Published online Jul 26, 2019. doi: 10.12998/wjcc.v7.i14.1825
Peer-review started: March 11, 2019
First decision: May 10, 2019
Revised: May 28, 2019
Accepted: June 27, 2019
Article in press: June 27, 2019
Published online: July 26, 2019
Processing time: 141 Days and 0.8 Hours
Increasing numbers of total joint arthroplasties and consecutive revision surgery are associated with the risk of periprosthetic joint infections (PPJI). Treatment of PPJI is complex and associated with immense socio-economic burden. One treatment aspect is parenteral antiinfective therapy, which usually requires an inpatient setting [Inpatient parenteral antibiotic therapy (IPAT)]. An alternative is outpatient parenteral treatment [Outpatient parenteral antibiotic therapy (OPAT)]. To conduct a health economic cost-benefit analysis of OPAT, a detailed cost analysis of IPAT and OPAT is required. So far, there is a lack of knowledge on the health economic effects of IPAT and OPAT for PPJI.
To review an economic comparison of IPAT and OPAT.
A systematic literature review was performed through Medline following the PRISMA guidelines.
Of 619 identified studies, 174 included information of interest and 21 studies were included for quantitative analysis of OPAT and IPAT costs. Except for one study, all showed relevant cost savings for OPAT compared to IPAT. Costs for IPAT were between 1.10 to 17.34 times higher than those for OPAT.
There are only few reports on OPAT for PPJI. Detailed analyses to support economic or clinical guidelines are therefore limited. There is good clinical evidence supporting economic benefits of OPAT, but more high quality studies are needed for PPJI.
Core tip: Periprosthetic joint infection of total joint replacement poses a significant socio-economic burden. One factor is the need for prolonged parenteral antibiotic therapy. Outpatient parenteral antibiotic therapy (OPAT) might reduce costs compared to inpatient (IPAT) settings. A systematic literature review was performed to compare economic impact of OPAT and IPAT. Twenty-one articles were identified of which 20 reported economic benefits of OPAT. While the heterogeneity of studies limited the interpretation and generalization, overall beneficial cost effects of OPAT were shown. Future studies should focus on specific economic outcomes of OPAT for PPJI.
- Citation: Boese CK, Lechler P, Frink M, Hackl M, Eysel P, Ries C. Cost-analysis of inpatient and outpatient parenteral antimicrobial therapy in orthopaedics: A systematic literature review. World J Clin Cases 2019; 7(14): 1825-1836
- URL: https://www.wjgnet.com/2307-8960/full/v7/i14/1825.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i14.1825
There is a continuous increase in numbers of total joint arthroplasties (TJA) and an expansion of indications has been noted in recent years[1,2]. In addition, predictions show a growing demand for the coming years[1,2]. On the one hand, more older and multi-morbid patients undergo TJA, on the other hand, indications have been extended to younger and more active patients with high functional demands and longer life expectancy[1,2]. Due to a general increase in life expectancy the consecutive risk for revision surgery rises. Every revision surgery is associated with a risk for infections as are co-morbidities (e.g., diabetes mellitus, obesity, immune suppression)[3]. Due to increasing numbers of joint replacements, a subsequent increase in numbers of periprosthetic joint infections (PPJI) can be expected. Treatment of PPJI usually consists of surgical intervention and a long-term antimicrobial therapy[4]. Surgical intervention includes one-stage revision with either retention or revision of components in combination with debridement and irrigation. Alternatively, patients can undergo a staged revision with explantation of the prosthesis combined with debridement and irrigation and potentially reimplantation after several weeks to months. Both surgical principles are combined with antimicrobial treatment – mostly antibiotics[4,5].
Empiric antimicrobial therapy is followed by calculated therapy as soon as the pathogenic agent is identified and a resistogram is available. In PPJI, a bone-infection must be assumed and therefore antiinfective therapy lasts for several weeks (usually 6-12 wk)[4]. Initially, antimicrobial therapy is started as parenteral therapy to achieve high plasma concentrations as well as sufficient concentrations in the targeted bone and joint as fast as possible (e.g., time to peak serum concentration and peak serum concentration). Additionally, there are reports indicating an increase in multi-resistant bacteria. Here, extended parenteral antiinfective therapy is often required and no oral antibiotics are available. Another advantage of parenteral therapy is the fast (immediate) absorption and bio-availability of the drug. Potential risks of mal-absorption do not occur. Usually, parenteral therapy requires an inpatient setting [Inpatient parenteral antibiotic therapy (IPAT)] due to monitoring, need for intravenous lines and administration of detergents by healthcare providers. This inpatient therapy goes along with high direct as well as indirect costs for the health system[6]. An alternative to IPAT is outpatient parenteral treatment [Outpatient parenteral antibiotic therapy (OPAT)]. To conduct a health economic cost-benefit analysis of OPAT, a detailed cost analysis of IPAT and OPAT is required. While calculation of direct costs hospital and outpatient settings is generally possible, there are no standards for methods and reporting. Additionally, exact cost-benefit analyses are a difficult endeavour as they should take into account direct cost savings (e.g., hospital stay, physician visits, drugs and supplies, childcare, housekeeping, transportation, etc.) as well as indirect benefits (lost wages, both for patient and family members, etc.)[7]. So far, there is a lack of knowledge regarding health economic effects of IPAT and OPAT for PPJI.
The aim of this study is an economic comparison of IPAT and OPAT. A systematic literature review was performed for this purpose.
A systematic literature review was performed. Medline was searched via PubMed after a previous pilot-search to identify relevant search terms and strategies. The literature search and presentation of results followed the most recent version of the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-Analyses). A PRISMA checklist was used in a modified form for this study. This systematic literature review was registered in the international, prospective registry for systematic literature reviews (PROSPERO) at the Centre for Reviews and Dissemination of the University of York (York, United Kingdom) (No. 71005).
The exact search strategy using Medical Subject Headings (MeSH) is presented in Figure 1. Identified hits were entered into proprietary literature management software (EndNote v. 7.7.1 for Mac). All articles were subsequently screened based on titles and abstracts.
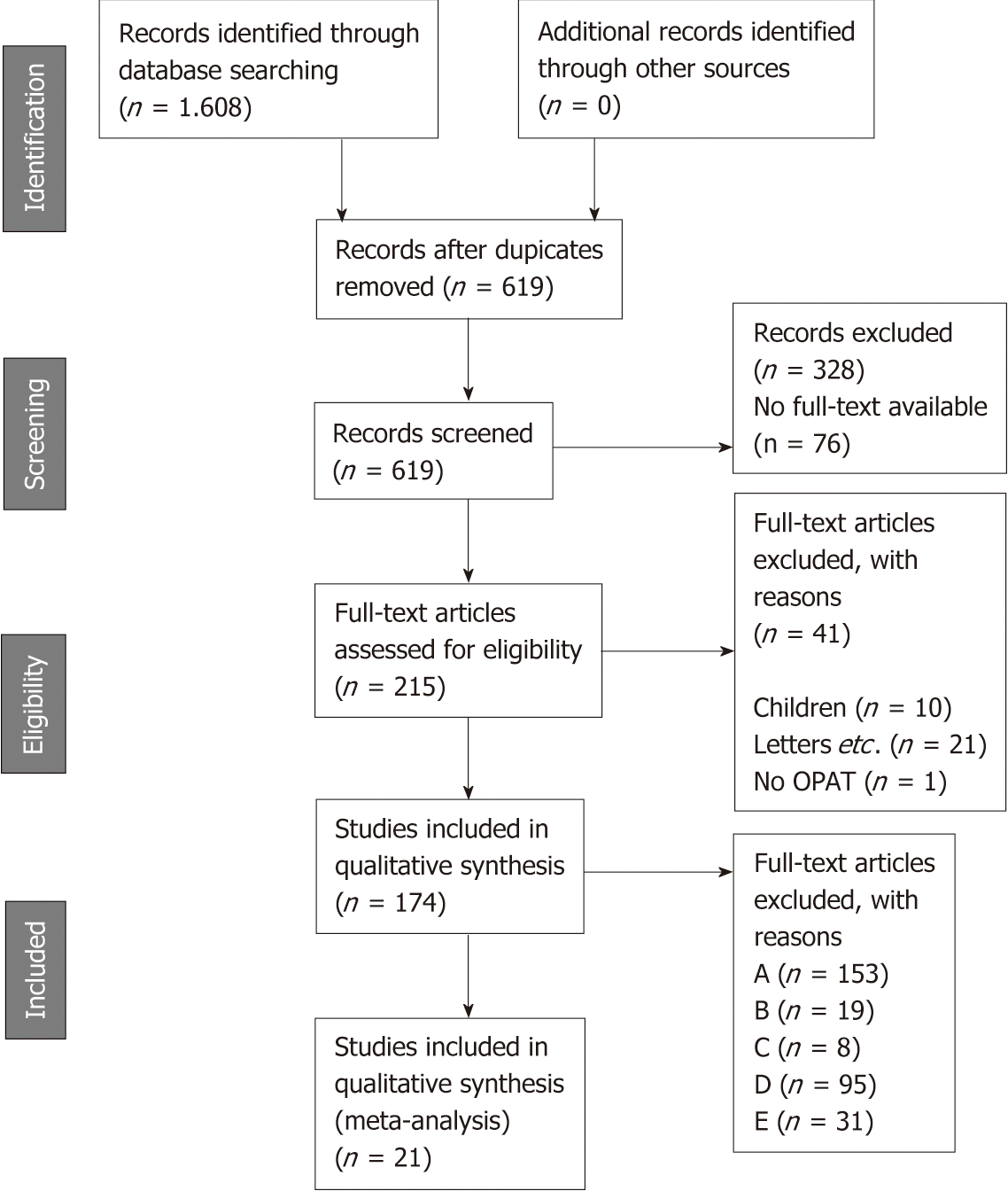
All studies reporting comparative costs of OPAT and IPAT were deemed eligible for inclusion in the systematic review. Exclusion was performed based on criteria outlined in Table 1. After screening, the full texts of all included articles were subjected to an in-depth analysis for inclusion and exclusion criteria. Finally, all eligible articles were stratified into five groups (Table 2). Studies were grouped into the highest possible category (A to E). Only category A studies were included into quantitative data analysis. Category B to E studies were used for background analysis and discussion. The inclusion process is depicted in Figure 2.
| Exclusion criteria |
| No relation to OPAT |
| No bacterial infections |
| Only prophylactic antibiotic therapy |
| No distinction between oral and parenteral outpatient antibiotic therapy |
| Parenteral therapy with orally available antibiotics |
| Non-English language publication |
| No original study (e.g., comments, letters, etc.) |
| Combination of IPAT and OPAT |
| Only patients < 18 yr |
| Case reports and case series with < 15 cases |
| Poster-publications/Congress abstracts etc. |
| “Specific infections” or tropical diseases |
| Study protocols (without original clinical data) |
| Simulations (without original clinical data) |
| CME publications (without original clinical data) |
| Non-peer-reviewed journals |
| No full text available |
| A | Original research with health economic data on OPAT (costs) |
| B | Secondary literature with health economic data on OPAT in PPJI |
| C | Pro-/Retrospective study on OPAT (PPJI of hip or knee) |
| D | Pro-/Retrospective study on OPAT (other infections) |
| E | Guidelines, reviews on OPAT with health economic data |
| F | Exclusion of publication |
Extraction of data followed a standardized protocol. First authors, publication year, country of origin, study design, sample size, diagnosis, anti-infective therapy, costs of IPAT and OPAT and differences were extracted and entered into a digital spreadsheet.
The systematic literature search resulted in 524 hits for “outpatient parenteral antibiotic therapy”, 570 hits for “outpatient parenteral anti-infective therapy”, and 514 for “outpatient parenteral antimicrobial therapy”. After deleting duplicates, 619 potential studies of interest remained. Of these, 328 were excluded based on title and abstract during screening. For the rest, 215 full texts were available for in-depth analysis and 174 were categorized into group A-E. For quantitative analysis of OPAT and IPAT cost, 21 studies were included (Table 3[8-28]). A detailed description of these studies can be found in Table 4[8-28].
| ID | First author | Ref. | Year | Country | Design | Diagnosis | Cases/Patients (n) | Antibiotic agent |
| 1 | Al Alawi | [8] | 2015 | Bahrain | Retrospective | Bacterial tonsillitis | 97 | Ceftriaxon |
| 2 | Antoniskis | [9] | 1978 | United States | Retrospective | Endocarditis + Osteomyelitis | 13/7 (Controls) | Multiple |
| 3 | Bernard | [10] | 2001 | Switzerland | Prospective | Osteomyelitis (incl. 10 PPJI) | 39 | Multiple |
| 4 | Chapman | [11] | 2009 | United Kingdom | Retrospective | multiple | 334/296 | Multiple |
| 5 | Connors | [12] | 2017 | Canada | Prospective | Dental | 110/. | Multiple |
| 6 | Gonzales | [13] | 2017 | Spain | Retrospective | Multiple | 1324/1190 | Multiple |
| 7 | Gray | [14] | 2012 | United Kingdom | Retrospective | Multiple | 291/. | Multiple |
| 8 | Grizzard | [15] | 1991 | United States | Retrospective | Multiple | 46/. | Multiple |
| 9 | Harrison | [16] | 2015 | United Kingdom | Retrospective | Kidney transplant | 12/9 | n. a. |
| 10 | Heintz | [17] | 2011 | United States | Prospective | Multiple | 569/536 | Multiple |
| 11 | Kieran | [18] | 2009 | Ireland | Retrospective | Multiple | 60/56 | Multiple |
| 12 | Lacroix | [19] | 2014 | France | Retrospective | Endocarditis | 18/. | Multiple |
| 13 | Malone | [20] | 2015 | Australia | Retrospective | Diabetic foot | 59/. | n. a. |
| 14 | Nathwani | [21] | 2003 | United Kingdom | Retrospective | Bone and joint | 55/. | Teicoplanin |
| 15 | Nguyen | [22] | 2010 | United States | NA | Cellulitis | 80/. | Multiple |
| 16 | Ruh | [23] | 2015 | United States | Retrospective | Multiple | 96/85 | Multiple |
| 17 | Seaton | [24] | 2014 | United Kingdom | Retrospective | MRSA skin and soft tissue | 173/. | Multiple |
| 18 | Sims | [25] | 2013 | United Kingdom | Retrospective | PPJI | 96/85 | Multiple |
| 19 | Theocharis | [26] | 2012 | Greece | Retrospective | Multiple | 173/. | Multiple |
| 20 | Wai | [27] | 2000 | Canada | Retrospective | Multiple | 80/. | Multiple |
| 21 | Yong | [28] | 2009 | Singapore | Retrospective | Multiple | 96/85 | Multiple |
| ID | First author | Ref. | Cost IPAT | Cost OPAT | Cost-difference | Comment |
| 1 | Al Alawi1 | [8] | €43152.41 / €50343.67 €143.37/d2 | €17261.45 €57.35/d2 | €25890.96 €86.02/d2 | 301 bed days were saved by OPAT. Exact cost reduction not clear in text |
| 2 | Antoniskis | [9] | US $234.22/d US $10022.23 /pat | US $69.35/d US $6357.52/pat | US $229.70/d US $3664.71/pat | Historical data/prices from 1977/78. Average day costs calculated |
| 3 | Bernard | [10] | US $710/d US $2233945 | US $129/d US $360060 | US $581/d US $1873885 | 2.147 patient-days of 39 patients |
| 4 | Chapman | [11] | £372.53/d £1502769 | £151.79/d £612306 | £220.74/d £890,463 | Calculation based on 4034 bed-days |
| 5 | Connors1 | [12] | CAN $1720.70 / d CAN $717530 €1063451.21 | CAN $1094/d CAN $120096 €177994.28 | CAN $288/d CAN $597434 €884919.24 | 110 patients with 417 bed-days. Average: 3.8 d with CAN $1094 / case |
| 6 | Gonzales | [13] | €4357/pat €518.70/d | €2350/pat €98.30/d | €420.40/d | Very detailed calculation. Methods presented. IPAT for an average of 8.4 days. OPAT included IPAT for readmission within 30 d |
| 7 | Gray | [14] | £192635 £662/patient | Simulation of cost analysis based on potentially saved bed days. Calculation include OPAT as well as other treatment changes. 18/429 patients in the calculation would have been eligible for OPAT | ||
| 8 | Grizzard | [15] | US $159.54/d US $101314 | US $112.68/d US $29763 | US $112.68/d US $71551 | Historical data (1988). Cost-Charge Ratio. Additional Homecare-treatment (OPHAT) of center based OPAT and IPAT differentiated. 635 bed days |
| 9 | Harrison | [16] | £264 / d £84480 | £109.59 / d £35070 | £154.41 / d £49,410 | Simplified calculation. 320 bed days |
| 10 | Heintz | [17] | US $424080 US $658.50/visit | Simplified calculation with relevant limitations. 228 bed days were saved by OPAT | ||
| 11 | Kieran | [18] | €342862 | €167.60/d €558912 | €-216.050 | Prospective study on 60 cases. 1289 bed days saved. Community nurses included in OPAT calculation |
| 12 | Lacroix | [19] | €1125/d €335250 | €228/d €67943 | €897/d €14850/pat €267307 | Simulated cost analysis. 298 bed days saved |
| 13 | Malone | [20] | US $829/d US $1143.957 | US $278848 | US $14661/pat US $864997 | IPAT costs based on Council of Australian Governments. 1,569 bed days saved |
| 14 | Nathwani | [21] | £300 / d £11400 / pat £627000 | £1,749.15/pat £96,203.25 | £9650.85/pat £530796.75 | Three groups: IPAT, OPAT (Teicoplanin) and outpatient oral therapy (Linezolid). Oral group not included |
| 15 | Nguyen | [22] | US $1180/d US $556960 | US $385/d US $181720 | US $795/d US $375240 | 472 OPAT visits |
| 16 | Ruh | [23] | US $7540135.35 | US $607583.32 | US $6932552.03 | Complex cost calculation with multiple factors |
| 17 | Seaton | [24] | £13019.57/pat £455685 | £6,532.89/pat £228651 | £6,487/pat £227034 | Three groups: IPAT, OPAT (Teicoplanin) and outpatient oral therapy (Linezolid). Oral group not included. Calculation based on 37 cases |
| 18 | Sims | [25] | £250/d £14500/pat | £24/d 1392/pat | £24/d £13108/pat | For two weeks OPAT cost reduction of 2108 £/Pat |
| 19 | Theocharis | [26] | €167.50-195.50/d | €164/d €637/pat | Re-admission rates (14.2%) and costs not included in OPAT calculation | |
| 20 | Wai | [27] | €20728/pat €2901983 | €2774/pat €388402 | €17954/pat €2513580 | Very detailed cost analysis for IPAT and OPAT. Perspectives of payer and hospitals were presented |
| 21 | Yong | [28] | US $457/d US $12403/pat | US $278/d US $12736/pat | Includes calculation for opportunity-costs |
Comparisons were performed based costs per day and costs per case. Table 5[8-28] shows costs per day and per case of IPAT and OPAT of included studies.
| ID | First author | Year | Ref. | IPAT | OPAT | ||
| € per case | € per day | € per case | € per day | ||||
| 1 | Al Alawi | 2015 | [8] | 143.361 | 57.351 | ||
| 2 | Antoniskis | 1978 | [9] | 251.26 | 74.09 | ||
| 3 | Bernand | 2001 | [10] | 755.88 | 137.34 | ||
| 4 | Chapman | 2009 | [11] | - | 418.92 | 170.71 | |
| 5 | Connors | 2017 | [12] | 2550.69 | 1621.42 | ||
| 6 | Gonzales | 2017 | [13] | 4357 | 519 | 2350 | 98 |
| 7 | Gray | 2012 | [14] | ||||
| 8 | Grizzard | 1991 | [15] | 110.29 | 77.89 | ||
| 9 | Harrison | 2015 | [16] | 337.26 | 140.53 | ||
| 10 | Heintz | 2011 | [17] | 492.12 | 28.38 | ||
| 11 | Kieran | 2009 | [18] | 168 | |||
| 12 | Lacroix | 2014 | [19] | 1125 | 228 | ||
| 13 | Malone | 2015 | [20] | 68524 | |||
| 14 | Nathwani | 2003 | [21] | 17502.42 | 460.59 | 2685.24 | |
| 15 | Nguyen | 2010 | [22] | 823.73 | 268.76 | ||
| 16 | Ruh | 2015 | [23] | ||||
| 17 | Seaton | 2014 | [24] | 15670.97 | 7863.77 | ||
| 18 | Sims | 2013 | [25] | 17881.4 | 308.3 | 1716.61 | 29.6 |
| 19 | Theocharis | 2012 | [26] | 180 | 637 | 164 | |
| 20 | Wai | 2000 | [27] | 20278 | 9188 | ||
| 21 | Yong | 2009 | [28] | 8873.23 | 326.94 | 9111.46 | 198.88 |
Five (24%) of the studies were performed in the United States, six (29%) in the United Kingdom, another five in the rest of Europe (24%) and the remainder in other countries. Except for one study, all showed relevant cost savings by OPAT compared to IPAT. Costs for IPAT were between 1.10 to 17.34 times higher than those for OPAT (Table 6 [8-28]).
| ID | First author | Year | Ref. | IPAT/OPAT | |
| Ratio per case | Ratio per day | ||||
| 1 | Al Alawi | 2015 | [8] | 2.501 | |
| 2 | Antoniskis | 1978 | [9] | 3.39 | |
| 3 | Bernand | 2001 | [10] | 5.50 | |
| 4 | Chapman | 2009 | [11] | 2.45 | |
| 5 | Connors | 2017 | [12] | 1.57 | |
| 6 | Gonzales | 2017 | [13] | 1.85 | 5.30 |
| 7 | Gray | 2012 | [14] | ||
| 8 | Grizzard | 1991 | [15] | 1.42 | |
| 9 | Harrison | 2015 | [16] | 2.40 | |
| 10 | Heintz | 2011 | [17] | 17.34 | |
| 11 | Kieran | 2009 | [18] | ||
| 12 | Lacroix | 2014 | [19] | 4.93 | |
| 13 | Malone | 2015 | [20] | ||
| 14 | Nathwani | 2003 | [21] | 6.52 | |
| 15 | Nguyen | 2010 | [22] | 3.06 | |
| 16 | Ruh | 2015 | [23] | ||
| 17 | Seaton | 2014 | [24] | 1.99 | |
| 18 | Sims | 2013 | [25] | 10.42 | 10.42 |
| 19 | Theocharis | 2012 | [26] | 1.10 | |
| 20 | Wai | 2000 | [27] | 2.21 | |
| 21 | Yong | 2009 | [28] | 0.97 | 1.64 |
PPJI of large joints remains a multidisciplinary challenge. They are associated with significant socio-economic costs. Haddad et al[6] estimate the costs of PPJI to be approximately $1.62 billion (approx. €1.4 billion). Additionally, Parisi et al[29] used a Markov model including direct and indirect costs, and calculated costs per case to be $390000 to $474000 (circa €337000 to €409700). An increase in PPJI in recent years could be shown by the Nationwide Inpatient Sample[30]. While 1104 cases were documented in 1990, a continuous increase was noted and reached 5838 cases in 2004. Interestingly, direct costs were stable at $55.000 per case. However, in the same timeframe, the length of hospital stay was significantly reduced from 22.2 to 7.6 d[30]. Kurtz et al[31] performed a follow-up study and calculated costs of infected total knee arthroplasties until the year 2020. The authors estimated costs of $330 billion in 2011; reaching $527 billion in 2020. Interestingly, the costs per case were stable in the calculation. This estimation was supported by the study and thus highlights the relevance of PPJI[30,31].
While avoiding PPJI is the best method to reduce costs, increasing numbers of total joint replacements makes this an illusionary aspiration. Occurrence of PPJI is associated with high costs and long-term therapy. By replacement of inpatient treatment with outpatient treatment, a significant cost reduction seems possible[32-34]. Already in the 1970s, studies showed higher levels of patient satisfaction with OPAT in comparison to IPAT[8,35]. Furthermore, no increase in risks and complications was noted by OPAT[36,37].
The first identified cost analysis with potential cost reduction were reported in the 1970s[9,38]. Antoniskis et al[9] reported the first detailed outpatient antibiotic therapy in 1978. Cost structures of OPAT as well as IPAT showed significant variability. This may result from the large timeframe of the included studies. For example, studies of Antoniskis et al[9] and Grizzard et al[15] were much older than comparable studies from 2000 to 2017. To allow for a better comparison of studies, therapy costs were calculated for per-day-costs and transformed using the exchange rate for the mid of the year of publication. Correction for inflation was not performed. Additionally, analysis and interpretation of costs per case are severely influenced by individual cases and as such demonstrate immense heterogeneity. Thus, the analysis of costs per day was deemed more suitable for comparison. Costs per day ranged from €110 to €1125 for IPAT and from €28 to €269 for OPAT. Comparability is, however, restricted by specific peculiarities of each healthcare system. The complexity of the healthcare system in the United States adds to these limitations as does reimbursement in each of the included countries. The highest comparability was probably achieved in United Kingdom studies. Here, the NHS National Institute for Health Research supports OPAT research and therapy. This is highlighted by “The Community Intra Venous Antibiotic Study” (CIVAS) by Minton et al[39]. One part of this research project was a systematic literature review on economic aspects of OPAT[39]. Cost-effectiveness was one of the five main research questions of the project and showed potential reduction in costs. Good acceptance of OPAT by patients and treating healthcare personnel was identified. Furthermore, safety and clinical effectiveness of OPAT were shown to be acceptable and comparable to IPAT. So far, no other comprehensive systematic literature review has been performed on cost comparisons of OPAT and IPAT. The aim of this study was the identification of costs of IPAT compared to OPAT. This systematic literature review identified 21 studies with sufficient information on costs of IPAT and OPAT. While the studies were published over a long time period (1978-2017) with wide geographical distribution, some generalized conclusions may be drawn. Here, the IPAT costs per day ranged from €110 to €1125 since 2001, OPAT costs were between €28 to €228. More comparable might be the ratio between IPAT and OPAT costs for each study. As shown in Table 6[8-28], the mean cost ratio was 3.6 (1.0-10.4) per case and 4.8 (1.1-17.3) per day. Thus, the assumption of cost reduction by OPAT seems reasonable. Additional effects by indirect opportunity costs were not included in this study and might therefore increase the beneficial effect of OPAT.
Notably, all studies had some limitations in design and data analysis. Overall, all studies used simplified methods to calculate IPAT costs. OPAT costs were mostly calculated in a more complex fashion. Cost reductions were beneficial in most cases and most publications did not differentiate between cost-effects for payers/insurances and hospitals. Therefore, it cannot be conclusively demonstrated whether cost reductions led to reduced costs to the health care system (e.g., insurance) or increased profit to the hospitals; mixed effects are possible as well. Additionally, the information regarding to PPJI was limited. Thirteen publications mentioned treatment of bone and joint infections (Table 7[9-11,13-15,17,18,20,21,23,25-28]). Of those, only two defined the affected bones or joints in detail. One study (Sims et al[25]) specifically looked at PPJI of the hip and knee. Therefore, it was the only study to provide economic information for this particular subset of patients.
| ID | First author | Ref. | Year | Diagnosis | |
| 2 | Antoniskis | [9] | 1978 | 5 acute OM, 6 chronic OM | No information regarding affected joints |
| 3 | Bernard | [10] | 2001 | 39 OM (13 non-union fracture; 16 chronic OM; 10 PPJI) | Sites of OM: femur (n = 11), hip (n = 9); tibia (n = 5), ankle (n = 4), mastoid, calcaneum, vertebra, knee (each n = 2), wrist and phalange (each n = 1). No information on outcome per affected joints |
| 4 | Chapman | [11] | 2009 | Of 334 infections, approx. 20 (6%) were bone and joint associated; bed days saved were approx. 481 of 4034 (12%) | No information regarding affected joints |
| 6 | Gonzales | [13] | 2017 | Underlying diagnosis not mentioned | No information regarding affected joints |
| 7 | Gray | [14] | 2012 | 291 cases; 14 in orthopaedics (4.8%) | No information regarding affected joints |
| 8 | Grizzard | [15] | 1991 | OM and septic arthritis most frequent diagnosis in OPAT (30% of patient days) | No information regarding affected joints |
| 10 | Heintz | [17] | 2011 | 569 cases; 190 (33.4%) bone and joint associated | No information regarding affected bones or joints |
| 11 | Kieran | [18] | 2009 | 60 cases; OM (n = 25, 41.7%), PPJI (n = 2; 3.3%) and septic arthritis (n = 3, 5.1%) accounting for 50% | No information regarding affected bones or joints |
| 13 | Malone | [20] | 2015 | Diabetic foot syndrome. Cellulites with OM (n = 14; 24%) and OM alone (n = 11; 19%) were documented (n = 25; 43%) | No information regarding affected bones or joints |
| 14 | Nathwani | [21] | 2003 | 4 septic arthritis; 3 acute and 48 chronic OM (40%/19 = PPJI) | No information regarding affected bones or joints |
| 16 | Ruh | [23] | 2015 | 96 cases; bone and joint infection in 14 (39.5%) | No information regarding affected bones or joints |
| 18 | Sims | [25] | 2013 | 10 primary total knee replacements and 4 primary total hip replacement | No economic analysis was performed by affected joint |
| 19 | Theocharis | [26] | 2012 | No bone or joint infection mentioned | No information regarding affected bones or joints |
| 20 | Wai | [27] | 2000 | 140 cases; 55 bone/joint (39%) infections | No information regarding affected bones or joints |
| 21 | Yong | [28] | 2009 | 7/72 cases of OPAT and 9/93 IPAT patient bone and joint associated | No information regarding affected bones or joints |
In the United States and the United Kingdom, OPAT is already well accepted. Efficacy and safety of OPAT was comparable to IPAT and associated with higher patient satisfaction (11, 18-21). Still, in many countries (e.g., Germany) there is none or only very limited availability of facilities to support OPAT.
In conclusion, a large number of publications on OPAT is available. Notably, there are only few reports on the specific subject of PPJI. Detailed analyses to support economical or clinical guidelines are therefore limited. This systematic literature review could identify only three studies that explicitly covered PPJI (category C)[32-34]. Overall, OPAT showed comparable efficacy, safety and success rates as did IPAT.
Increasing numbers of total joint arthroplasties worldwide are noted. This is associated with rising risk for revision surgery. Periprosthetic joint infections (PPJI) play a significant role in revisions. Treatment of PPJI often requires long-term antimicrobial therapy. In PPJI, a bone-infection must be assumed and therefore antiinfective therapy lasts for 6-12 wk or longer. Parenteral antiinfective therapy is often required. Usually, parenteral therapy requires an inpatient setting [Inpatient parenteral antibiotic therapy (IPAT)] and goes along with high direct as well as indirect costs. An alternative is outpatient parenteral treatment [Outpatient parenteral antibiotic therapy (OPAT)]. So far, there is a lack of knowledge regarding health economic effects of IPAT and OPAT in general and for PPJI specifically.
To identify the proposed economical benefits of OPAT in comparison to IPAT health economic cost-benefit analysis are needed. While various publications dealt with OPAT, generalization of assumptions requires input from multiple studies. We aimed to perform a systematic literature review of published literature on cost comparisons of OPAT and IPAT to better delineate the effects. The motivation was generating evidence to support OPAT for PPJI and create awareness for this alternative treatment option.
The aim of this study was an economic comparison of IPAT and OPAT. A systematic literature review was performed for this purpose.
For this purpose, a search strategy was developed and we performed a systematic review of published literature by searching the Medline database via PubMed. All abstracts meeting the inclusion criteria were identified, and relevant articles were analyzed in detail. Relevant data was extracted and homogenized.
The literature search identified 619 potential studies of interest. 328 were excluded during screening. 215 full texts were available for in-depth analysis. For quantitative analysis of OPAT and IPAT cost, 21 studies were included. Costs for IPAT were between 1.10 to 17.34 times higher than those for OPAT. Only one study showed marginally lesser costs for IPAT. Only one study focused specifically on PPJI.
To the best of our knowledge, this is the first comprehensive systematic literature review outside the CIVAS report on cost effectiveness of OPAT. The review provides a wide overview over the exiting literature with minimal exclusion criteria. The presentation of extracted data allows for detailed understanding of included studies. Limitations of the study were the heterogeneity of studies from different health care systems and a wide time interval. Still, this open inclusion allows for better understanding of the available data worldwide.
While the beneficial cost effect of OPAT has been shown, there is need to provide more specific studies. In particular, there is need to analyze cost structures for PPJI treatment in different health care systems. With such studies, guidelines to implement OPAT into the standard of care might be created.
Manuscript source: Invited Manuscript
Specialty type: Medicine, research and experimental
Country of origin: Germany
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Emara KM, Malik H S-Editor: Cui LJ L-Editor: A E-Editor: Liu JH
| 1. | Pabinger C, Lothaller H, Geissler A. Utilization rates of knee-arthroplasty in OECD countries. Osteoarthritis Cartilage. 2015;23:1664-1673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 152] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 2. | Pabinger C, Geissler A. Utilization rates of hip arthroplasty in OECD countries. Osteoarthritis Cartilage. 2014;22:734-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 137] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 3. | Ramkumar PN, Chu CT, Harris JD, Athiviraham A, Harrington MA, White DL, Berger DH, Naik AD, Li LT. Causes and Rates of Unplanned Readmissions After Elective Primary Total Joint Arthroplasty: A Systematic Review and Meta-Analysis. Am J Orthop (Belle Mead NJ). 2015;44:397-405. [PubMed] |
| 4. | Aboltins CA, Berdal JE, Casas F, Corona PS, Cuellar D, Ferrari MC, Hendershot E, Huang W, Kuo FC, Malkani A, Reyes F, Rudelli S, Safir O, Seyler T, Tan TL, Townsend R, Tuncay I, Turner D, Winkler H, Wouthuyzen-Bakker M, Yates AJ, Zahar A. Hip and Knee Section, Prevention, Antimicrobials (Systemic): Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S279-S288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 5. | Anemüller R, Belden K, Brause B, Citak M, Del Pozo JL, Frommelt L, Gehrke T, Hewlett A, Higuera CA, Hughes H, Kheir M, Kim KI, Konan S, Lausmann C, Marculescu C, Morata L, Ramirez I, Rossmann M, Silibovsky R, Soriano A, Suh GA, Vogely C, Volpin A, Yombi J, Zahar A, Zimmerli W. Hip and Knee Section, Treatment, Antimicrobials: Proceedings of International Consensus on Orthopedic Infections. J Arthroplasty. 2019;34:S463-S475. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 34] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 6. | Haddad FS, Ngu A, Negus JJ. Prosthetic Joint Infections and Cost Analysis? Adv Exp Med Biol. 2017;971:93-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Beguin Y, Benoit Y, Crokaert F, Selleslag D, Vandercam B; National Fund for Scientific Research. Outpatient and home parenteral antibiotic therapy (OHPAT) in low-risk febrile neutropenia: consensus statement of a Belgian panel. Acta Clin Belg. 2002;57:309-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 8. | Al Alawi S, Abdulkarim S, Elhennawy H, Al-Mansoor A, Al Ansari A. Outpatient parenteral antimicrobial therapy with ceftriaxone for acute tonsillopharyngitis: efficacy, patient satisfaction, cost effectiveness, and safety. Infect Drug Resist. 2015;8:279-285. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 9. | Antoniskis A, Anderson BC, Van Volkinburg EJ, Jackson JM, Gilbert DN. Feasibility of outpatient self-administration of parenteral antibiotics. West J Med. 1978;128:203-206. [PubMed] |
| 10. | Bernard L, El-Hajj, Pron B, Lotthé A, Gleizes V, Signoret F, Denormandie P, Gaillard JL, Perronne C. Outpatient parenteral antimicrobial therapy (OPAT) for the treatment of osteomyelitis: evaluation of efficacy, tolerance and cost. J Clin Pharm Ther. 2001;26:445-451. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 11. | Chapman AL, Dixon S, Andrews D, Lillie PJ, Bazaz R, Patchett JD. Clinical efficacy and cost-effectiveness of outpatient parenteral antibiotic therapy (OPAT): a UK perspective. J Antimicrob Chemother. 2009;64:1316-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 193] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 12. | Connors WJ, Rabie HH, Figueiredo RL, Holton DL, Parkins MD. Acute dental infections managed in an outpatient parenteral antibiotic program setting: prospective analysis and public health implications. BMC Infect Dis. 2017;17:202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | González-Ramallo VJ, Mirón-Rubio M, Mujal A, Estrada O, Forné C, Aragón B, Rivera AJ. Costs of outpatient parenteral antimicrobial therapy (OPAT) administered by Hospital at Home units in Spain. Int J Antimicrob Agents. 2017;50:114-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 14. | Gray A, Dryden M, Charos A. Antibiotic management and early discharge from hospital: an economic analysis. J Antimicrob Chemother. 2012;67:2297-2302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 15. | Grizzard MB, Harris G, Karns H. Use of outpatient parenteral antibiotic therapy in a health maintenance organization. Rev Infect Dis. 1991;13 Suppl 2:S174-S179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Harrison J, Hossain MA, Morsy M, Ghazanfar A. Outpatient parenteral antibiotic therapy in a renal transplant population: A single-center experience. Saudi J Kidney Dis Transpl. 2015;26:1121-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 17. | Heintz BH, Halilovic J, Christensen CL. Impact of a multidisciplinary team review of potential outpatient parenteral antimicrobial therapy prior to discharge from an academic medical center. Ann Pharmacother. 2011;45:1329-1337. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 18. | Kieran J, O'Reilly A, Parker J, Clarke S, Bergin C. Self-administered outpatient parenteral antimicrobial therapy: a report of three years experience in the Irish healthcare setting. Eur J Clin Microbiol Infect Dis. 2009;28:1369-1374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 19. | Lacroix A, Revest M, Patrat-Delon S, Lemaître F, Donal E, Lorléac'h A, Arvieux C, Michelet C, Tattevin P. Outpatient parenteral antimicrobial therapy for infective endocarditis: a cost-effective strategy. Med Mal Infect. 2014;44:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 20. | Malone M, West D, Xuan W, Lau NS, Maley M, Dickson HG. Outcomes and cost minimisation associated with outpatient parenteral antimicrobial therapy (OPAT) for foot infections in people with diabetes. Diabetes Metab Res Rev. 2015;31:638-645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Nathwani D, Barlow GD, Ajdukiewicz K, Gray K, Morrison J, Clift B, France AJ, Davey P. Cost-minimization analysis and audit of antibiotic management of bone and joint infections with ambulatory teicoplanin, in-patient care or outpatient oral linezolid therapy. J Antimicrob Chemother. 2003;51:391-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 22. | Nguyen HH. Hospitalist to home: outpatient parenteral antimicrobial therapy at an academic center. Clin Infect Dis. 2010;51 Suppl 2:S220-S223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 23. | Ruh CA, Parameswaran GI, Wojciechowski AL, Mergenhagen KA. Outcomes and Pharmacoeconomic Analysis of a Home Intravenous Antibiotic Infusion Program in Veterans. Clin Ther. 2015;37:2527-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 24. | Seaton RA, Johal S, Coia JE, Reid N, Cooper S, Jones BL. Economic evaluation of treatment for MRSA complicated skin and soft tissue infections in Glasgow hospitals. Eur J Clin Microbiol Infect Dis. 2014;33:305-311. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 25. | Sims AL, Baker P, Bellamy R, McMurtry IA. Outpatient parenteral antibiotic therapy in primary hip and knee arthroplasty infection managed with debridement and retention of prosthesis: a retrospective cohort study. Surg Infect (Larchmt). 2013;14:293-296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 26. | Theocharis G, Rafailidis PI, Rodis D, Kontopidis I, Barbas SG, Falagas ME. Outpatient parenteral antibiotic therapy (OPAT) at home in Attica, Greece. Eur J Clin Microbiol Infect Dis. 2012;31:2957-2961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Wai AO, Frighetto L, Marra CA, Chan E, Jewesson PJ. Cost analysis of an adult outpatient parenteral antibiotic therapy (OPAT) programme. A Canadian teaching hospital and Ministry of Health perspective. Pharmacoeconomics. 2000;18:451-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 71] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Yong C, Fisher DA, Sklar GE, Li SC. A cost analysis of Outpatient Parenteral Antibiotic Therapy (OPAT): an Asian perspective. Int J Antimicrob Agents. 2009;33:46-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 29. | Parisi TJ, Konopka JF, Bedair HS. What is the Long-term Economic Societal Effect of Periprosthetic Infections After THA? A Markov Analysis. Clin Orthop Relat Res. 2017;475:1891-1900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 103] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 30. | Kurtz SM, Lau E, Schmier J, Ong KL, Zhao K, Parvizi J. Infection burden for hip and knee arthroplasty in the United States. J Arthroplasty. 2008;23:984-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 708] [Cited by in RCA: 763] [Article Influence: 44.9] [Reference Citation Analysis (0)] |
| 31. | Kurtz SM, Ong KL, Schmier J, Zhao K, Mowat F, Lau E. Primary and revision arthroplasty surgery caseloads in the United States from 1990 to 2004. J Arthroplasty. 2009;24:195-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 139] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 32. | Esposito S, Leone S, Noviello S, Ianniello F, Fiore M, Russo M, Foti G, Carpentieri MS, Cellesi C, Zanelli G, Cellini A, Girmenia C, De Lalla F, Maiello A, Maio P, Marranconi F, Sabbatani S, Pantaleoni M, Ghinelli F, Soranzo ML, Vigano P, Re T, Viale P, Scudeller L, Scaglione F, Vullo V. Outpatient parenteral antibiotic therapy for bone and joint infections: an italian multicenter study. J Chemother. 2007;19:417-422. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 43] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 33. | Zeller V, Durand F, Kitzis MD, Lhotellier L, Ziza JM, Mamoudy P, Desplaces N. Continuous cefazolin infusion to treat bone and joint infections: clinical efficacy, feasibility, safety, and serum and bone concentrations. Antimicrob Agents Chemother. 2009;53:883-887. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Muldoon EG, Switkowski K, Tice A, Snydman DR, Allison GM. A national survey of infectious disease practitioners on their use of outpatient parenteral antimicrobial therapy (OPAT). Infect Dis (Lond). 2015;47:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 41] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 35. | Saillen L, Arensdorff L, Moulin E, Voumard R, Cochet C, Boillat-Blanco N, Gardiol C, de Vallière S. Patient satisfaction in an outpatient parenteral antimicrobial therapy (OPAT) unit practising predominantly self-administration of antibiotics with elastomeric pumps. Eur J Clin Microbiol Infect Dis. 2017;36:1387-1392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 40] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 36. | Felder KK, Marshall LM, Vaz LE, Barnes PD. Risk Factors for Complications during Outpatient Parenteral Antimicrobial Therapy for Adult Orthopedic and Neurosurgical Infections. South Med J. 2016;109:53-60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 37. | Le J, San Agustin M, Hernandez EA, Tran TT, Adler-Shohet FC. Complications associated with outpatient parenteral antibiotic therapy in children. Clin Pediatr (Phila). 2010;49:1038-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 38. | Rucker RW, Harrison GM. Outpatient intravenous medications in the management of cystic fibrosis. Pediatrics. 1974;54:358-360. [PubMed] |
| 39. | Minton J, Murray CC, Meads D, Hess S, Vargas-Palacios A, Mitchell E, Wright J, Hulme C, Raynor DK, Gregson A, Stanley P, McLintock K, Vincent R, Twiddy M. The Community IntraVenous Antibiotic Study (CIVAS): a mixed-methods evaluation of patient preferences for and cost-effectiveness of different service models for delivering outpatient parenteral antimicrobial therapy. Southampton. 2017;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 2.0] [Reference Citation Analysis (0)] |