Published online Nov 26, 2019. doi: 10.12998/wjcc.v7.i22.3881
Peer-review started: July 23, 2019
First decision: September 9, 2019
Revised: September 17, 2019
Accepted: October 5, 2019
Article in press: October 5, 2019
Published online: November 26, 2019
Processing time: 128 Days and 17.7 Hours
Pseudomyxoma peritonei (PMP) is a rare benign, but progressive, disease according to myxoma histopathology. Surgical resection is the preferred and most effective treatment, but the outcomes are often unsatisfactory.
A 63-year-old Chinese woman with PMP received apatinib at a daily dose of 0.5 mg for 15 d per cycle and at a daily dose of 0.4 mg to date for recurrent abdominal distension after surgical treatment and hyperthermic intraperitoneal chemotherapy. During the follow-up period, apatinib was the maintenance treatment with a progression-free period of 10 mo and the toxicity of apatinib was controllable and tolerable. Unfortunately, recurrence occurred 10 mo after administration. After two operations, the patient gave up treatment at the 18th mo and eventually died of intestinal obstruction and multiple organ failure.
Apatinib may be an option for recurrent PMP after surgical treatment, but this conclusion remains to be confirmed.
Core tip: The preferred treatment for pseudomyxoma peritonei (PMP) is surgery, but it is easy to relapse after surgery. There is currently no good treatment for patients with postoperative recurrence. In this work, a PMP patient received apatinib for recurrent abdominal distension after surgical treatment and hyperthermic intraperitoneal chemotherapy. After treatment with apatinib, the recurrence time was significantly prolonged. Apatinib, a small-molecule oral inhibitor with anti-angiogenic function, may be an option for recurrent PMP after surgical treatment.
- Citation: Huang R, Shi XL, Wang YF, Yang F, Wang TT, Peng CX. Apatinib for treatment of a pseudomyxoma peritonei patient after surgical treatment and hyperthermic intraperitoneal chemotherapy: A case report. World J Clin Cases 2019; 7(22): 3881-3886
- URL: https://www.wjgnet.com/2307-8960/full/v7/i22/3881.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i22.3881
Pseudomyxoma peritonei (PMP) is a rare disease with an estimated incidence of 1 per million per year, and the appendix and ovaries are reported as the primary sites[1]. Surgical resection is the preferred and most effective treatment. However, according to Sugarbaker[2], the recurrence rate after tumor reduction can be as high as 100%. After aggressive cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC), patient outcomes remain unsatisfactory due to recurrent abdominal distension. Thus, the development of effective novel agents, especially targeted therapy, is required to improve patient outcome.
Tumor angiogenesis has been found to be essential for tumor cell proliferation[3]. Vascular endothelial growth receptor-2 (VEGFR-2) is an important factor involved in tumor angiogenesis.
Apatinib, a small-molecule inhibitor with anti-angiogenic function, acts mainly by the highly selective inhibition of vascular endothelial growth factor receptor-2 and is used in clinical practice for metastatic breast cancer, esophageal cancer, non-small-cell lung cancer, and hepatocellular carcinoma[4,5]. This new oral drug can efficiently suppress tumor angiogenesis.
Here, we report on a patient with PMP who was treated with apatinib after CRS and HIPEC. This patient did not exhibit symptoms of abdominal distention during the 6 mo of follow-up, which demonstrated the potential of apatinib in the treatment of PMP.
In November 2015, a 63-year-old woman arrived at our hospital with a 2-mo history of persistent abdominal distention along with an increased abdominal girth.
The patient’s symptoms started two month ago with recurrent abdominal distention along with an increased abdominal girth.
The patient had a free previous medical history.
The patient’s abdomen was bulging, but no obvious hard mass could be touched.
The results of hematological examinations were: White blood cell count, 7.44 × 109/L; C-reactive protein, 44.00 mg/L; hemoglobin, 87 g/L; platelets, 254 × 109/L; no abnormalities in liver and kidney function tests. The tumor markers were not elevated (CEA, 138 ng/mL; CA19-9, 99.67 U/mL; CA125, 133.1 U/mL).
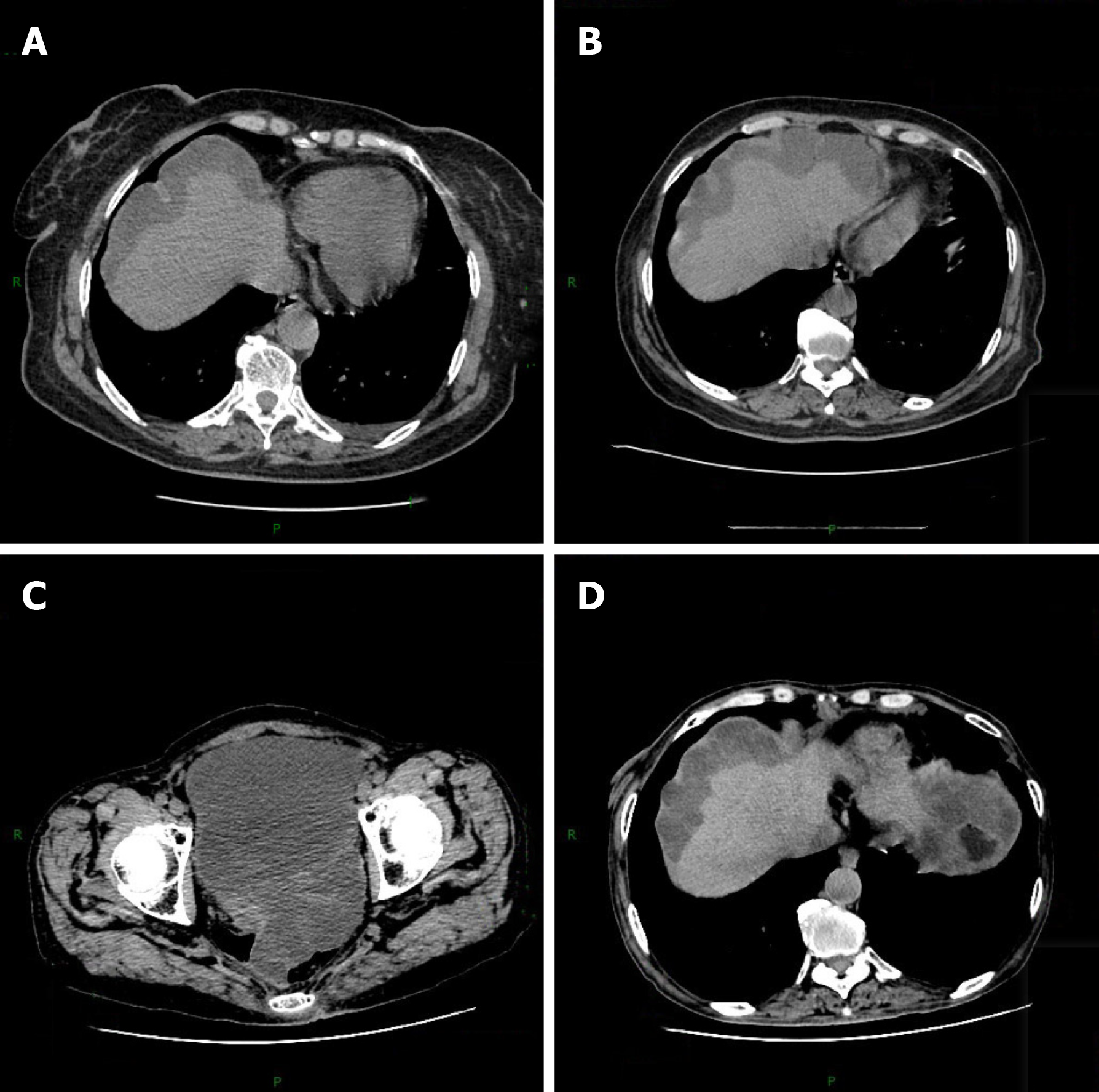
Computed tomography (CT) shows a large amount of fluid, and the lesion has affected the liver surface (Figure 1A).
Ovarian cancer.
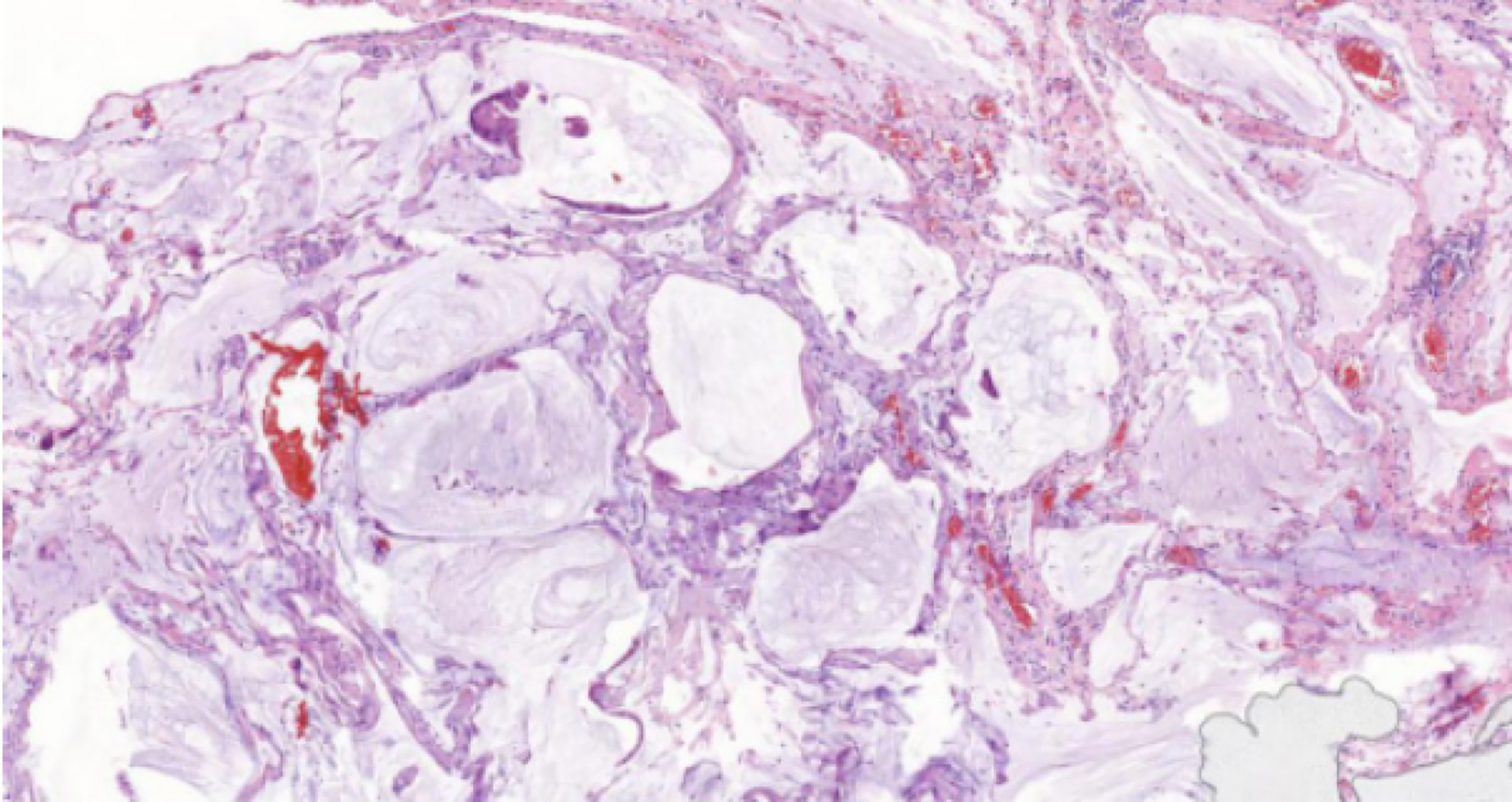
The patient underwent intraperitoneal laparoscopic exploration on November 26, 2015. Intraperitoneal laparoscopic exploration revealed dense light-red ascitic fluid in the abdominopelvic cavity. The peritoneum, intestinal tube, liver, and diaphragm surfaces displayed disseminated gelatinous tumor implants, approximately 1 millimeter to 1 centimeter. The surface of the right ovary and fallopian tubes contained dense gelatinous nodules. Subsequently, some lesions, including those in the right ovary, peritoneum, and omentum, were excised for biopsy. Biopsy of the lesions showed low-grade mucinous neoplasm in the right ovary and pseudomyxoma in the peritoneum and omentum. The patient underwent debulking surgery on December 10, 2015, including total hysterectomy, left adnexectomy, greater omental resection, and appendectomy. Pathology confirmed low-grade mucinous neoplasm in the right ovary with metastatic implants in the peritoneum, intestinal tube, liver, and diaphragm (Figure 2).
Postoperative review CT showed that the patient’s condition was temporarily improved (Figure 1B). However, on April 26, 2016, the patient presented at the Aero Space Center Hospital with a 1-mo history of recurrent abdominal distension. The patient underwent myxoma reduction surgery. Exploratory laparotomy revealed approximately 6000 mL of dense yellow ascites, peritoneal thickening, and gelatinous masses on the surface of the descending colon, sigmoid colon, mesentery, and pelvic peritoneal space. Then, the patient underwent enterolysis, peritoneal tumor resection, and small omental resection of the stomach. Meanwhile, the patient underwent HIPEC with 5-FU (1000 mg) and mitomycin (10 mg). After that, the patient received four cycles of HIPEC.
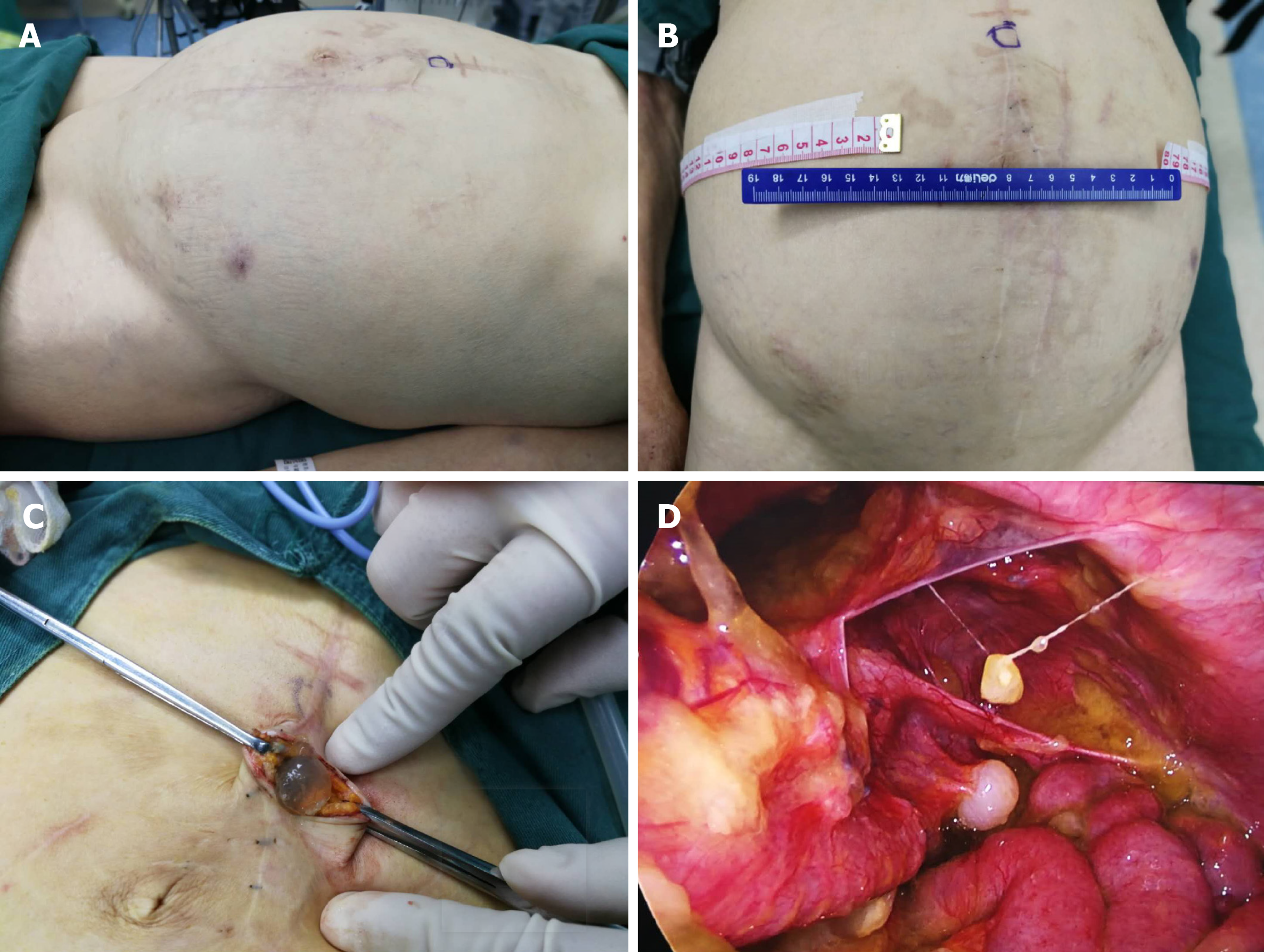
On June 18, 2017, the patient arrived at Yuncheng God Faith Hospital with the same complaint, recurrent abdominal distension. At this time, the patient had a poor appetite. The patient underwent two rounds of laparoscopic ascites removal by pumping. On June 20, 2017, the first laparoscopic ascites removal from the patient’s abdominal cavity produced approximately 7000 mL of light yellow viscous gelatinous substance. After 20 d, the patient developed abdominal distension, which gradually increased. On August 22, 2017, the second laparoscopic ascites removal from the patient’s abdominal cavity produced approximately 5000 mL of dark red gelatinous substance (Figure 3). By this time, the patient had become agitated about the multiple operative treatments. Therefore, we recommended her to receive apatinib as anti-angiogenesis therapy. After we explained the function and possible risks of apatinib, the patient signed an informed consent form for the administration of apatinib, which was approved by the Medical Ethics Committee of Yuncheng God Faith Hospital. Meanwhile, the patient provided her written informed consent for the accompanying images of the examination result to be published with this case study. On August 31, 2017, the ninth day after surgery, the patient received apatinib (0. 25 mg bid) as the main treatment. After 15 d, the patient had high blood pressure of 160/100 mmHg. Since then, the patient has received valsartan and apatinib (0.45 mg qd).
Postoperative pathology showed low-grade mucinous neoplasm in the right ovary and pseudomyxoma in the peritoneum and omentum. The patient received apatinib at a daily dose of 0.5 mg for 15 d per cycle and at a daily dose of 0.4 mg to date for recurrent abdominal distension after surgical treatment and HIPEC. During apatinib treatment, the primary side effect developed by the patient was hypertension. This side effect was well controlled with drug treatment with valsartan.
On December 18, 2017, the patient visited our hospital for review. An abdominal CT scan revealed no obvious mucinous lesion. The patient’s abdominal distention did not reappear. CT review results showed no progression (Figure 1C and D).
At the follow-up visit, the patient adhered to the above medication until the 10th mo after the medication, and the patient relapsed and was treated again with abdominal distension. In the 18th mo after the administration, the patient developed abdominal distension again with intestinal obstruction and multiple organ failure. After 15 d of maintenance treatment in an external hospital, he abandoned the treatment. After leaving the hospital for 20 d, he died at home.
To the best of our knowledge, this is the first case report of apatinib treatment for PMP. PMP is a rare benign, but progressive, condition associated with myxoma histopathology. The typical clinical manifestation is an enlarged tumor encroaching into the organs of the abdominal cavity, resulting in abdominal distension and an increase in abdominal circumference[6]. The current best treatment for PMP is CRS combined with HIPEC[7]. The literature reports that CRS combined with HIPEC can achieve a 5-year survival rate of 62.5% to 100.0% for low-grade malignancy and a 5-year survival rate of 0.0% to 65.0% for high-grade malignancy[8,9]. Despite combination therapy, some patients will experience repeated relapse. For example, for this patient, although pathology indicated a low-grade disease, repeated recurrence was observed. Thus, new treatments are urgently needed, and new drugs and targeted therapy might be good options.
Angiogenesis is an important process for tumor growth and metastasis[10]. VEGF plays an important role in angiogenesis and displays broad vascular functions by binding and activating VEGFR, a key regulator of the tyrosine kinase signaling pathway, especially VEGFR-2, which is mainly expressed in vascular endothelial cells[11]. Therefore, VEGFR may be a target of anticancer therapy.
Apatinib, a small-molecule oral inhibitor with anti-angiogenic function, mainly targets VEGFR-2 through an intracellular ATP-binding site and inhibits all VEGF-stimulated endothelial cell migration and proliferation, decreases tumor microvascular density, and promotes apoptosis[12]. In a phase II randomized clinical trial, a total of 144 patients with advanced metastatic gastric cancer who had failed two or more lines of chemotherapy were randomly assigned to a placebo (PL) group or two groups with different doses of apatinib[13]. The results showed that the median overall survival of the three groups was 2.50 mo, 4.83 mo, and 4.27 mo, and the median progression-free survival of the three groups was 1.40 mo, 3.67 mo, and 3.20 mo, respectively, demonstrating a significant difference between the apatinib and PL groups, but no significant difference between the two apatinib groups. On October 17, 2014, apatinib was recommended for the treatment of advanced gastric adenocarcinoma of the gastroesophageal junction by the Food and Drug Administration of the People’s Republic of China. Moreover, other clinical trials indicated that apatinib can be effective without severe additional toxicity in patients with hepatocellular carcinoma, ovarian cancer, and advanced non-squamous and non-small-cell lung cancer[14,15].
In this case, the patient who had failed CRS and HIPEC received apatinib treatment. During treatment with apatinib, the patient did not experience severe side effects except hypertension, which was well controlled by drugs. Although the patient eventually relapsed, the time to relapse was extended from the original 2 mo to 10 mo, which significantly improved the quality of life of the patient. Although this is a single case, apatinib did have an effect on PMP.
Here, we report the first case of PMP treated with apatinib, which demonstrated improvement in the patient’s condition. Apatinib may be an additional treatment option for PMP. Nevertheless, there are many questions: (1) Does apatinib affect all PMP types? (2) How to overcome rapid drug resistance if apatinib is effective? and (3) When is the best time to use apatinib for PMP patients? Thus, future clinical trials are still required.
Apatinib may be an option for recurrent PMP after surgical treatment, but this conclusion remains to be confirmed.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Morris DL, Nag DS S-Editor: Dou Y L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Ghosh RK, Somasundaram M, Ravakhah K, Hassan C. Pseudomyxoma peritonei with intrathoracic extension: a rare disease with rarer presentation from low-grade mucinous adenocarcinoma of the appendix. BMJ Case Rep. 2016;2016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Sugarbaker PH. Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol. 2001;27:239-243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 148] [Cited by in RCA: 159] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 3. | Carmeliet P, Jain RK. Angiogenesis in cancer and other diseases. Nature. 2000;407:249-257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6437] [Cited by in RCA: 6488] [Article Influence: 259.5] [Reference Citation Analysis (0)] |
| 4. | Hu X, Zhang J, Xu B, Jiang Z, Ragaz J, Tong Z, Zhang Q, Wang X, Feng J, Pang D, Fan M, Li J, Wang B, Wang Z, Zhang Q, Sun S, Liao C. Multicenter phase II study of apatinib, a novel VEGFR inhibitor in heavily pretreated patients with metastatic triple-negative breast cancer. Int J Cancer. 2014;135:1961-1969. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 209] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 5. | Zhang H. Apatinib for molecular targeted therapy in tumor. Drug Des Devel Ther. 2015;9:6075-6081. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 171] [Article Influence: 17.1] [Reference Citation Analysis (0)] |
| 6. | Jacquemin G, Laloux P. Pseudomyxoma peritonei: review on a cluster of peritoneal mucinous diseases. Acta Chir Belg. 2005;105:127-133. [PubMed] |
| 7. | Moran B, Baratti D, Yan TD, Kusamura S, Deraco M. Consensus statement on the loco-regional treatment of appendiceal mucinous neoplasms with peritoneal dissemination (pseudomyxoma peritonei). J Surg Oncol. 2008;98:277-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 169] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 8. | Misdraji J. Mucinous epithelial neoplasms of the appendix and pseudomyxoma peritonei. Mod Pathol. 2015;28 Suppl 1:S67-S79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 9. | Ramaswamy V. Pathology of Mucinous Appendiceal Tumors and Pseudomyxoma Peritonei. Indian J Surg Oncol. 2016;7:258-267. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 48] [Article Influence: 5.3] [Reference Citation Analysis (2)] |
| 10. | Cowey CL. Profile of tivozanib and its potential for the treatment of advanced renal cell carcinoma. Drug Des Devel Ther. 2013;7:519-527. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Kieran MW, Kalluri R, Cho YJ. The VEGF pathway in cancer and disease: responses, resistance, and the path forward. Cold Spring Harb Perspect Med. 2012;2:a006593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 158] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 12. | Li F, Liao Z, Zhao J, Zhao G, Li X, Du X, Yang Y, Yang J. Efficacy and safety of Apatinib in stage IV sarcomas: experience of a major sarcoma center in China. Oncotarget. 2017;8:64471-64480. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 53] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Li J, Qin S, Xu J, Guo W, Xiong J, Bai Y, Sun G, Yang Y, Wang L, Xu N, Cheng Y, Wang Z, Zheng L, Tao M, Zhu X, Ji D, Liu X, Yu H. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219-3225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 416] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 14. | Li J, Qin S, Xu J, Xiong J, Wu C, Bai Y, Liu W, Tong J, Liu Y, Xu R, Wang Z, Wang Q, Ouyang X, Yang Y, Ba Y, Liang J, Lin X, Luo D, Zheng R, Wang X, Sun G, Wang L, Zheng L, Guo H, Wu J, Xu N, Yang J, Zhang H, Cheng Y, Wang N, Chen L, Fan Z, Sun P, Yu H. Randomized, Double-Blind, Placebo-Controlled Phase III Trial of Apatinib in Patients With Chemotherapy-Refractory Advanced or Metastatic Adenocarcinoma of the Stomach or Gastroesophageal Junction. J Clin Oncol. 2016;34:1448-1454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 551] [Cited by in RCA: 732] [Article Influence: 81.3] [Reference Citation Analysis (1)] |
| 15. | Ledermann JA, Embleton AC, Raja F, Perren TJ, Jayson GC, Rustin GJS, Kaye SB, Hirte H, Eisenhauer E, Vaughan M, Friedlander M, González-Martín A, Stark D, Clark E, Farrelly L, Swart AM, Cook A, Kaplan RS, Parmar MKB; ICON6 collaborators. Cediranib in patients with relapsed platinum-sensitive ovarian cancer (ICON6): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2016;387:1066-1074. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 184] [Article Influence: 20.4] [Reference Citation Analysis (0)] |