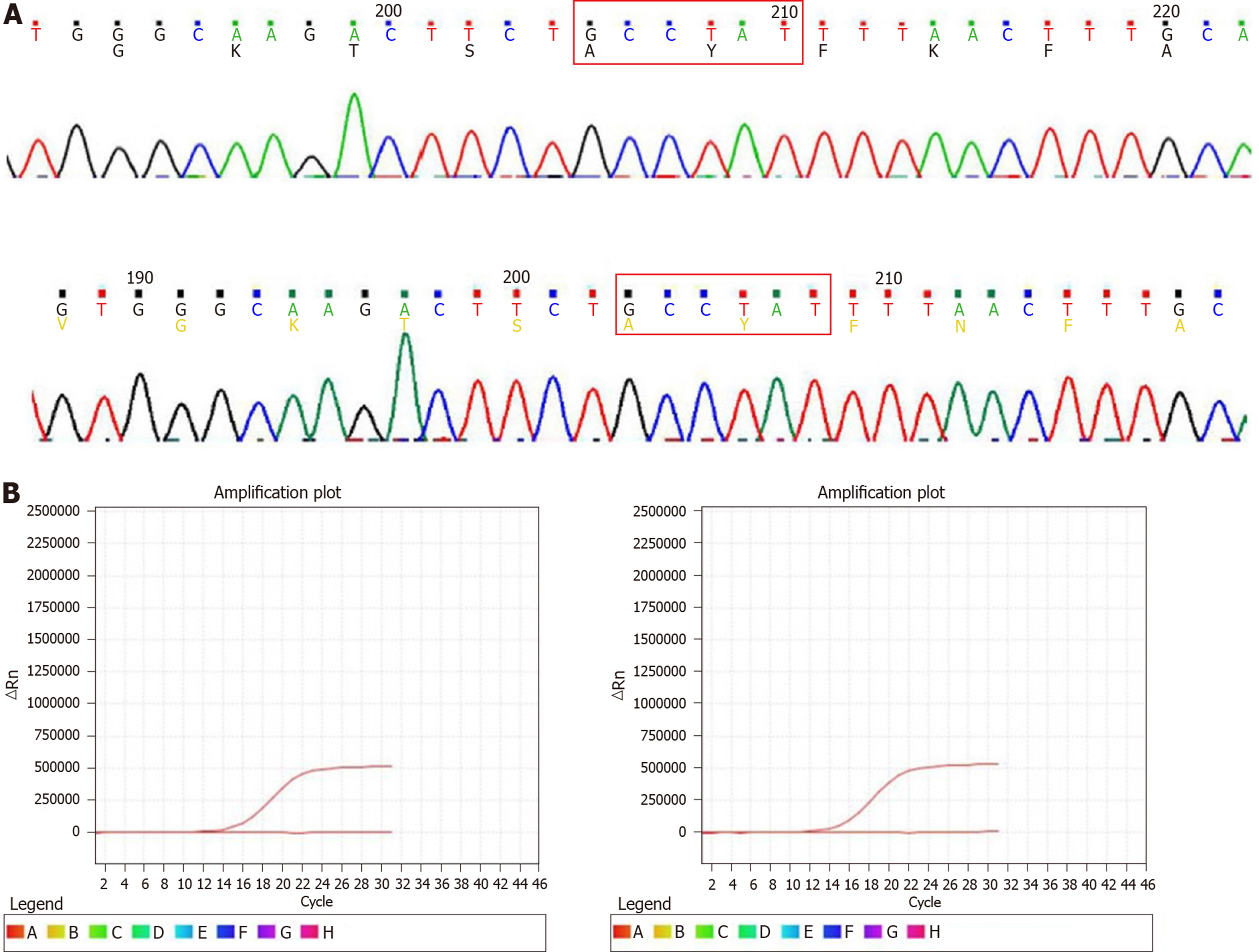
No significant mitotic cells were found in the above tissues. KIT, PDFGRA, and BRAFV600E mutation status were analyzed in GIST tissues. None of the tumors had a KIT exon 9, 11, 13, or 17, PDGFRA exon 12 or 18, or BRAFV600E mutation (Figure 5).
Case 2: Pathology diagnosis and molecular analysis were performed after the operation. Histologically, the multiple tumors from the duodenum were typically composed of relatively uniform spindle cells. Immunohistochemical staining of the tumors revealed the following: CD117 (+), DOG-1 (+), CD34 (+), SDHB (+), SMA (-), S-100 (-), desmin (-), β-catenin (-), and Ki67 (2%) (Figure 6). Scant mitotic cells were noted in all of the above tissues. The mutation status of KIT, PDFGRA, and BRAFV600E were analyzed in tumors. None of the four tumors had a KIT exon 9, 11, 13, or 17, PDGFRA exon 12 or 18, or BRAFV600E mutation (Figure 5).
Imatinib treatment was not recommended post-operatively.
Because the tumors were shown by molecular analysis to be wide-type GIST, the patient was not recommended for imatinib treatment post-operatively.
No tumor recurrence and metastasis were found on regular CT scan review after 0.6 years of follow-up.
No tumor recurrence or metastasis was demonstrated on regular CT scan review after 5 years of follow-up.
NF-1 is the most common inheritable disease. The birth incidence is estimated to be 1:3000 via autosomal dominant transmission[5]. The underlying pathogenesis is thought to be based on biallelic loss of the NF-1 tumor suppressor gene that leads to loss of neurofibromin, a negative regulator of RAS signaling[6].
In addition to cutaneous, soft tissue, and visceral (plexiform) neurofibromas, this syndrome is associated with several types of gastrointestinal and abdominal tumors, including neuronal hyperplasia (neuromas), ampullary carcinoids, pheochromo-cytomas, and GISTs. Indeed, GISTs have been suggested to be the most common NF-1-associated gastrointestinal tumor.
Neurofibromatosis with GISTs is rare. The pathogenesis of NF-1-associated GISTs has not been established. Several studies have confirmed that NF-1-associated GISTs are different from sporadic GISTs with respect to clinicopathologic characteristics, and KIT and PDGFRA gene mutations[1,5]. Moreover, the age and location of onset differ. Specifically, the median age of 45 patients with NF-1-associated GISTs reported by Miettinen et al[3] was 49 years, which is younger than patients with sporadic GIST (median age: 56 years). Women are more likely to have tumors in the small intestine, especially the jejunum, which differs from sporadic GISTs (most likely in the stomach). Most of these tumors occur in the small bowel with multifocal tumors, which is rare in sporadic GISTs.
The six tumors from our two cases were located in the duodenum and jejunum. The immunophenotypes also differed. Approximately 90% of NF-1-associated GISTs are CD34-positive, which is higher than sporadic GISTs. The S-100 protein-positive rate was higher than sporadic small intestinal GISTs. Moreover, the genotypes are different. Approximately 90% of sporadic GISTs have KIT gene mutations and 5% have PDGFRA gene mutations, while most NF-1-related GISTs have no KIT and PDGFRA mutations, suggesting that the pathogenesis of the two kinds of GISTs is different. It has been reported that < 5% of sporadic GISTs have SDHB or BRAFV600E gene mutations. The tumor cells from our two cases had strong positivity for KIT (CD117), DOG-1, CD34, and SDHB, and were negative for SMA, desmin, β-catenin and S-100.
The two patients signed an informed consent form before molecular analysis. The data revealed that none of the six tumors from the two patients had a KIT exon 9, 11, 13, or 17, PDGFRA exon 12 or 18, or BRAFV600E mutation, which typically occurs in sporadic GISTs. It is reported most of the GISTs associated with NF-1 are well differentiated histologically, small in size, exhibit low proliferative activity, are often benign, and have a good prognosis[1,4]. Surgical resection of GIST is still the preferred method. So far, the prognosis in these two cases is similar to that reported in the literature. No recurrences or metastases were found after long-term follow-up, but there are a few exceptions. Momani et al[6] reported a recurrent GIST in a patient with neurofibromatosis. The tumor cells from our two cases were well differentiated and low proliferative activity, and tumors were small in size. The GISTs in both patients did not recur or metastasize after surgery during the follow-up period.
GISTs are considered pathologic oddities and are somewhat neglected by oncologists as chemoresistant sarcomas. However, GISTs have now emerged as distinct pathogenetic entities that reinforce the concept of molecular targeted therapy. Imatinib therapy has produced dramatic results in the management of metastatic and unresectable GISTs. However, the response to imatinib is usually confined to GISTs harboring detectable KIT or PDGFRA gene mutations. Interestingly, nearly all GISTs associated with NF-1 lack a detectable KIT gene mutation[7]. Despite these observations, there have been only a limited number of studies on NF-1-associated GISTs, and a few studies have reported on the GIST response to imatinib in such patients[8,9]. Although all of the reported cases in two large series expressed CD117, the patients with metastatic tumors had a poor response to imatinib. NF-1-associated GISTs are usually KIT/PDGFRA wild-type, although sporadic KIT/PDGFRA mutations have been reported in some cases[4,10,11].
NF-1-associated GISTs exhibit increased signaling through the mitogen-activated protein kinase signaling cascade, raising the possibility that treatment with MEK inhibitors could be promising. Comprehensive genomic profiling studies have identified a number of gene fusions in KIT/PDGFRA wild-type GISTs that involve neurotrophic tyrosine kinase receptor type 3 and fibroblast growth factor receptor 1, some of which might represent actionable alterations[12]. A study reported two cases of metastatic GISTs in NF-1 patients who responded favorably to imatinib despite the absence of mutations in the KIT and PDGFRA genes.
Tyrosine kinase inhibitors, such as imatinib or sunitinib, were not recommended in our two cases. The GISTs in both patients did not recur or metastasize after surgery. Furthermore, patients with NF-1 are at high risk of developing neurogenic, neuroendocrine, and mesenchymal intra-abdominal tumors. Patients with NF-1 and abdominal symptoms should be treated with a high index of clinical suspicion and thoroughly evaluated to rule out multiple tumors[13-15]. Ultrasonography is an effective and safe method for the diagnosis of abdominal diseases[16-18]. If the symptoms of gastrointestinal bleeding occur in patients with NF-1, then endoscopic ultrasonography should be performed first[19-22].
The limitations of this study are the small number of patients enrolled and the relatively short period of follow-up. The follow-up is still in progress, and a further study with a larger number of cases is needed.
Our cases illustrate the increased prevalence and association of GISTs in patients with NF-1. GISTs typically occur in the small intestine, are multiple, do not have SDHB, BRAFV600E, KIT, or PDGFRA mutations, and exhibit favorable clinical behavior. A medical history of NF-1 in a patient who has gastrointestinal bleeding or anemia and an intra-abdominal mass with nonspecific CT features may help in diagnosing GISTs by virtue of the well-known association of the two entities.
Manuscript source: Unsolicited manuscript
Accepted: November 26, 2019
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bystrom P, Jaggi M S-Editor: Wang JL L-Editor: Filipodia E-Editor: Liu JH
| 1. | Hemming ML, Lawlor MA, Andersen JL, Hagan T, Chipashvili O, Scott TG, Raut CP, Sicinska E, Armstrong SA, Demetri GD, Bradner JE. Enhancer Domains in Gastrointestinal Stromal Tumor Regulate KIT Expression and Are Targetable by BET Bromodomain Inhibition. Cancer Res. 2019;79:994-1009. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 759] [Reference Citation Analysis (0)] |
| 2. | Han SH, Park SH, Cho GH, Kim NR, Oh JH, Nam E, Shin DB. Malignant gastrointestinal stromal tumor in a patient with neurofibromatosis type 1. Korean J Intern Med. 2007;22:21-23. [PubMed] |
| 3. | Miettinen M, Fetsch JF, Sobin LH, Lasota J. Gastrointestinal stromal tumors in patients with neurofibromatosis 1: a clinicopathologic and molecular genetic study of 45 cases. Am J Surg Pathol. 2006;30:90-96. [PubMed] |
| 4. | Gutmann DH, Aylsworth A, Carey JC, Korf B, Marks J, Pyeritz RE, Rubenstein A, Viskochil D. The diagnostic evaluation and multidisciplinary management of neurofibromatosis 1 and neurofibromatosis 2. JAMA. 1997;278:51-57. [PubMed] |
| 5. | Yamamoto H, Tobo T, Nakamori M, Imamura M, Kojima A, Oda Y, Nakamura N, Takahira T, Yao T, Tsuneyoshi M. Neurofibromatosis type 1-related gastrointestinal stromal tumors: a special reference to loss of heterozygosity at 14q and 22q. J Cancer Res Clin Oncol. 2009;135:791-798. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 6. | Al Momani LA, Abughanimeh O, Shipley LC, Phemister J, Swenson J, Young M. Recurrent Gastric Gastrointestinal Stromal Tumor in a Patient with Neurofibromatosis. Cureus. 2018;10:e2854. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Wu CE, Tzen CY, Wang SY, Yeh CN. Clinical Diagnosis of Gastrointestinal Stromal Tumor (GIST): From the Molecular Genetic Point of View. Cancers (Basel). 2019;11:E679. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 66] [Article Influence: 11.0] [Reference Citation Analysis (1)] |
| 8. | Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 190] [Article Influence: 11.9] [Reference Citation Analysis (0)] |
| 9. | Mussi C, Schildhaus HU, Gronchi A, Wardelmann E, Hohenberger P. Therapeutic consequences from molecular biology for gastrointestinal stromal tumor patients affected by neurofibromatosis type 1. Clin Cancer Res. 2008;14:4550-4555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 121] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 10. | Langabeer SE. Detecting CALR Mutations in Splanchnic Vein Thrombosis: Who and How? J Transl Int Med. 2018;6:55-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 11. | Xue A, Yuan W, Gao X, Fang Y, Shu P, Xu C, Li H, Xu Y, Song Q, Hou Y, Shen K. Gastrointestinal stromal tumors (GISTs) with remarkable cystic change: a specific subtype of GISTs with relatively indolent behaviors and favorable prognoses. J Cancer Res Clin Oncol. 2019;145:1559-1568. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 12. | Shi E, Chmielecki J, Tang CM, Wang K, Heinrich MC, Kang G, Corless CL, Hong D, Fero KE, Murphy JD, Fanta PT, Ali SM, De Siena M, Burgoyne AM, Movva S, Madlensky L, Heestand GM, Trent JC, Kurzrock R, Morosini D, Ross JS, Harismendy O, Sicklick JK. FGFR1 and NTRK3 actionable alterations in "Wild-Type" gastrointestinal stromal tumors. J Transl Med. 2016;14:339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 163] [Article Influence: 18.1] [Reference Citation Analysis (0)] |
| 13. | Abdessayed N, Gupta R, Mestiri S, Bdioui A, Trimech M, Mokni M. Rare triad of periampullary carcinoid, duodenal gastrointestinal stromal tumor and plexiform neurofibroma at hepatic hilum in neurofibromatosis type 1: a case report. BMC Cancer. 2017;17:579. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Teixeira F, Menegozzo CAM, Couto Netto SDD, Scapini G, Akaishi EH, Vasconcelos MPS, Utiyama EM. Pancreaticoduodenectomy in patients with type 1 Neurofibromatosis: Report of two cases and literature review. Int J Surg Case Rep. 2016;27:36-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 15. | Liu Q, Kong F, Zhou J, Dong M, Dong Q. Management of hemorrhage in gastrointestinal stromal tumors: a review. Cancer Manag Res. 2018;10:735-743. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 34] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Barresi L, Tacelli M, Tarantino I, Cipolletta F, Granata A, Traina M. Improving the yield of EUS-guided histology. Endosc Ultrasound. 2018;7:301-305. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Gottschalk U, Dietrich CF, Jenssen C. Ectopic pancreas in the upper gastrointestinal tract: Is endosonographic diagnosis reliable? Data from the German Endoscopic Ultrasound Registry and review of the literature. Endosc Ultrasound. 2018;7:270-278. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | Abad-Belando R, Varas-Lorenzo MJ, Pons-Vilardell C, Puig-Torrus X, Pla-Alcaraz M, Monleón-Getino A, Sánchez-Vizcaíno-Mengual E. Canalization technique to obtain deep tissue biopsy of gastrointestinal subepithelial tumors as an alternative to conventional known techniques. Endosc Ultrasound. 2018;7:184-190. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 19. | Alkhormi AM, Memon MY, Alqarawi A. Gastric Antral Vascular Ectasia: A Case Report and Literature Review. J Transl Int Med. 2018;6:47-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Cartana ET, Gheonea DI, Cherciu IF, Streaţa I, Uscatu CD, Nicoli ER, Ioana M, Pirici D, Georgescu CV, Alexandru DO, Şurlin V, Gruionu G, Săftoiu A. Assessing tumor angiogenesis in colorectal cancer by quantitative contrast-enhanced endoscopic ultrasound and molecular and immunohistochemical analysis. Endosc Ultrasound. 2018;7:175-183. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 21. | Chong CCN, Teoh AYB, Tang RSY, Chan AWH, Ng EKW, Lai PBS. EUS-FNA using 22G nitinol or ProCore needles without on-site cytopathology. Endosc Ultrasound. 2018;7:56-60. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 22. | Nam K, Kim DU, Lee TH, Iwashita T, Nakai Y, Bolkhir A, Castro LA, Vazquez-Sequeiros E, de la Serna C, Perez-Miranda M, Lee JG, Lee SS, Seo DW, Lee SK, Kim MH, Park DH. Patient perception and preference of EUS-guided drainage over percutaneous drainage when endoscopic transpapillary biliary drainage fails: An international multicenter survey. Endosc Ultrasound. 2018;7:48-55. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 46] [Article Influence: 6.6] [Reference Citation Analysis (0)] |