Published online Dec 6, 2019. doi: 10.12998/wjcc.v7.i23.4036
Peer-review started: August 13, 2019
First decision: September 23, 2019
Revised: November 8, 2019
Accepted: November 14, 2019
Article in press: November 14, 2019
Published online: December 6, 2019
Processing time: 114 Days and 22 Hours
We report the first case, to the best of our knowledge, of massive ascites due to recurrent malignant pleural mesothelioma that was controlled using KM-cell-free and concentrated ascites reinfusion therapy (KM-CART). The tumor cells derived via KM-CART were utilized secondarily in an in vitro cell growth assay using the collagen gel droplet-embedded culture drug sensitivity test (CD-DST) to investigate anticancer drug susceptibility.
A 56-year-old man presented with recurrent malignant mesothelioma with massive ascites; more than 4000 mL of ascitic fluid was removed, filtered, and concentrated using KM-CART, and the cell-free ascitic fluid was reinfused into the patient to improve quality of life. Cancer cells isolated secondarily in an in vitro proliferation assay using CD-DST exhibited low sensitivity to pemetrexed and high sensitivity to gemcitabine. Treatment with gemcitabine maintained stable disease for 4 mo.
The combination of KM-CART and CD-DST may be a promising treatment option for malignant ascites associated with malignant mesothelioma.
Core tip: Massive ascites due to recurrent malignant mesothelioma was controlled with innovative cell-free and concentrated ascites reinfusion therapy, and the derived tumor cells were utilized secondarily in an in vitro cell growth assay that contributed to the personalized chemotherapy for the patient.
- Citation: Anayama T, Taguchi M, Tatenuma T, Okada H, Miyazaki R, Hirohashi K, Kume M, Matsusaki K, Orihashi K. In-vitro proliferation assay with recycled ascitic cancer cells in malignant pleural mesothelioma: A case report. World J Clin Cases 2019; 7(23): 4036-4043
- URL: https://www.wjgnet.com/2307-8960/full/v7/i23/4036.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i23.4036
Malignant mesothelioma is a rare and insidious neoplasm with a poor prognosis. Pemetrexed-cisplatin (PEM-CDDP) combination therapy was established as a standard treatment with level I evidence and grade A recommendation[1]. Only patients with favorable prognostic features, histology, and staging are referred for radical treatment involving extensive cytoreductive surgery consideration[2]. For cure, two radical surgical procedures, extra-pleural pneumonectomy (EPP) and pleurectomy/decortication, are performed in combination with chemotherapy and/or radiotherapy. A systematic review showed that the median overall survival after EPP was 9.4–27.5 mo, with a 5-year survival rate of 0%–24%[3].
Ascites is a unique form of malignant pleural mesothelioma (MPM) recurrence[4]. Although puncture and aspiration can improve the symptoms of ascites, repeated procedures result in loss of large amounts of electrolytes and albumin. Cell-free and concentrated ascites reinfusion therapy (CART) was developed as a palliative therapeutic option for controlling massive ascites and improving the patient’s quality of life[5,6]. KM-CART, a novel CART system developed in 2011, is a highly efficient method of processing tumor cell-rich malignant ascites[7,8]. In this report, we described a case of massive ascites due to recurrent MPM that was controlled using KM-CART. Here, the tumor cells derived via KM-CART were utilized secondarily in an in-vitro cell growth assay using the collagen gel droplet-embedded culture drug sensitivity test (CD-DST) to investigate anticancer drug susceptibility[9,10].
A 56-year-old man presented to our hospital with right pleural effusion.
The patient had a long history of outpatient treatment for hypertension. Although he was asymptomatic, a routine chest computed tomography (CT) scan revealed the presence of pleural effusion in the right lung.
He had no prior history of hospitalization, operations, or injuries.
He had no significant childhood illnesses. His deceased parents had no history of malignancy. He had two healthy siblings. He had a 30-year smoking history of 1 pack/d between the ages of 20 and 56 years (30 pack/year). He was occupationally exposed to asbestos from the age of 24 to 30 years.
Upon physical examination, his respiratory sounds were slightly attenuated in the right back. His cervical, supraclavicular, and axillary lymph nodes were not palpable.
Preoperative complete blood count and biochemical examination of blood showed almost normal findings. The levels of serum tumor markers such as squamous cell carcinoma related antigen, cytokeratin fraction, and carbohydrate antigen 19-9 were within normal limits, while elevation of carcinoembryonic antigen level to 10.5 ng/mL (< 5.0 ng/mL) was observed.
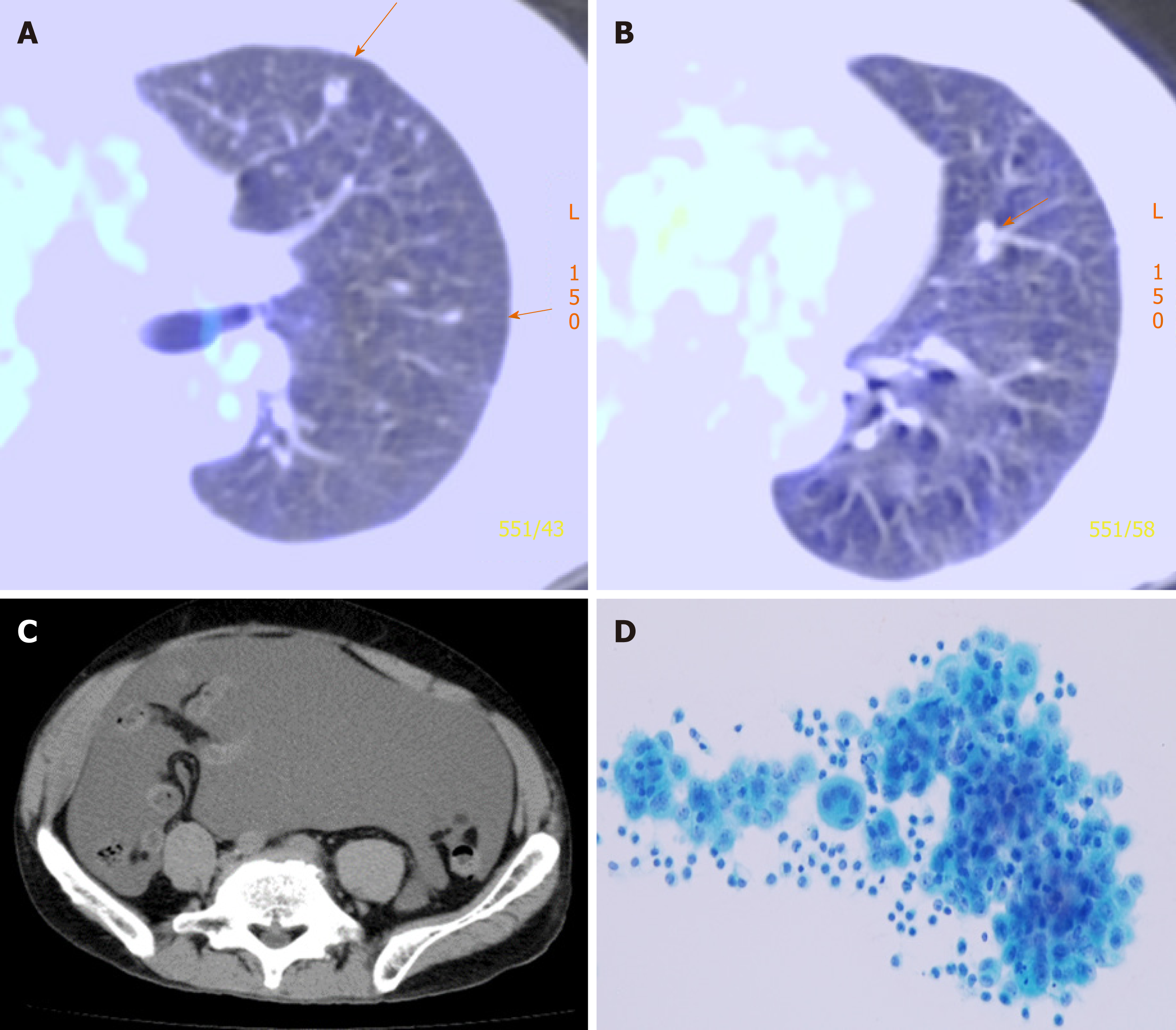
A chest radiograph and chest CT revealed right pleural effusion, but no tumorous shadow was pointed out in the chest. Cytological examination of the right pleural effusion, sampled via thoracentesis, revealed malignant mesothelioma cells. He had been diagnosed with c-T1aN0M0 c-stage IA[11] malignant pleural mesothelioma..
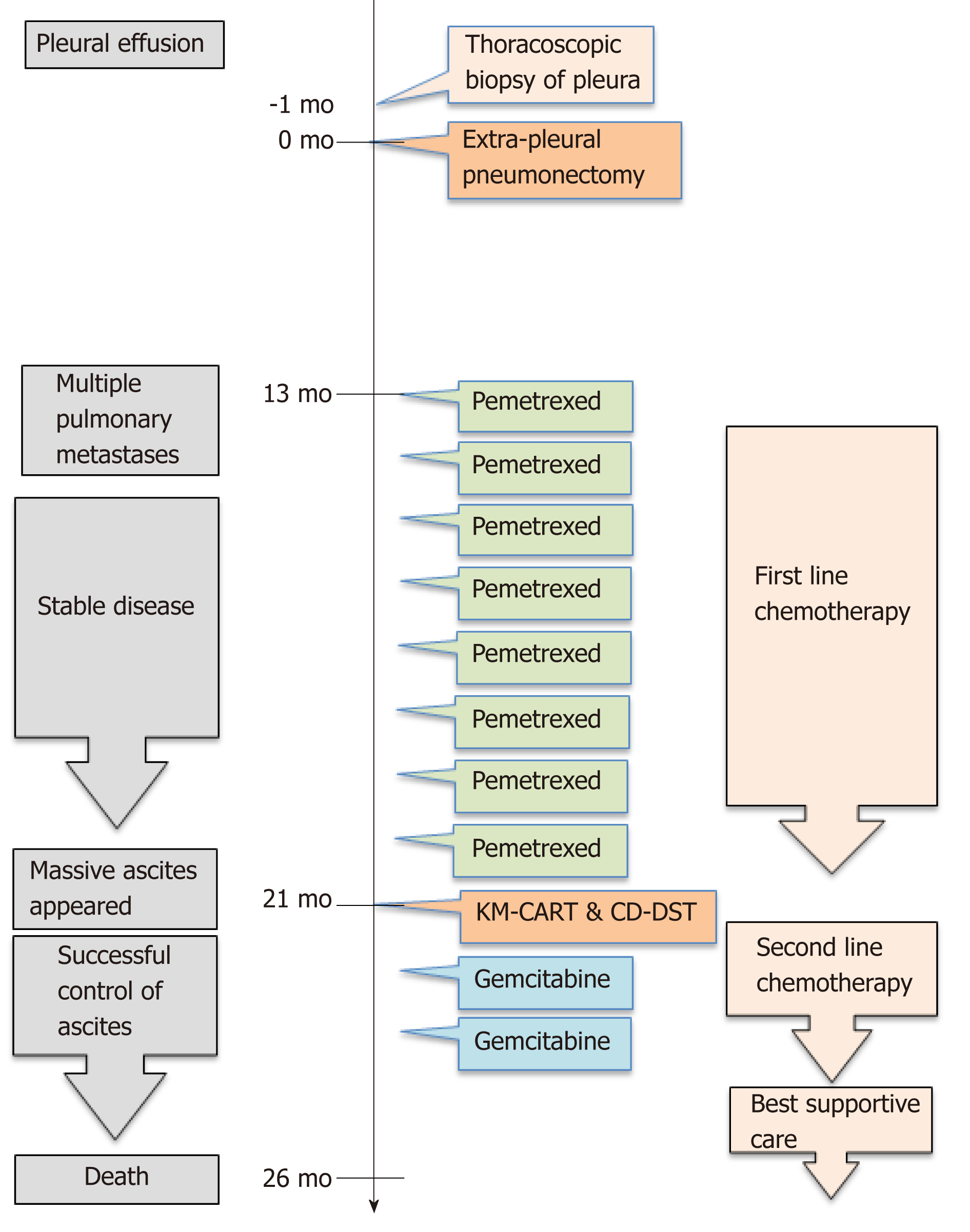
He underwent right EPP and lymph node dissection with curative intent. Pathological examination of the resected tissue yielded a diagnosis of diffuse MPM, epithelioid type, p-T2N2M0, and p-Stage III. He did not receive postoperative adjuvant chemotherapy. Thirteen months after operation, multiple pulmonary metastases were detected on F-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) (Figure 1). The patient refused CDDP/PEM treatment because of the anticipated side effects of CDDP; he received 8 mo courses of single-agent PEM therapy (500 mg/m2) and achieved stable disease[12].
Twenty-one months post-surgery, the patient was re-hospitalized because of abdominal pain and tightness, dyspnea, and anorexia due to massive ascites. Accordingly, we diagnosed recurrent mesothelioma presenting with massive malignant ascites.
The final diagnosis was recurrent mesothelioma presenting with massive malignant ascites.
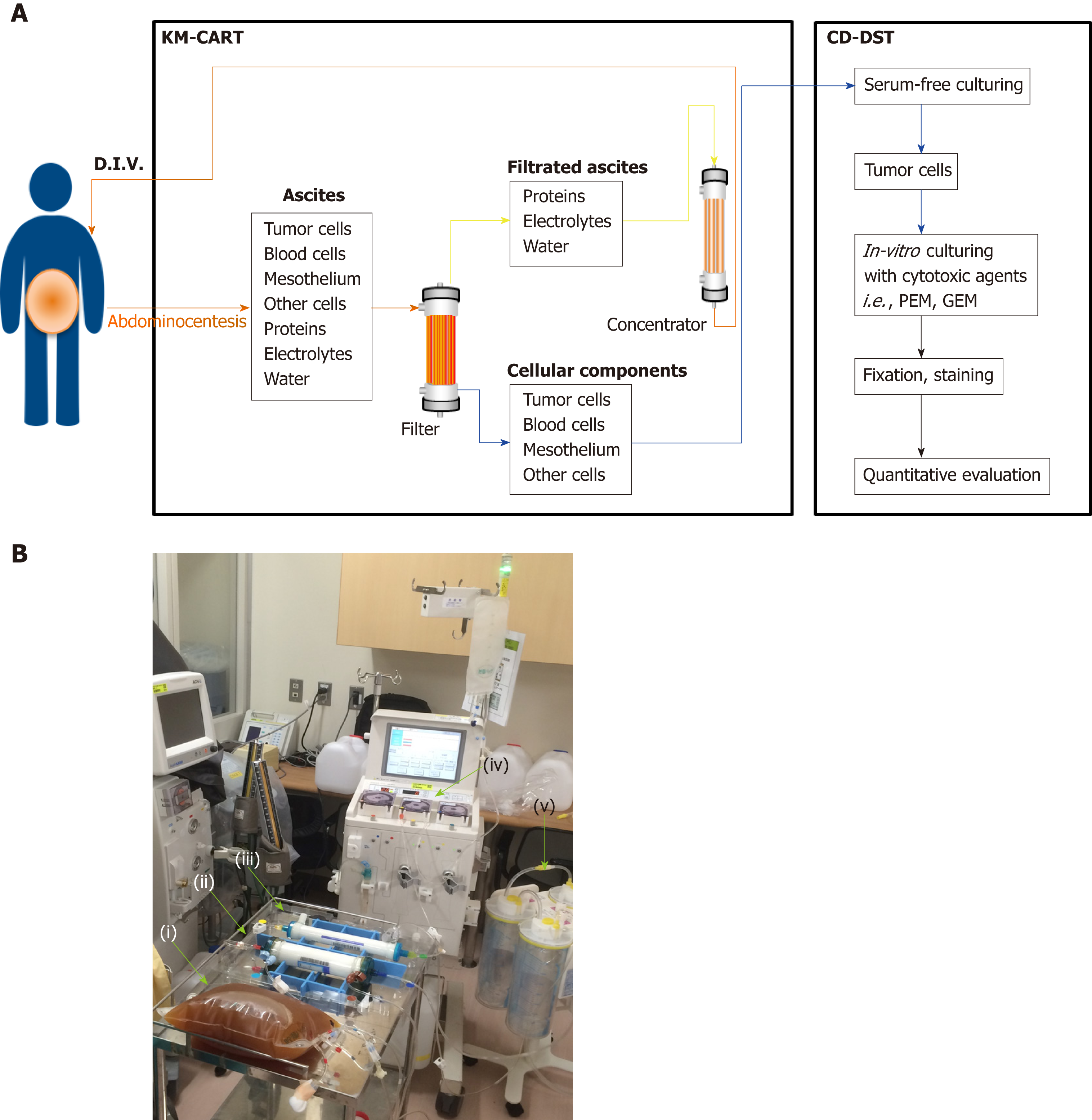
KM-CART was performed as a palliative treatment (Figure 2). An 8-Fr trocar tube was inserted into the Douglas pouch under local anesthesia for ascitic fluid discharge into a dedicated bag. The discharged fluid, with a total volume of 4050 mL, was filtered and concentrated, while raw ascites was drained from the body. The filtrate concentrate, with a fluid volume of 610 mL and protein concentration of 8.0 g/dL, was reinfused intravenously into the patient. After KM-CART therapy, the patient's body weight decreased by 1.7 kg, his abdomen flattened, and the abdominal pain and dyspnea quickly disappeared. He was able to ingest a normal amount of food the following day.
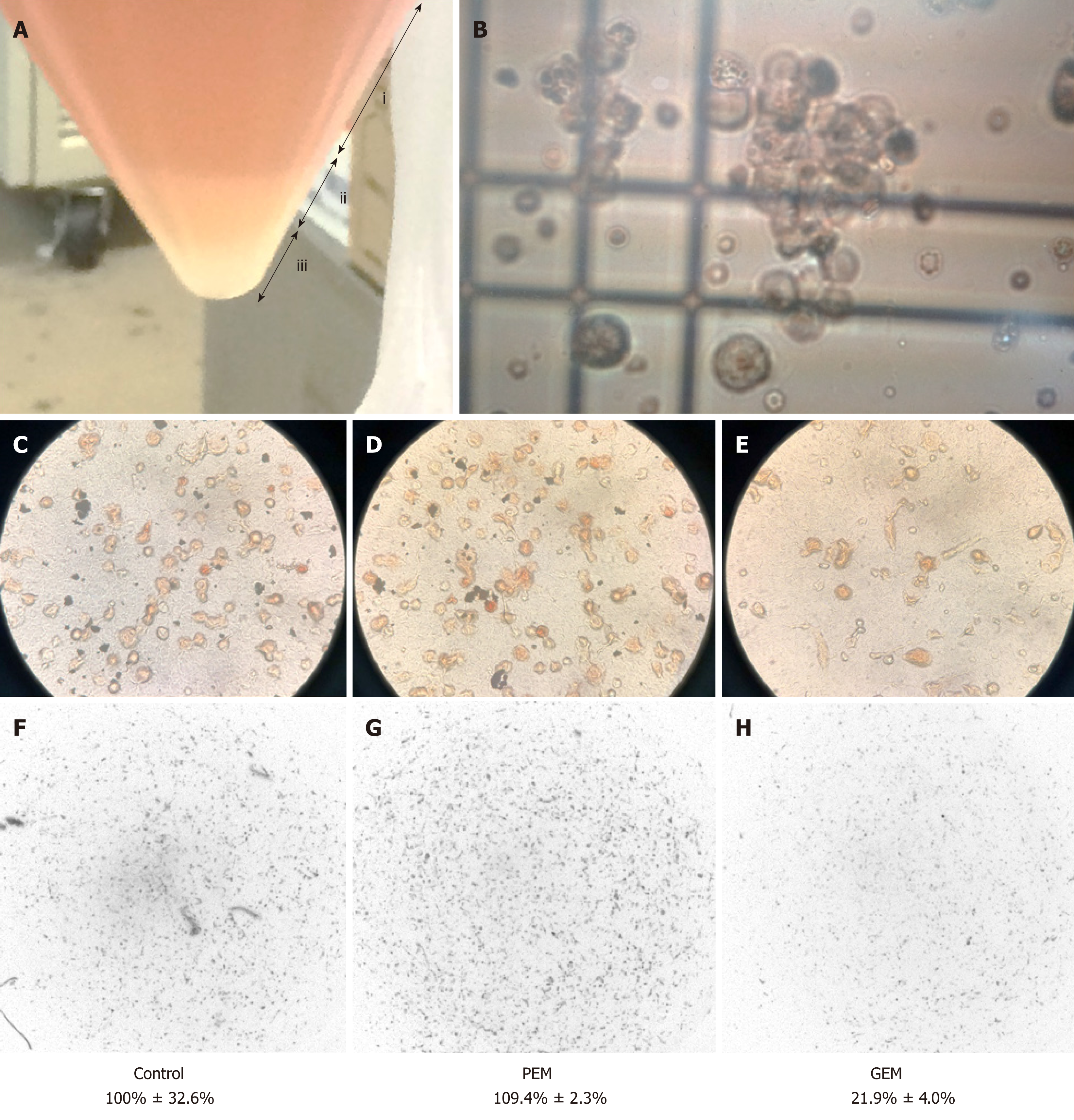
Then the recycled 5.4 × 108 tumor cells were subjected to CD-DST according to a previously reported method[10-13]. The isolated malignant mesothelioma cells were less susceptible to previously used systemic chemotherapy agent PEM (109.4% growth rate relative to the normal control). The other agents such as CDDP, carboplatin, nedaplatin, paclitaxel, docetaxel, and vinorelbine also exhibited less susceptible. However, the tumor cells were sensitive to gemcitabine (GEM) (growth rate reduction to 21.9% relative to the normal control) (Figure 3). Therefore, we administered two courses of second-line GEM chemotherapy (1000 mg/m2 on days 1, 8, and 15).
Although the patient received palliative treatment, he died 2 years and 4 mo after the initial surgery. However, we observed no increase in the amount of ascites following KM-CART, during GEM therapy, or until 4 mo before death. The overall timeline of treatments and outcomes is presented in Figure 4.
MPM may spread to various locations. Local recurrence is generally associated with lymph node disease (65%), pleural effusion (64%), chest wall involvement (43%), contralateral lung disease (36%), pericardial infiltration (29%), and pericardial effusion (12%). Patterns of distal spreading are characterized by parenchymal lung metastasis (27%) and peritoneal/omental disease (24%) with malignant ascites in 16% of cases. These conditions are often followed by bone metastasis (20%) and the development of subcutaneous metastatic nodules (19%)[14].
The patient exhibited recurrent mesothelioma presenting with massive malignant ascites during systemic chemotherapy with PEM. Generally, the fluid associated with ascites is removed by paracentesis in order to relieve abdominal pain as needed as part of palliative care. KM-CART, a unique approach to CART developed by Dr. Matsusaki et al[8] involves a simple and innovative system consisting of an infusion pump and a suction system. Here, the ascitic fluid is filtered from the outside to the inside of the filtration membrane, in the opposite direction to the hemodialysis system. Therefore, clogging of the filtration membrane can be eliminated by a reverse-direction (i.e. inside to outside) bolus flush of physiological saline in the CART system circuit. The flushed cancer cells can then be stored in an isolated bag and utilized for in vitro growth assay.
CD-DST is a culture-based anticancer drug susceptibility test that uses fresh tumor tissues obtained from various excised solid tumors that have a high probability of growth in in vitro environments. The usefulness of CD-DST has been demonstrated in lung cancer[15-17], gastric cancer[18], colorectal cancer[19,20], and MPM studies[21]. In our case, the cultured cancer cells were resistant to PEM but highly sensitive to GEM. Therefore, second-line chemotherapy with GEM enabled the patient to maintain stable disease for 4 mo.
This case report had one important limitation. CD-DST is an in vitro growth assay that originally utilized highly viable fresh cancer cells excised surgically; malignant mesothelioma cells extracted from ascites may exhibit lower viability than to those extracted from a surgical specimen. It is possible that the low viability may have affected the in vitro growth rate success of the assay. We recommend further comparisons to clarify this point.
To the best of our knowledge, this is the first report of the use of KM-CART and CD-DST of recycled massive ascitic cancer cells. The combination of these two techniques may provide a unique option for personalized treatment of massive malignant ascites.
We would like to express our special thanks and gratitude to Ms. Yoko Asakura and Miss Ami Yamanaka, who provided technical assistance with the in vitro CD-DST assay, and to Mr. Masahiro Yokoi, the technical adviser of the Japanese CART study group, who provided technical assistance with KM-CART.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Köker IH, Su CC S-Editor: Ma YJ L-Editor: Filipodia E-Editor: Xing YX
| 1. | Baas P, Fennell D, Kerr KM, Van Schil PE, Haas RL, Peters S; ESMO Guidelines Committee. Malignant pleural mesothelioma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2015;26 Suppl 5:v31-v39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 233] [Article Influence: 23.3] [Reference Citation Analysis (0)] |
| 2. | Kindler HL, Ismaila N, Armato SG, Bueno R, Hesdorffer M, Jahan T, Jones CM, Miettinen M, Pass H, Rimner A, Rusch V, Sterman D, Thomas A, Hassan R. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol. 2018;36:1343-1373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 286] [Article Influence: 40.9] [Reference Citation Analysis (0)] |
| 3. | Cao CQ, Yan TD, Bannon PG, McCaughan BC. A systematic review of extrapleural pneumonectomy for malignant pleural mesothelioma. J Thorac Oncol. 2010;5:1692-1703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 4. | Moore AJ, Parker RJ, Wiggins J. Malignant mesothelioma. Orphanet J Rare Dis. 2008;3:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 5. | Inoue N, Yamazaki Z, Oda T, Sugiura M, Wada T. Treatment of intractable ascites by continuous reinfusion of the sterilized, cell-free and concentrated ascitic fluid. Trans Am Soc Artif Intern Organs. 1977;23:699-702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 37] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 6. | Katoh S, Tatsukawa H, Kondoh M, Inoue M, Ida K, Miyagawa F. Prevention of the febrile reaction occurring on reinfusion of cell-free and concentrated autogenous ascites. Jpn J Med. 1991;30:311-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Kitayama J, Emoto S, Yamaguchi H, Ishigami H, Onoyama H, Yamashita H, Seto Y, Matsuzaki K, Watanabe T. Flow Cytometric Quantification of Intraperitoneal Free Tumor Cells is a Useful Biomarker in Gastric Cancer Patients with Peritoneal Metastasis. Ann Surg Oncol. 2015;22:2336-2342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 8. | Japanese CART Study Group. Matsusaki K, Ohta K, Yoshizawa A, Gyoda Y. Novel cell-free and concentrated ascites reinfusion therapy (KM-CART) for refractory ascites associated with cancerous peritonitis: its effect and future perspectives. Int J Clin Oncol. 2011;16:395-400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 9. | Kobayashi H. Development of a new in vitro chemosensitivity test using collagen gel droplet embedded culture and image analysis for clinical usefulness. Recent Results Cancer Res. 2003;161:48-61. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 54] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 10. | Kobayashi H. Collagen gel droplet culture method to examine in vitro chemosensitivity. Methods Mol Med. 2005;110:59-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 11. | Pass H, Giroux D, Kennedy C, Ruffini E, Cangir AK, Rice D, Asamura H, Waller D, Edwards J, Weder W, Hoffmann H, van Meerbeeck JP, Nowak A, Rusch VW; IASLC Staging and Prognostic Factors Committee, Advisory Boards and Participating Institutions. The IASLC Mesothelioma Staging Project: Improving Staging of a Rare Disease Through International Participation. J Thorac Oncol. 2016;11:2082-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 12. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M, Rubinstein L, Shankar L, Dodd L, Kaplan R, Lacombe D, Verweij J. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15860] [Cited by in RCA: 21529] [Article Influence: 1345.6] [Reference Citation Analysis (1)] |
| 13. | Nagai N, Minamikawa K, Mukai K, Hirata E, Komatsu M, Kobayashi H. Predicting the chemosensitivity of ovarian and uterine cancers with the collagen gel droplet culture drug-sensitivity test. Anticancer Drugs. 2005;16:525-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Collins DC, Constantinidou A, Sundar R, Chenard-Poirier M, Yap TA, Banerji U, deBono J, Lopez JS, Tunariu N. Patterns of metastases in malignant pleural mesothelioma in the modern era: Redefining the spread of an old disease. J Clin Oncol. 2017;35:8556–8556. [RCA] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Kawamura M, Gika M, Abiko T, Inoue Y, Oyama T, Izumi Y, Kobayashi H, Kobayashi K. Clinical evaluation of chemosensitivity testing for patients with unresectable non-small cell lung cancer (NSCLC) using collagen gel droplet embedded culture drug sensitivity test (CD-DST). Cancer Chemother Pharmacol. 2007;59:507-513. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Miyazaki R, Anayama T, Hirohashi K, Okada H, Kume M, Orihashi K. In Vitro Drug Sensitivity Tests to Predict Molecular Target Drug Responses in Surgically Resected Lung Cancer. PLoS One. 2016;11:e0152665. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Inoue M, Maeda H, Takeuchi Y, Fukuhara K, Shintani Y, Funakoshi Y, Funaki S, Nojiri T, Kusu T, Kusumoto H, Kimura T, Okumura M; Representing the Thoracic Surgery Study Group of Osaka University. Collagen gel droplet-embedded culture drug sensitivity test for adjuvant chemotherapy after complete resection of non-small-cell lung cancer. Surg Today. 2018;48:380-387. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 18. | Kanazawa Y, Yamada T, Fujita I, Kakinuma D, Matsuno K, Arai H, Shimoda T, Ko K, Kato S, Matsutani T, Hagiwara N, Nomura T, Uchida E. In Vitro Chemosensitivity Test for Gastric Cancer Specimens Predicts Effectiveness of Oxaliplatin and 5-Fluorouracil. Anticancer Res. 2017;37:6401-6405. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 19. | Araki Y, Isomoto H, Matsumoto A, Kaibara A, Yasunaga M, Hayashi K, Yatsugi H, Yamauchi K. An in vitro chemosensitivity test for colorectal cancer using collagen-gel droplet embedded cultures. Kurume Med J. 1999;46:163-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Ochiai T, Nishimura K, Watanabe T, Kitajima M, Nakatani A, Nagayasu K, Sakuyama N, Sato T, Kishine K, Abe Y, Nagaoka I. Impact of primary tumor location as a predictive factor in patients suffering from colorectal cancer treated with cytotoxic anticancer agents based on the collagen gel droplet-embedded drug sensitivity test. Oncol Lett. 2019;17:1842-1850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Higashiyama M, Oda K, Okami J, Maeda J, Kodama K, Takami K, Morinaga K, Takano T, Kobayashi H. In vitro-chemosensitivity test using the collagen gel droplet embedded culture drug test (CD-DST) for malignant pleural mesothelioma: possibility of clinical application. Ann Thorac Cardiovasc Surg. 2008;14:355-362. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |