Published online Dec 26, 2019. doi: 10.12998/wjcc.v7.i24.4285
Peer-review started: September 2, 2019
First decision: November 13, 2019
Revised: November 17, 2019
Accepted: November 30, 2019
Article in press: November 30, 2019
Published online: December 26, 2019
Processing time: 113 Days and 20.2 Hours
Heparin is commonly recommended for warfarin-induced skin necrosis; however, there is currently no established therapy for this disease. We present a serious case of warfarin-induced skin necrosis that was successfully treated with oral rivaroxaban, a factor Xa inhibitor.
A 48-year-old woman was admitted to the hospital for cellulitis of the right lower extremity. After antibiotic treatment, she developed pain and swelling of the left lower extremity, and deep vein thrombosis of both lower extremities was diagnosed. She was treated with a continuous heparin injection; subsequently, oral warfarin was concomitantly administered. Heparin was terminated after the therapeutic range was reached. On the following day, the patient had swelling and pain in the left lower extremity. In addition to decrease in protein S activity due to systemic lupus erythematosus, warfarin also reduced protein C activity, resulting in further hypercoagulation and skin necrosis. Warfarin was discontinued, and continuous heparin injection was resumed. Although the patient had to undergo amputation of the distal end of her left foot, continuous heparin injection was switched to oral rivaroxaban, and she was eventually discharged from the hospital in remission.
Administration of direct oral anticoagulants instead of warfarin is important in patients with decreased protein S and C activity.
Core tip: We present a serious case of warfarin-induced skin necrosis that was successfully treated with oral rivaroxaban, a factor Xa inhibitor. Administration of direct oral anticoagulants instead of warfarin is important in patients with decreased protein S and C activity.
- Citation: Kamada M, Kenzaka T. Successful treatment of warfarin-induced skin necrosis using oral rivaroxaban: A case report. World J Clin Cases 2019; 7(24): 4285-4291
- URL: https://www.wjgnet.com/2307-8960/full/v7/i24/4285.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i24.4285
In warfarin-induced skin necrosis, the production of protein C and S is inhibited in an early stage after warfarin administration, which increases coagulability and thrombosis formation in the capillaries and venules of the dermis or subcutaneous tissue, leading to skin ischemia or necrosis[1]. Although there is currently no established treatment for warfarin-induced skin necrosis, heparin is commonly recommended[1]. However, the use of non-vitamin K antagonist anticoagulants is recommended in some case reports[2-6].
We report the case of a patient who developed serious warfarin-induced skin necrosis as well as protein S deficiency caused by systemic lupus erythematosus (SLE), who was then successfully treated with oral rivaroxaban.
A 48-year-old woman presented to the emergency room with chief complaints of swelling of the right lower extremity and pyrexia.
Regarding her present illness, pyrexia and redness, and swelling of the right lower extremity developed 10 and 5 d before hospitalization, respectively. She visited our hospital because the pyrexia was unresolved, and the symptoms of the lower extremity worsened.
Her medical history included paronychia of the right big toe. She was gravida 3 and para 3, with no history of abortion.
Her father had a history of cerebral infarction.
The patient’s physical examination findings during examination were as follows: Body temperature, 39.4 °C; blood pressure, 107/65 mmHg; pulse rate, 81 beats/min and regular; respiratory rate, 13 breaths/min; and oxygen saturation, 97% (room air). Physical findings included swelling, warmth, redness, and pain in the right lower extremity as well as tinea unguium in the right foot.
Blood test findings on admission were as follows: White blood cell count, 4800 cells/μL; C-reactive protein, 9.51 mg/dL; prothrombin time (PT), 13.5 seconds; activated partial thromboplastin time, 34.5 s; and D-dimer, 6.9 μg/mL (Table 1).
| Parameter | Recorded value | Standard value |
| White blood cell count | 4800/µL | 4500-7500/µL |
| Lymphocyte count | 1300/µL | |
| Red blood cell count | 327 × 103/µL | 380-480 × 103/µL |
| Hemoglobin | 9.4 g/dL | 11.3-15.2 g/dL |
| Hematocrit | 31.3% | 36%-45% |
| Platelet count | 10.6 × 103/µL | 13-35 × 103/µL |
| International normalized ratio | 1.05 | 0.80-1.20 |
| Activated partial thromboplastin time | 34.5 s | 26.9-38.1 s |
| Fibrinogen | 374 mg/L | 150-400 mg/dL |
| D-dimer | 6.9 µg/mL | ≤ 1.0 µg/mL |
| C-reactive protein | 9.51 mg/L | ≤ 1.0 mg/L |
| Total protein | 7.5 g/dL | 6.9-8.4 g/dL |
| Albumin | 3.6 g/dL | 3.9-5.1 g/dL |
| Total bilirubin | 0.6 mg/dL | 0.2-1.2 mg/dL |
| Aspartate aminotransferase | 22 U/L | 11-30 U/L |
| Alanine aminotransferase | 24 U/L | 4-30 U/L |
| Lactate dehydrogenase | 262 U/L | 109-216 U/L |
| Creatine phosphokinase | 75 U/L | 40-150 U/L |
| Blood urea nitrogen | 7.6 mg/dL | 8-20 mg/dL |
| Creatinine | 0.43 mg/dL | 0.63-1.03 mg/dL |
| Sodium | 137 mEq/L | 136-148 mEq/L |
| Potassium | 3.6 mEq/L | 3.6-5.0 mEq/L |
| Chloride | 103 mEq/L | 98-108 mEq/L |
| Glucose | 146 mg/dL | 70-109 mg/dL |
| Hemoglobin A1c | 5.7% | ≤ 5.8% |
The patient was admitted to the hospital for cellulitis of the right lower extremity. Cefazolin (1 g) was administered every 8 h; subsequently, pyrexia declined. However, she redeveloped pyrexia (temperature, 39 °C). Considering the possibility of drug fever, we switched the antibiotic to clindamycin (600 mg) and administered it every 8 h from day 4 of hospitalization. As skin findings improved, the treatment of cellulitis was completed after 8 d.
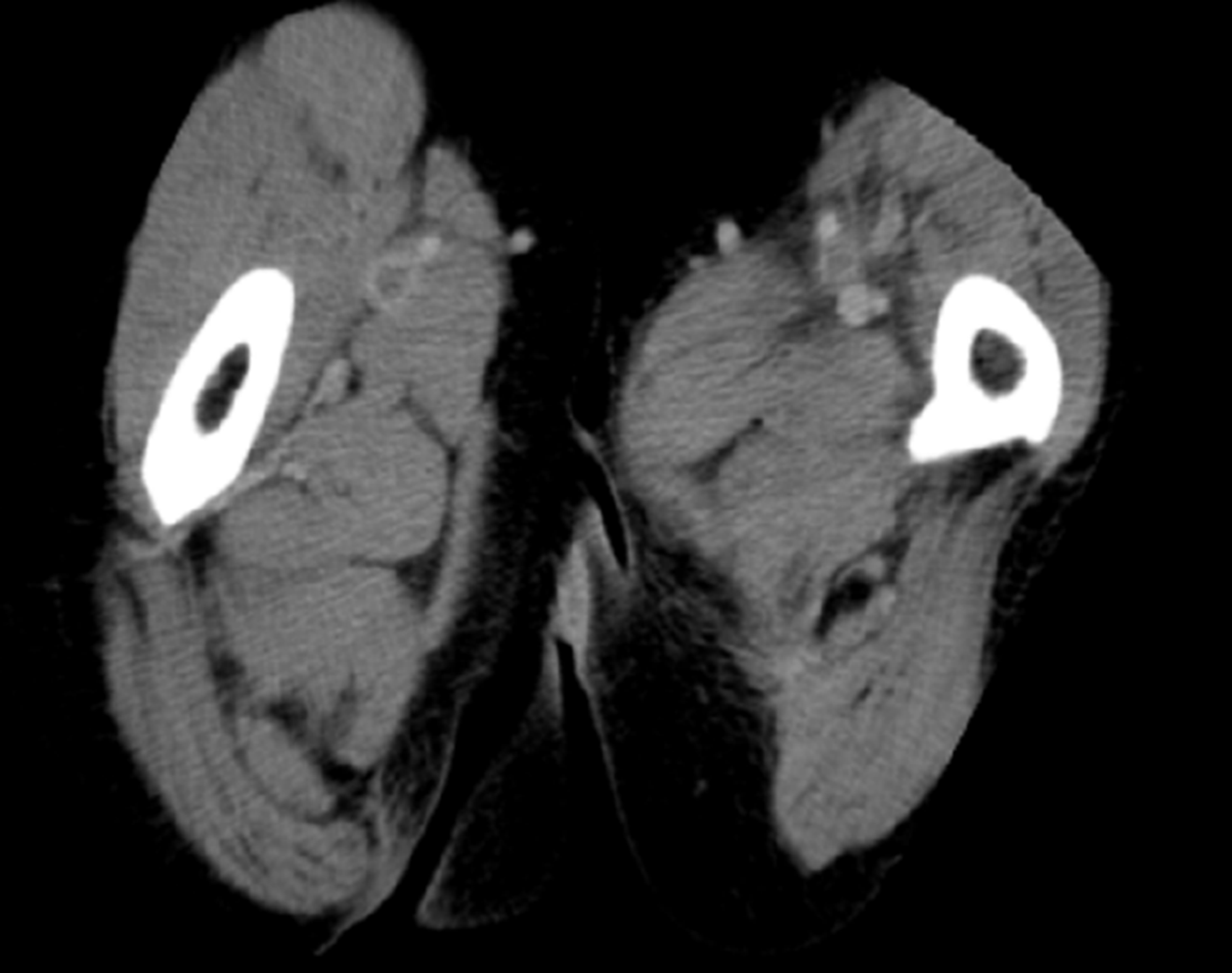
On day 8 of hospitalization, the patient developed swelling of the left lower extremity. Deep vein thrombosis was suspected because she was on bed rest for the treatment of cellulitis. Contrast-enhanced computed tomography revealed deep vein thrombi in both femoral veins (Figure 1); she was diagnosed with bilateral deep vein thrombosis of the lower extremities. Blood tests for the evaluation of thrombophilia as a risk factor for the development of deep vein thrombosis revealed decreased protein S activity (Table 2); therefore, the patient was diagnosed with deep vein thrombosis caused by protein S deficiency.
| Parameter | Recorded value | Standard value |
| Anti-cardiolipin β2-glycoprotein I complex antibody | 2.0 U/mL | < 3.5 U/mL |
| Lupus anticoagulant | 1.3 | < 1.3 |
| Protein S activity | < 10% | 56%-126% |
| Protein C activity | 83% | 64%-146% |
| Antithrombin III | 79% | 79%-121% |
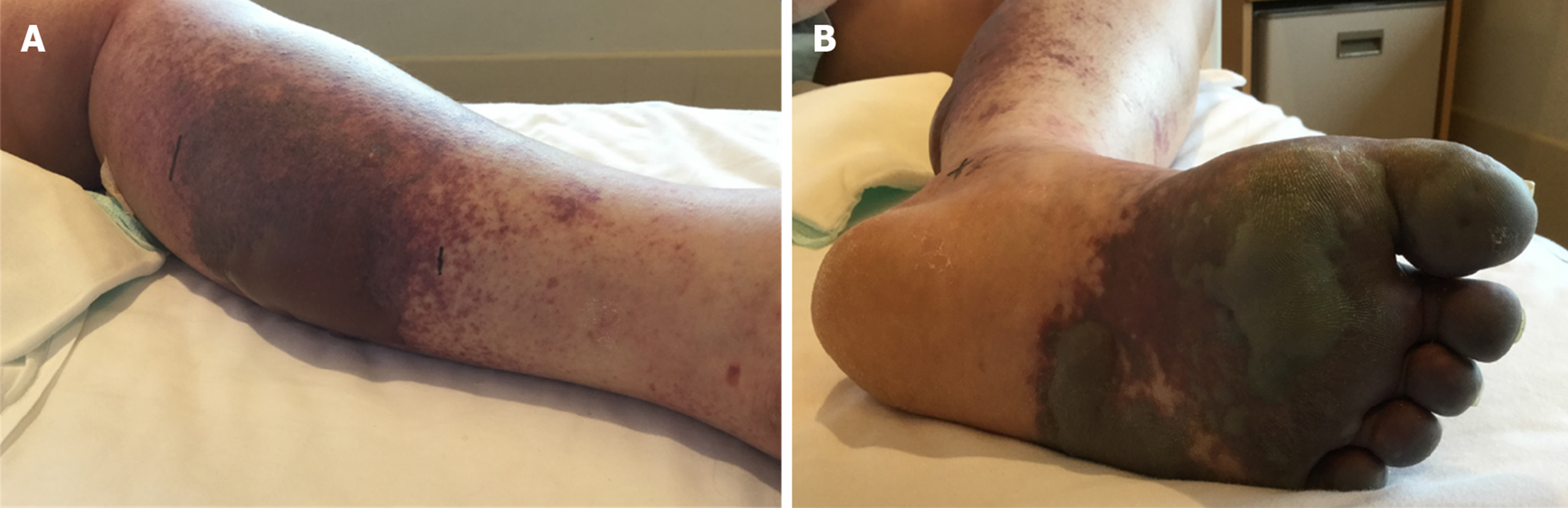
Continuous intravenous infusion of heparin was initiated for deep vein thrombosis on day 8 of hospitalization, and oral warfarin was concomitantly administered from day 14 of hospitalization. Continuous heparin injection was completed on day 19 of hospitalization when the PT-international normalized ratio (INR) reached the therapeutic target range (1.6-2.5). Immediately after heparin cessation, the patient developed pain, swelling, redness, and blisters in her left lower extremity, as well as cold peripheries and skin necrosis (Figure 2). The activities of protein S and C at this time were both < 10%. Warfarin administration during the period of low protein S activity subsequently led to decreased protein C activity, and discontinuation of the heparin injection caused hypercoagulation, leading to the onset of skin necrosis. The patient had a score of 7 points on the Naranjo adverse drug reaction probability scale[7]. Accordingly, we diagnosed her with warfarin-induced skin necrosis.
When evaluating the cause of decrease in protein S activity in this patient, we observed that she developed pleurisy approximately at the same time as the onset of bilateral deep vein thrombosis of the lower extremities. Along with lymphopenia, the patient fulfilled 2 clinical and 3 immunologic criteria of the Systemic Lupus International Collaborating Clinics classification[7]: Antinuclear antibody-positive (640-fold, homogeneous); low complement C4 (10 mg/dL; reference value, 17-45 mg/dL), C3 (56 mg/dL; reference value, 86-160 mg/dL) and CH50 (25 U/mL; reference value, 30-45 U/mL) levels; and direct Coombs test-positive. Therefore, the patient was diagnosed with SLE.
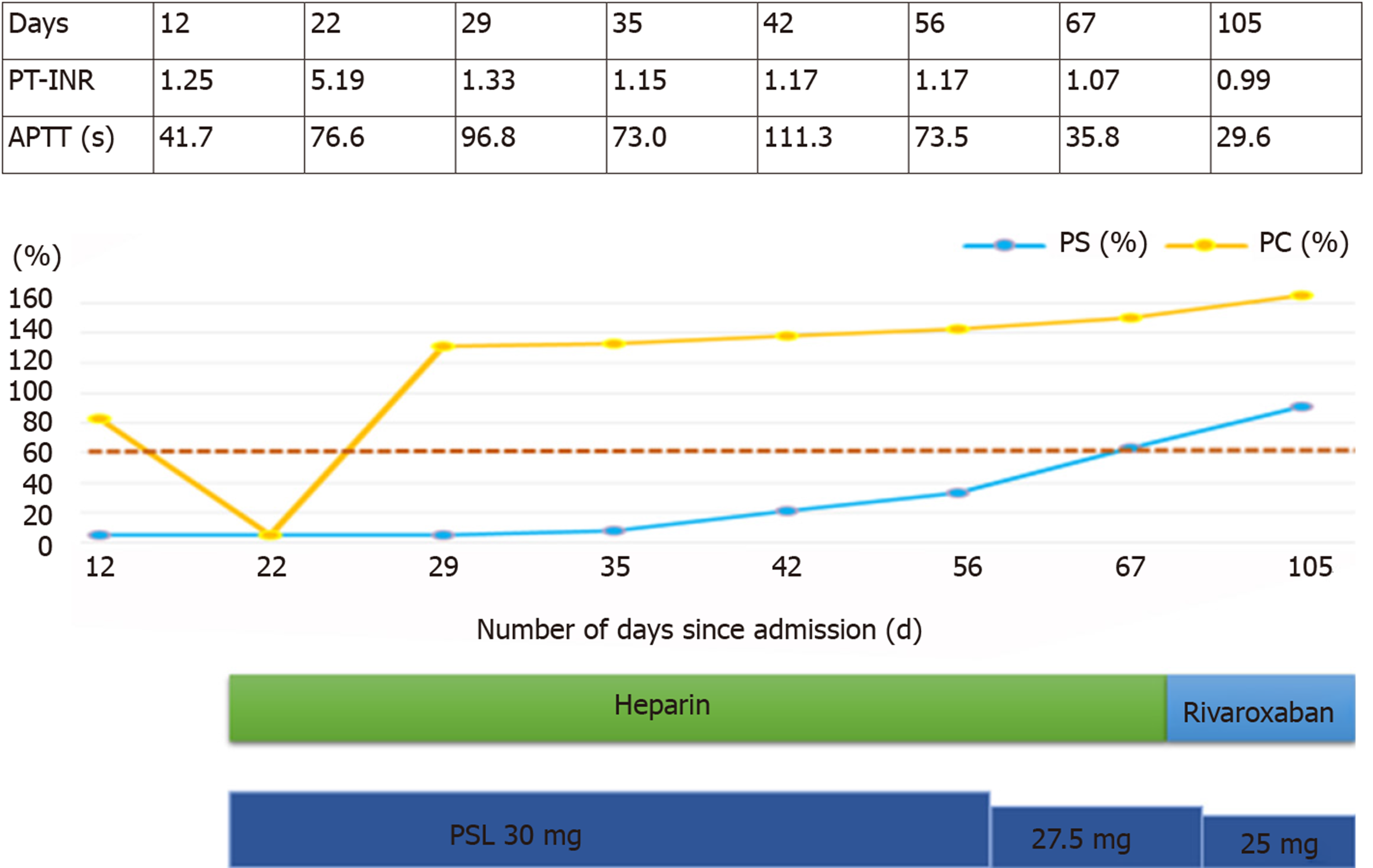
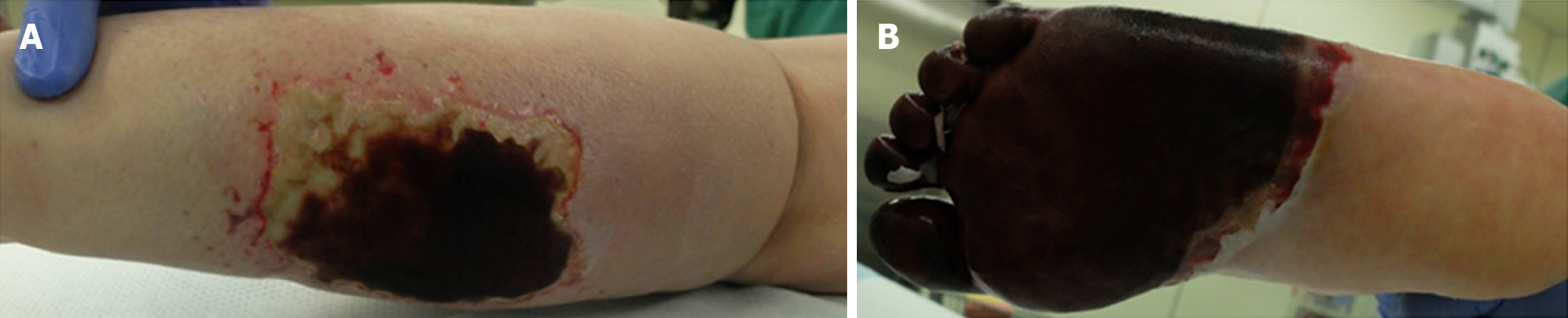
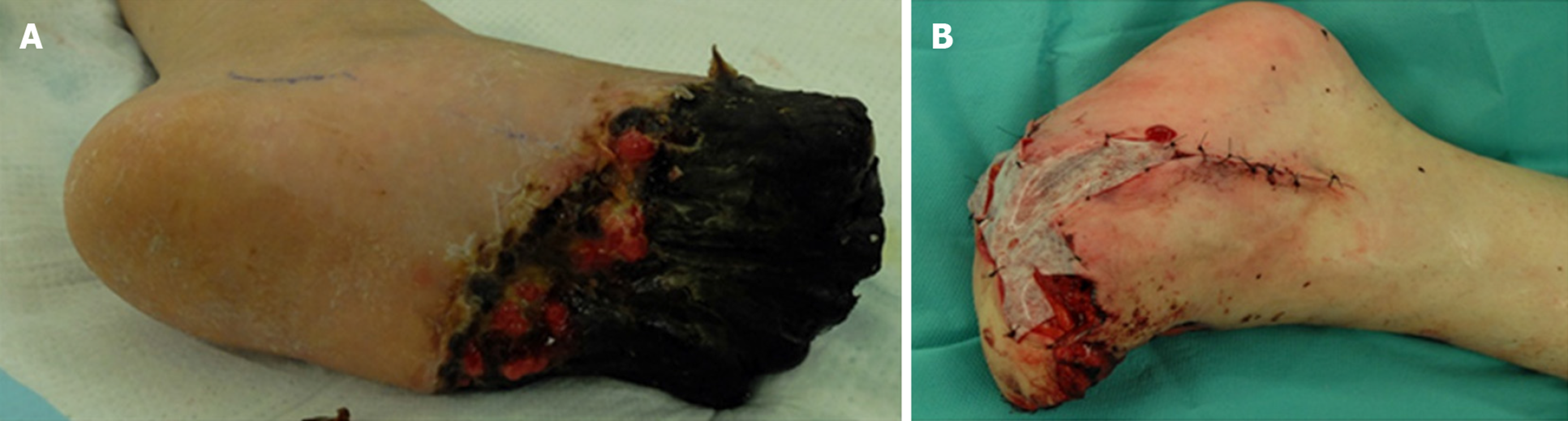
The activities of protein S and C over time are shown in Figure 3. Protein S activity was normalized with 30 mg of prednisolone (PSL). Based on these results, the decrease in protein S activity in this patient was attributed to SLE. Warfarin was discontinued on the day 22 of hospitalization, and continuous heparin injection was resumed for warfarin-induced skin necrosis, and protein C activity was normalized. Necrotic tissue debridement was performed on day 35 of hospitalization (Figure 4), and left side toe amputation and free flap surgery were performed on day 67 of hospitalization (Figure 5). Subsequently, heparin was discontinued, and the patient was switched to oral rivaroxaban (15 mg/d) on day 68 of hospitalization. She was admitted to another hospital for rehabilitation on day 86 of hospitalization. At the 2-year follow-up, no aggravations of skin necrosis were observed, and the activities of both proteins S and C remained normal.
Decreased protein S activity caused by systemic lupus erythematosus, and warfarin-induced skin necrosis decreased with protein C activity.
Prednisolone and rivaroxaban.
At the 2-year follow-up, no aggravations of skin necrosis were observed, and the activities of both proteins S and C remained normal. Activity of systemic lupus erythematosus was stable.
In this report, the patient developed warfarin-induced skin necrosis as well as decreased protein S activity secondary to SLE. Warfarin-induced skin necrosis occurred after discontinuation of heparin; however, the skin necrosis was not aggravated by the SLE treatment or oral administration of rivaroxaban, a direct factor Xa inhibitor. Only three other cases showing response to rivaroxaban for warfarin-induced skin necrosis have been previously reported[4-6].
Our patient developed bilateral deep vein thrombosis of the lower extremities during the treatment for cellulitis. A detailed examination for thrombophilia revealed markedly decreased protein S activity. Protein S deficiency can occur due to the following: Congenital factors, pregnancy, oral hormonal contraceptive use, disseminated intravascular coagulation, acute thromboembolism, human immunodeficiency virus infection, nephrotic syndrome, liver disease, L-asparaginase chemotherapy, varicella recovery, antiphospholipid antibody syndrome, oral steroid use, and vitamin K deficiency (i.e., decreased food intake, biliary obstruction, and oral warfarin administration)[8]. Protein S deficiency has been rarely reported in patients with SLE[9,10]. Our patient developed pleurisy during the clinical course. She was diagnosed with SLE after she fulfilled the Systemic Lupus International Collaborating Clinics classification criteria (i.e., clinical criteria of pleurisy and lymphopenia as well as the immunologic criteria of positive antinuclear antibody, low complement, and positive direct Coombs-test)[7]. She had no family history, history of abortion, hepatic dysfunction, or renal dysfunction suggestive of congenital diseases. After initiating PSL treatment, protein S activity returned to normal as SLE improved, indicating that decreased protein S activity was caused by SLE.
Warfarin induced skin necrosis develops when proteins C and S production is inhibited in the early stage after the administration of warfarin, a vitamin K antagonist, resulting in increased coagulability and hypercoagulation[11]. Our case demonstrated that serious skin necrosis can occur in the early stage of treatment despite achieving a PT-INR within the therapeutic range. Onset of skin necrosis can occur within a few hours or several weeks after warfarin administration[11]. There are no currently established anticoagulant therapies for patients with decreased proteins S and C activities. Heparin, which is not a vitamin K antagonist, is commonly used for the treatment of warfarin-induced necrosis[11]. However, direct oral anticoagulants, such as dabigatran and rivaroxaban, have been found to be more effective for our patient based on previous findings[2-6]. We could find only three studies reporting that oral rivaroxaban was effective[7-9]. Heparin requires continuous intravenous injection, and its administration is not suitable for patients with deep vein thrombosis who require long-term anticoagulant therapy, such as our patient, because of the route of administration and necessity of hospitalization.
We report the case of a patient with warfarin-induced skin necrosis who was successfully treated with oral rivaroxaban, a factor Xa inhibitor. It is important to administer DOACs instead of warfarin in patients with decreased activities of protein S and C, which are vitamin K-dependent coagulation inhibitors. During treatment of bilateral deep vein thrombosis of the lower extremities, it is necessary to closely examine the patient for the presence of thrombophilia and avoid warfarin administration until confirmation of the examination results. Furthermore, continuous heparin injections should be carefully discontinued when warfarin is administered.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ciccone MM, De Ponti F S-Editor: Ma YJ L-Editor: A E-Editor: Liu JH
| 1. | Kakagia DD, Papanas N, Karadimas E, Polychronidis A. Warfarin-induced skin necrosis. Ann Dermatol. 2014;26:96-98. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 89] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 2. | Tripodi A, Martinelli I, Chantarangkul V, Clerici M, Artoni A, Passamonti S, Peyvandi F. Thrombin generation and other coagulation parameters in a patient with homozygous congenital protein S deficiency on treatment with rivaroxaban. Int J Hematol. 2016;103:165-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 3. | Bakoyiannis C, Karaolanis G, Patelis N, Maskanakis A, Tsaples G, Klonaris C, Georgopoulos S, Liakakos T. Dabigatran in the Treatment of Warfarin-Induced Skin Necrosis: A New Hope. Case Rep Dermatol Med. 2016;2016:3121469. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 4. | Martinelli I, Bucciarelli P, Artoni A, Fossali EF, Passamonti SM, Tripodi A, Peyvandi F. Anticoagulant treatment with rivaroxaban in severe protein S deficiency. Pediatrics. 2013;132:e1435-e1439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 5. | Lai J, Ramai D, Alchi R, Bloomfield D. Anticoagulation therapy for thromboembolism prevention: a case of warfarin-induced skin necrosis in the setting of protein C deficiency. BMJ Case Rep. 2017;2017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 6. | Menon N, Sarode R, Zia A. Rivaroxaban dose adjustment using thrombin generation in severe congenital protein C deficiency and warfarin-induced skin necrosis. Blood Adv. 2018;2:142-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 7. | Petri M, Orbai AM, Alarcón GS, Gordon C, Merrill JT, Fortin PR, Bruce IN, Isenberg D, Wallace DJ, Nived O, Sturfelt G, Ramsey-Goldman R, Bae SC, Hanly JG, Sánchez-Guerrero J, Clarke A, Aranow C, Manzi S, Urowitz M, Gladman D, Kalunian K, Costner M, Werth VP, Zoma A, Bernatsky S, Ruiz-Irastorza G, Khamashta MA, Jacobsen S, Buyon JP, Maddison P, Dooley MA, van Vollenhoven RF, Ginzler E, Stoll T, Peschken C, Jorizzo JL, Callen JP, Lim SS, Fessler BJ, Inanc M, Kamen DL, Rahman A, Steinsson K, Franks AG, Sigler L, Hameed S, Fang H, Pham N, Brey R, Weisman MH, McGwin G, Magder LS. Derivation and validation of the Systemic Lupus International Collaborating Clinics classification criteria for systemic lupus erythematosus. Arthritis Rheum. 2012;64:2677-2686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2816] [Cited by in RCA: 3536] [Article Influence: 272.0] [Reference Citation Analysis (0)] |
| 8. | Bauer KA. Protein S deficiency. 2017. Available from: https://www.uptodate.com/contents/protein-s-deficiency?search=Protein%20S%20deficiencysource=search_resultselectedTitle=1~76usage_type=defaultdisplay_rank=1. |
| 9. | Lertnawapan R, Sakonlaya D. Lupus protein-losing enteropathy patient with protein C and protein S deficiency-induced thrombosis: A case report with review of the literature. Acta Reumatol Port. 2017;42:265-268. [PubMed] |
| 10. | Ginsberg JS, Demers C, Brill-Edwards P, Bona R, Johnston M, Wong A, Denburg JA. Acquired free protein S deficiency is associated with antiphospholipid antibodies and increased thrombin generation in patients with systemic lupus erythematosus. Am J Med. 1995;98:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 58] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 11. | Naranjo CA, Busto U, Sellers EM, Sandor P, Ruiz I, Roberts EA, Janecek E, Domecq C, Greenblatt DJ. A method for estimating the probability of adverse drug reactions. Clin Pharmacol Ther. 1981;30:239-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7061] [Cited by in RCA: 8196] [Article Influence: 186.3] [Reference Citation Analysis (0)] |