Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4377
Peer-review started: September 4, 2019
First decision: September 23, 2019
Revised: November 8, 2019
Accepted: November 23, 2019
Article in press: November 23, 2019
Published online: December 26, 2019
Processing time: 111 Days and 20.2 Hours
Fabry disease is a kind of lysosomal storage disease resulting from deficient activity of the lysosomal hydrolase alpha-galactosidase A (GLA). A mutation in the GLA gene leads to a loss of activity of alpha-galactosidase A. Some drugs, such as hydroxychloroquine, can cause pathological changes similar to those usually seen in Fabry disease.
We report the case of a 41-year-old female patient who was diagnosed with undifferentiated connective tissue disease in 2008. Hydroxychloroquine treatment started 2 years ago, and proteinuria and hematuria increased. Renal biopsy demonstrated renal phospholipidosis. Zebra bodies and myelin figures were found by renal electron microscopy and were initially thought to be indicators of Fabry disease. A genetic analysis of the patient and her family members did not reveal mutations in the GLA gene, supporting a diagnosis of hydroxychloroquine-induced renal phospholipidosis.
This report reveals one of the adverse effects of hydroxychloroquine. We should pay more attention to hydroxychloroquine-induced renal phospholipidosis.
Core tip: Hydroxychloroquine-induced renal phospholipidosis is characterised by zebra bodies and myelin figures, mimicking nephropathy of Fabry disease. It reminds that clinical application of hydroxychloroquine should be careful. Moreover, drug-induced renal phospholipidosis should be considered as a differential diagnosis, especially when zebra bodies and myelin figures are found in the kidney.
- Citation: Wu SZ, Liang X, Geng J, Zhang MB, Xie N, Su XY. Hydroxychloroquine-induced renal phospholipidosis resembling Fabry disease in undifferentiated connective tissue disease: A case report. World J Clin Cases 2019; 7(24): 4377-4383
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4377.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4377
Fabry disease is a genetically X chromosome linked disease that can affect many human organs, including the kidneys, heart, and skin[1-4]. Furthermore, Fabry disease is a kind of lysosomal storage disease[5]. In Fabry disease, deficient activity of the lysosomal hydrolase alpha-galactosidase A (GLA) is caused by a mutation in the GLA gene, resulting in the intracellular accumulation of enzyme substrates inside of lysosomes[1-4].
The symptoms of Fabry disease affect multiple systems and organs[1-4]. Early symptoms arise in the nervous system and are characterized by paresthesia and pain. Symptoms in the skin and eyes appear afterwards and include fever, angiokeratomas, and cornea verticillate. Kidney and heart dysfunction are the main symptoms in adults. Heart dysfunction includes cardiac hypertrophy, valvular abnormalities, and arrhythmias. Renal dysfunction usually includes hematuria, proteinuria, and nephrotic syndrome. In addition, such conditions in the kidney ultimately progress to end-stage kidney disease accompanied by various severe complications. Severe complications are ultimately the primary cause of death. A light microscopic examination of the kidney demonstrates that glomerular visceral epithelial cells are diffusely enlarged with vacuolar degeneration. Electron microscopy examination shows that all kinds of renal cells contain many dense lamellated structures, including glomerular visceral epithelial cells, endothelial cells, and mesangial cells. Such structures are widely called zebra bodies or myelin figures and are the typical characteristics of Fabry disease[6].
Zebra bodies or myelin figures were previously seen as the prime characteristics of Fabry disease. However, previous reports showed that some drugs, including amiodarone, chloroquine, and hydroxychloroquine, may lead to similar histological changes[7-10]. Here, we report the case of a 41-year-old female patient who was diagnosed with undifferentiated connective tissue disease in 2008. This patient had been on hydroxychloroquine therapy for two years until now. Renal biopsy revealed zebra bodies and myelin figures mimicking Fabry disease. However, the clinical symptoms of Fabry disease, a family history of Fabry disease, and a genetic evaluation of the GLA gene were negative.
A 41-year-old female patient was diagnosed with undifferentiated connective tissue disease in 2008. Since then, this patient received low doses of prednisone (Table 1). Because of facial erythema and a decrease in blood complement in 2016, hydroxychloroquine was added to 400 mg/d, and the dosage of prednisone was increased to 10 mg/d (Table 1). This patient had a loss of weight of approximately 3 kg by March 2018, as well as proteinuria and weakness. The patient was admitted to our hospital in April 2018.
| Time | Symptoms | Diagnoses | Treatments |
| 2008 | Canker sores | Undifferentiated connective tissue disease | Low doses of prednisone: 5 mg |
| White blood cell count: 1.8 × 109/L | |||
| ANA: 1:320(+) | |||
| Blood complements: Normal | |||
| Serum creatinine: 66 µmol/L | |||
| 2016 | Facial erythema | Undifferentiated connective tissue disease | Hydroxychloroquine; Prednisone |
| Decreased blood complements | |||
| April 2018 | Weight loss | Undifferentiated connective tissue disease; Hydroxychloroquine -induced renal phospholipidosis | Withdrawal of hydroxychloroquine; Prednisone |
| Weakness | |||
| White blood count: 2.7-4.7 × 109/L | |||
| Urine erythrocytes: 28/µL | |||
| Serum creatinine: 58 µmol/L | |||
| 24-h urinary protein, quantitative: 1120 mg | |||
| ANA: 1:80(+) | |||
| Complement C3: 0.58 g/L | |||
| Complement C4: 0.09 g/L | |||
| Renal biopsy: Renal phospholipidosis | |||
| Mutation of GLA gene: Negative |
The patient had a history of hypertension. There was no relevant family history.
There were a few of ulcers in the mouth.
The results of laboratory examinations are as follows: White blood cell count, 4.7 × 109/L; 24-h urine protein, 1120 mg and urine red blood cells, 28/µL; routine fecal tests and occult blood test, normal; blood albumin (ALB), 33.40 g/L; serum creatinine, 58 µmol/L; positive antinuclear antibody (ANA), 1/80+; anti-histone antibody, +/-; anti-nucleosome antibody, +/-; negative anti-dsDNA antibody, ANA and anti-GBM antibody; complement C3, 0.58 g/L; complement C4, 0.09 g/L (Table 1); erythrocyte sedimentation rate and C-reactive protein, normal.
Color Doppler ultrasound of the kidneys indicated a right renal nodule.
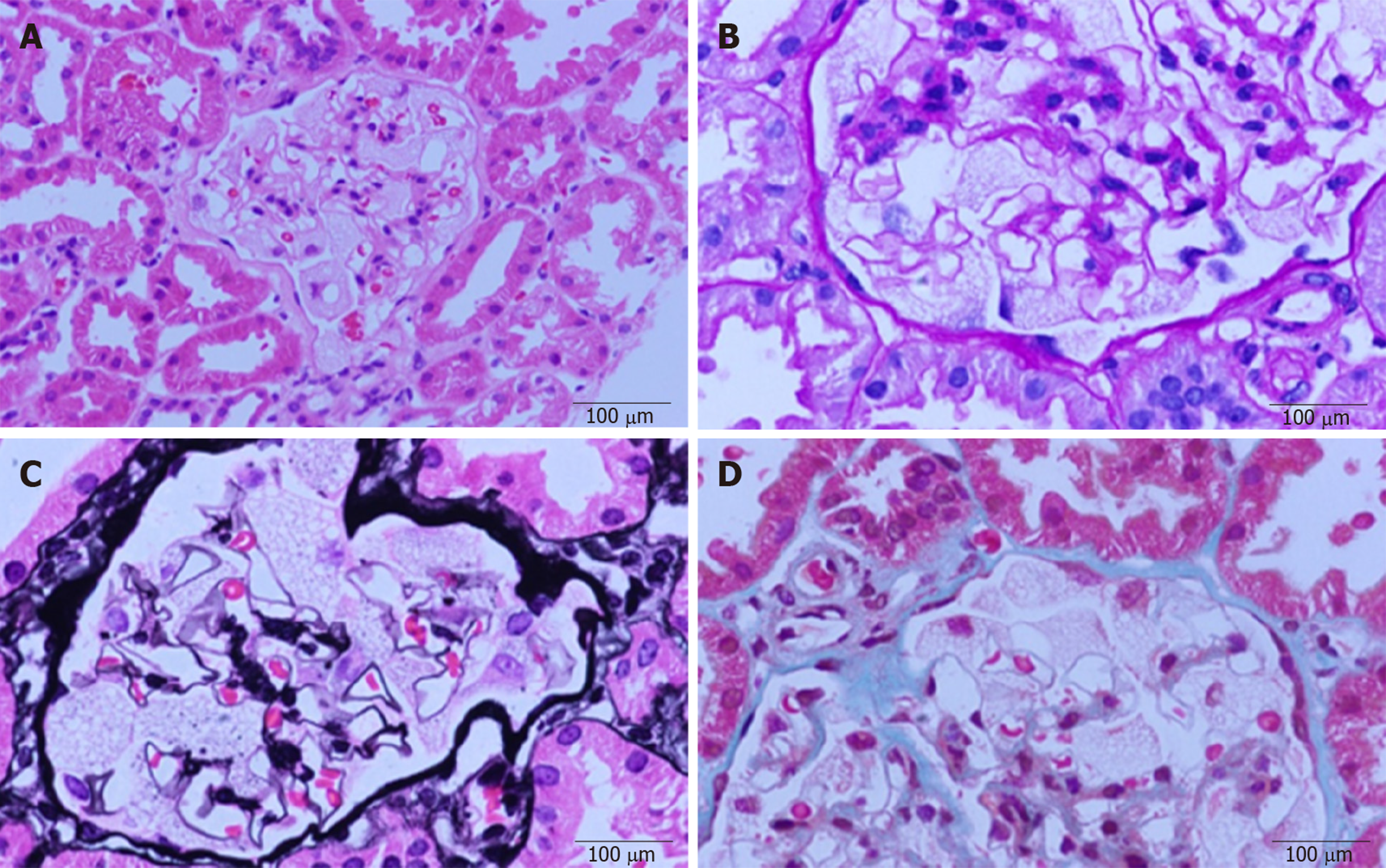
Renal biopsy was performed to evaluate nephropathy. A light microscopic examination (Figure 1) of paraffin-embedded sections stained with hematoxylin and eosin, periodic acid–Schiff, and Masson’s trichrome showed that glomerular visceral epithelial cells were diffusely enlarged with vacuolar degeneration, but segmental sclerosis and crescents were not observed in glomeruli. The mesangial matrix and cellularity were normal. Renal tubular epithelial cells presented granular degeneration without obvious atrophy. An infiltration of several inflammatory cells could be seen in the renal mesenchyme, but fibrosis was hardly found. Arterioles appeared to be thickened and narrow.
Immunofluorescence analysis revealed mild staining for IgM. Immunostaining for IgA; IgG; ALB; complement factors C3, C4, and C1q; and fibrinogen was negative.
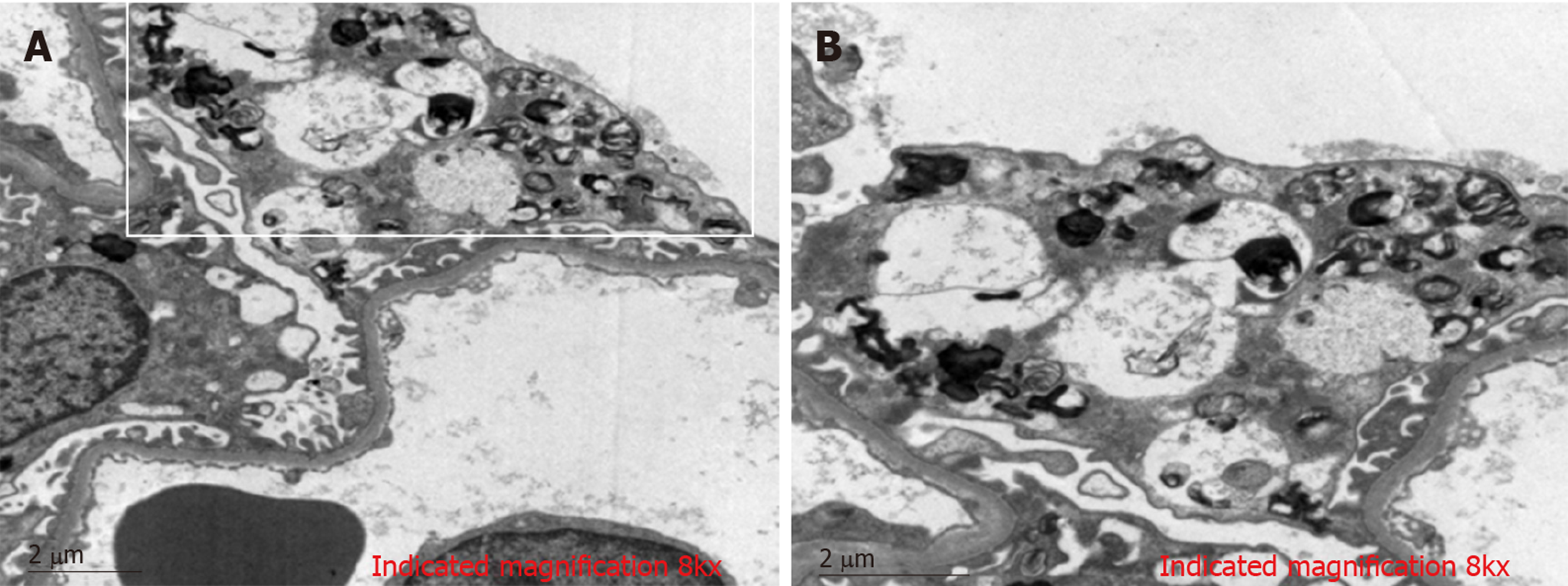
Electron microscopic analysis with toluidine blue staining showed (Figure 2) that glomerular visceral epithelial cells were swollen. Many vacuoles with dense lamellated structures were present in the cytoplasm of podocytes. A number of secondary lysosomes and foot process fusion could be seen in the cytoplasm of podocytes. Arterioles, mesangial matrix and cellularity were normal. Renal tubular epithelial cells manifested vacuolar degeneration. Some inflammatory cells had infiltrated the renal mesenchyme.
In conclusion, the renal biopsy demonstrated glomerular visceral epithelial cells containing zebra bodies and myelin figures.
A genetic evaluation of the patient and her family members was performed, and mutations of the GLA gene were not detected.
Hydroxychloroquine-induced renal phospholipidosis.
Withdrawal of hydroxychloroquine.
The patient had returned to her native place, and we keep in touch with her. The patient went to the local hospital for examination in April 2019, and urine tests showed that hematuria and proteinuria decreased. Although the patient had already decided not to repeat renal biopsy, we will continue to monitor the conditions of this patient.
Fabry disease is a rare X-linked genetic disease that can affect many human organs, including the kidney, heart, and skin[1-4]. Fabry disease is a kind of lysosomal storage disease[5]. A mutation in the GLA gene in Fabry disease leads to deficient activity of GLA, resulting in the accumulation of multiple hydrolase substrates inside of lysosomes, such as globotriaosylceramide and glycosphingolipids. Fabry disease affects multiple systems and organs, including the nervous system, skin, eyes, kidneys, and heart. The clinical manifestations of multiple systems and organs caused by Fabry disease are various and include paresthesia, fever, angiokeratomas, cornea verticillate, cardiovascular events, hematuria, proteinuria, and nephrotic syndrome. Zebra bodies or myelin figures detected by electron microscopy are typical characteristics of Fabry disease[6].
Zebra bodies and myelin figures are prime pathological changes of microscopic tests in Fabry disease, and some studies have shown that similar pathological changes can be caused by some drugs, such as amiodarone, chloroquine, and hydroxychloroquine[1,7-10]. Because of the amphiphilic nature of such drugs, similar pathological changes can be easily seen in various organs, such as the liver, lung, and kidney[7-11]. Some lysosomal enzymes, including GLA, can be suppressed by such drugs and lose their biological activity, resulting in a deposition of enzyme substrates inside of lysosomes[12]. Such enzyme substrates also include globotriaosylceramide and glycosphingolipid[1]. The deposition of enzyme substrates caused by such drugs in the kidney is usually known as renal phospholipidosis, which is characterized by zebra bodies or myelin figures[9]. Thus, renal phospholipidosis mimicking Fabry disease may be closely related to the toxicity of some drugs.
Phospholipidosis caused by drugs usually exhibits intracellular deposition of phospholipids and lamellar bodies, which are often regarded as the primary microscopic markers of lipid storage diseases. Lysosomes are a type of cellular organelle, and they contain all kinds of hydrolytic enzymes, including lipases, phospholipases, and proteases. The deposition of lipids in drug-induced phospholipidosis can be easily found in lysosomes. Hydroxychloroquine is capable of passing through the lysosomal membrane due to its particular chemical structure. Hydroxyhloroquine is able to maintain its structural integrity when it passes through the lysosomal membrane. With the continuous accumulation of hydroxychloroquine inside of lysosomes, some hydrolytic enzymes, including GLA, are suppressed and lose their biological activity. After that, the catabolic processes of numerous enzymatic substrates are blocked, which leads to the deposition of phospholipids and lamellar bodies[7-9]. Deposition of the substrates in the kidney leads to renal dysfunction, such as glomerulosclerosis, thickening of glomerular basement membrane, and increase of mesangial matrix. All of these renal pathological changes ultimately cause proteinuria and hematuria. Such characteristics are similar to those of Fabry disease. Therefore, renal phospholipidosis has a close relationship with hydroxychloroquine as well as similar chemical structures[7-9].
Previous studies have reported that some patients who were diagnosed with systemic lupus erythematosus or Sjogren’s syndrome were treated with hydroxychloroquine. After long-term treatment with hydroxychloroquine, renal phospholipidosis was detected by renal microscopic examination. Zebra bodies and myelin figures were found by electron microscopy and were similar to those found in nephropathy of Fabry disease. However, drug-induced renal phospholipidosis was ultimately confirmed based on the manifestations; the activity level of GLA; and the evaluation of the GLA gene, family history, and medication history[8,13,14]. A consensus regarding how to make a precise diagnosis of drug-induced renal phospholipidosis has not been established until now. Thus, when some findings resembling Fabry disease are detected during microscopic examination, drug-induced renal phospholipidosis should always be considered as a differential diagnosis, particularly in cases with no family history or relevant symptoms. Accordingly, in this case, a two-year treatment with hydroxychloroquine, an absence of symptoms of Fabry disease, a negative family history of Fabry disease, and an absence of mutations in the GLA gene largely pointed to a diagnosis of hydroxychloroquine-induced renal phospholipidosis.
Early diagnosis is widely acknowledged as an effective treatment for renal phospholipidosis caused by drugs[15]. In this case, hydroxychloroquine was withdrawn when the diagnosis of hydroxychloroquine-induced renal phospholipidosis was confirmed. Although the patient had returned to her native place, we keep in touch with her. The patient went to the local hospital for examination in April 2019, and urine tests showed that hematuria and proteinuria decreased. Even though the patient had already decided not to repeat renal biopsy, we will continue to monitor the conditions of this patient.
We have reported a case of hydroxychloroquine-induced renal phospholipidosis. Deposition of phospholipids caused by hydroxychloroquine in the kidney is characterized by zebra bodies and myelin figures similar to nephropathy of Fabry disease. Such pathological changes in the kidney gradually result in glomerulosclerosis, thickening of glomerular basement membrane, and increase of mesangial matrix. Finally, proteinuria or hematuria also appears as the first symptoms. Overall, our presentation provides further evidence of the side effects of hydroxychloroquine. It demonstrates that we should pay more attention to application of hydroxychloroquine. Furthermore, drug-induced renal phospholipidosis should be considered as a differential diagnosis, especially when zebra bodies and myelin figures are found in the kidney.
We would like to thank all members of our department for their helpful comments and general support. We would also like to thank Jian Geng for pathological and genetic evaluations.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Galvañ VG S-Editor: Gong ZM L-Editor: Wang TQ E-Editor: Liu JH
| 1. | Alroy J, Sabnis S, Kopp JB. Renal pathology in Fabry disease. J Am Soc Nephrol. 2002;13 Suppl 2:S134-S138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 158] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 2. | Waldek S, Feriozzi S. Fabry nephropathy: a review - how can we optimize the management of Fabry nephropathy? BMC Nephrol. 2014;15:72. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 3. | Ortiz A, Oliveira JP, Wanner C, Brenner BM, Waldek S, Warnock DG. Recommendations and guidelines for the diagnosis and treatment of Fabry nephropathy in adults. Nat Clin Pract Nephrol. 2008;4:327-336. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Wilcox WR, Oliveira JP, Hopkin RJ, Ortiz A, Banikazemi M, Feldt-Rasmussen U, Sims K, Waldek S, Pastores GM, Lee P, Eng CM, Marodi L, Stanford KE, Breunig F, Wanner C, Warnock DG, Lemay RM, Germain DP; Fabry Registry. Females with Fabry disease frequently have major organ involvement: lessons from the Fabry Registry. Mol Genet Metab. 2008;93:112-128. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 370] [Cited by in RCA: 391] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 5. | Azevedo DJ, D’Almeida LO, Silveira RA, Sachs K, Alexandrino M. Primeiros pacientes do Estado do Rio de Janeiro tratados com a enzima recombinante Agalsidase Beta (Fabrazime)/Fabryïs disease: First patients of the state of fio treated with agalsidase beta. Rev Bras Oftal. 2004;63:259-263. |
| 6. | Fogo AB, Bostad L, Svarstad E, Cook WJ, Moll S, Barbey F, Geldenhuys L, West M, Ferluga D, Vujkovac B, Howie AJ, Burns A, Reeve R, Waldek S, Noël LH, Grünfeld JP, Valbuena C, Oliveira JP, Müller J, Breunig F, Zhang X, Warnock DG; all members of the International Study Group of Fabry Nephropathy (ISGFN). Scoring system for renal pathology in Fabry disease: report of the International Study Group of Fabry Nephropathy (ISGFN). Nephrol Dial Transplant. 2010;25:2168-2177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 7. | Albay D, Adler SG, Philipose J, Calescibetta CC, Romansky SG, Cohen AH. Chloroquine-induced lipidosis mimicking Fabry disease. Mod Pathol. 2005;18:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 53] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 8. | Müller-Höcker J, Schmid H, Weiss M, Dendorfer U, Braun GS. Chloroquine-induced phospholipidosis of the kidney mimicking Fabry's disease: case report and review of the literature. Hum Pathol. 2003;34:285-289. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Bracamonte ER, Kowalewska J, Starr J, Gitomer J, Alpers CE. Iatrogenic phospholipidosis mimicking Fabry disease. Am J Kidney Dis. 2006;48:844-850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 41] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Woywodt A, Hellweg S, Schwarz A, Schaefer RM, Mengel M. A wild zebra chase. Nephrol Dial Transplant. 2007;22:3074-3077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 11. | Selvarajah M, Nicholls K, Hewitson TD, Becker GJ. Targeted urine microscopy in Anderson-Fabry disease: a cheap, sensitive and specific diagnostic technique. Nephrol Dial Transplant. 2011;26:3195-3202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 12. | Fredman P, Klinghardt GW, Svennerholm L. Effect of chloroquine on the activity of some lysosomal enzymes involved in ganglioside degradation. Biochim Biophys Acta. 1987;917:1-8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Costa RM, Martul EV, Reboredo JM, Cigarrán S. Curvilinear bodies in hydroxychloroquine-induced renal phospholipidosis resembling Fabry disease. Clin Kidney J. 2013;6:533-536. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 14. | de Menezes Neves PDM, Machado JR, Custódio FB, Dos Reis Monteiro MLG, Iwamoto S, Freire M, Ferreira MF, Dos Reis MA. Ultrastructural deposits appearing as "zebra bodies" in renal biopsy: Fabry disease?- comparative case reports. BMC Nephrol. 2017;18:157. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 15. | Zhao F, Dou Y, Liu D, Yuan W, Quan S, Wang X, Cheng G, Xiao J, Zhao Z. Hydroxychloroquine-induced lipidosis of the kidney mimicking Fabry disease: a case report. Int J Clin Exp Pathol. 2016;9:2591-2593. |