Published online Jul 6, 2019. doi: 10.12998/wjcc.v7.i13.1696
Peer-review started: March 25, 2019
First decision: April 11, 2019
Revised: April 29, 2019
Accepted: May 10, 2019
Article in press: May 11, 2019
Published online: July 6, 2019
Processing time: 104 Days and 21.6 Hours
Gastric adenocarcinoma of fundic gland type (GA-FG) has recently been proposed as a novel histological type of gastric cancer.
We report a case of GA-FG in a 77-year-old Chinese woman with epigastric distention who was referred to endoscopy for the management of an incidentally found submucosal tumor-like elevated lesion in the lower part of the gastric body. The tumor occurred after Helicobacter pylori (H. pylori) eradication therapy without long-term use of proton pump inhibitors. Complete and curable removal of the tumor was performed by endoscopic submucosal dissection. Histopathological findings showed numerous cells with basophilic cytoplasm and mildly atypical nuclei-like chief cells of the fundic gland. The tumor was observed to have the so-called “endless glands” pattern of the well-differentiated mixed phenotype. A safe resection margin without lymphatic and venous invasion was observed. As the tumor occurred after H. pylori eradication therapy, it is unknown whether there was a relationship with H. pylori eradication. The patient will be followed up by periodic gastroscopic observation.
In conclusion, we report a case of GA-FG after H. pylori eradication therapy without long-term proton pump inhibitors use. Further analysis of similar cases will reveal the clinical behavior of GA-FG.
Core tip: Gastric adenocarcinoma of fundic gland type (GA-FG) remains a rare disease despite a recent uptick in diagnoses. Here, we report a case of GA-FG with submucosal tumor-like appearance, located in the lower part of the gastric body. This case report discusses the probability-of-occurrence after Helicobacter pylori eradication therapy without long-term usage of proton pump inhibitors. Complete and curable removal of endoscopic submucosal dissection was performed. Further analysis of similar cases will reveal the clinical behavior of GA-FG.
- Citation: Yu YN, Yin XY, Sun Q, Liu H, Zhang Q, Chen YQ, Zhao QX, Tian ZB. Gastric adenocarcinoma of fundic gland type after Helicobacter pylori eradication: A case report. World J Clin Cases 2019; 7(13): 1696-1702
- URL: https://www.wjgnet.com/2307-8960/full/v7/i13/1696.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i13.1696
Traditionally, gastric carcinoma has been categorized into intestinal and diffuse types using the Lauren classification[1]. However, with progress made in histochemical techniques, it has been shown that gastric adenocarcinoma of the intestinal type comprises the gastric phenotype[2]. It was demonstrated that gastric phenotypes included the foveolar type, pyloric gland type, and fundic gland type. Of these phenotypes, gastric adenocarcinoma of fundic gland type (GA-FG) is a novel histological type of gastric cancer first proposed by Tsukamoto et al[3] in 2007. Based on their composition, the differentiation of characteristic oxyntic glands can be divided into chief cell predominant, parietal cell predominant, and mixed phenotype. Following a report in 2010 that included 10 cases of gastric adenocarcinoma showing chief cell differentiation, the concept of GA-FG was proposed[4], which has since been gradually recognized. However, the demographics, predisposing factors, prognosis, and how GA-FGs differ from conventional carcinomas are unclear. To date, only information on the early stages of GA-FG are available.
We report a patient with GA-FG who was treated with endoscopic submucosal dissection (ESD). This case report discusses the probability-of-occurrence after Helicobacter pylori (H. pylori) eradication without long-term use of proton pump inhibitors (PPIs).
A 77-year-old Chinese woman visited our hospital with epigastric discomfort for more than 4 years and worsened for 1 month.
Her medical history was notable for chronic atrophic gastritis.
She had a longstanding history of chronic atrophic gastritis in January 2014 with gastroscopy. According to the Kimura/Takemoto classification[5], atrophy of the gastric mucosa was classified as C-2. She had received recommended dose of H. pylori eradication therapy twice, once 7 d for omeprazole + amoxicillin + clarithromycin and once 14 d for pantoprazole + bismuthate + tetracycline + metronidazole. She was followed up with annual gastroscopy and had not taken any PPIs since then. In addition, she had a history of myoma of uterus for more than 40 years, without operation.
On physical examination upon admission, she was 1.54 meters in height and 60 kilogram in weight. She had a blood pressure of 117/68 mmHg with pulse rate of 65 beats per minute. Her jugular venous pressure was not elevated. She had clear lungs and normal heart sounds with no murmurs or gallops on auscultation. There were no other pathognomonic signs during physical examination, except for slight tenderness in the upper abdomen.
After admission, the patient was thoroughly evaluated with routine blood tests, routine urine tests, routine fecal tests and occult blood test, blood biochemistry, and infection indexes. No significant abnormal laboratory results were recorded in this patient.
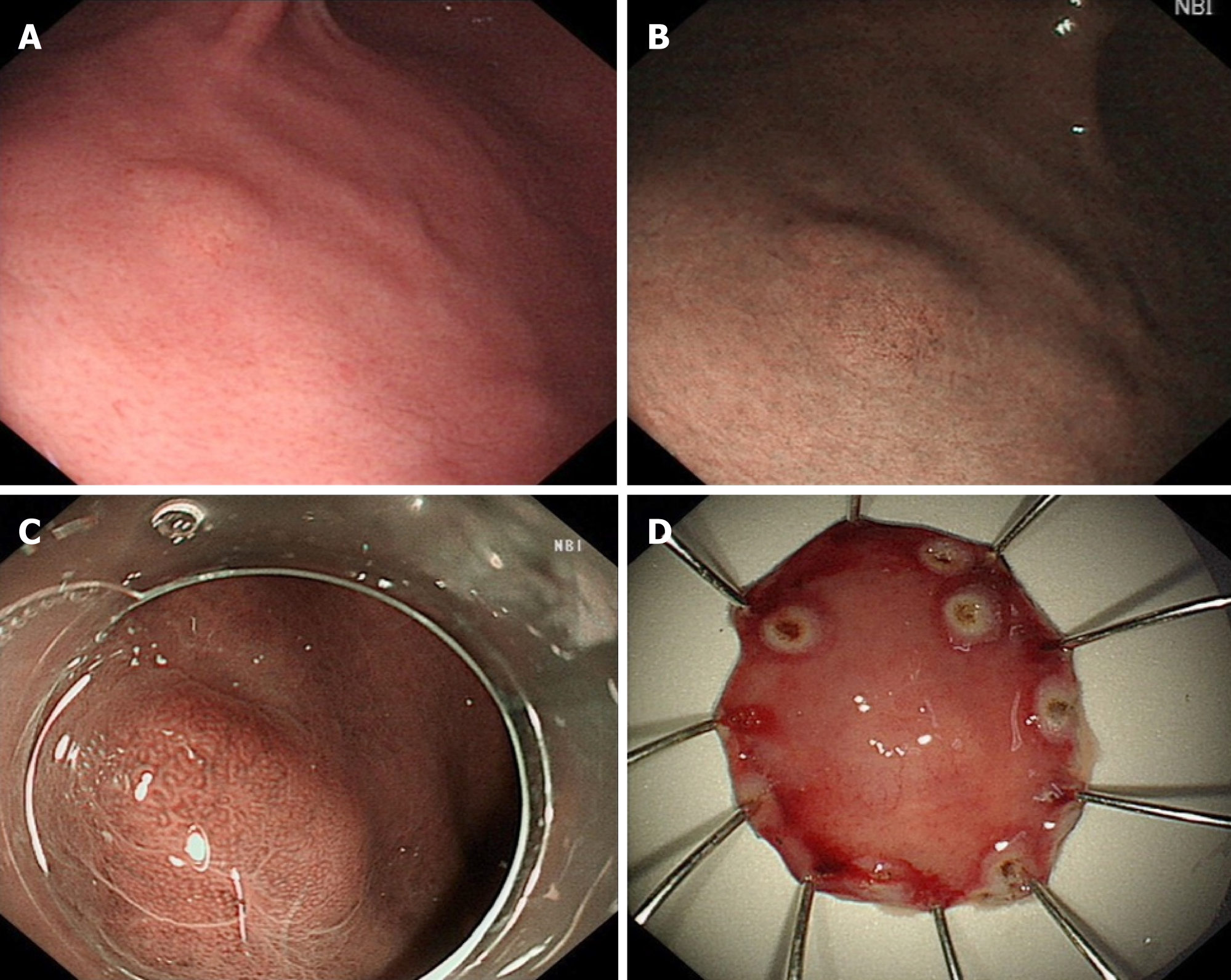
Gastroscopy revealed a 6 mm submucosal tumor (SMT)-like slightly elevated lesion in the anterior wall of the lower part of the gastric body, which had faint yellow discoloration (Figure 1A and B). Atrophy of the gastric mucosa was classified as C-2, yet the local background mucosa of the lesion showed no apparent atrophy, after eradication of H. pylori. No fundus gland polyp (FGP) was found in other sections of the stomach.
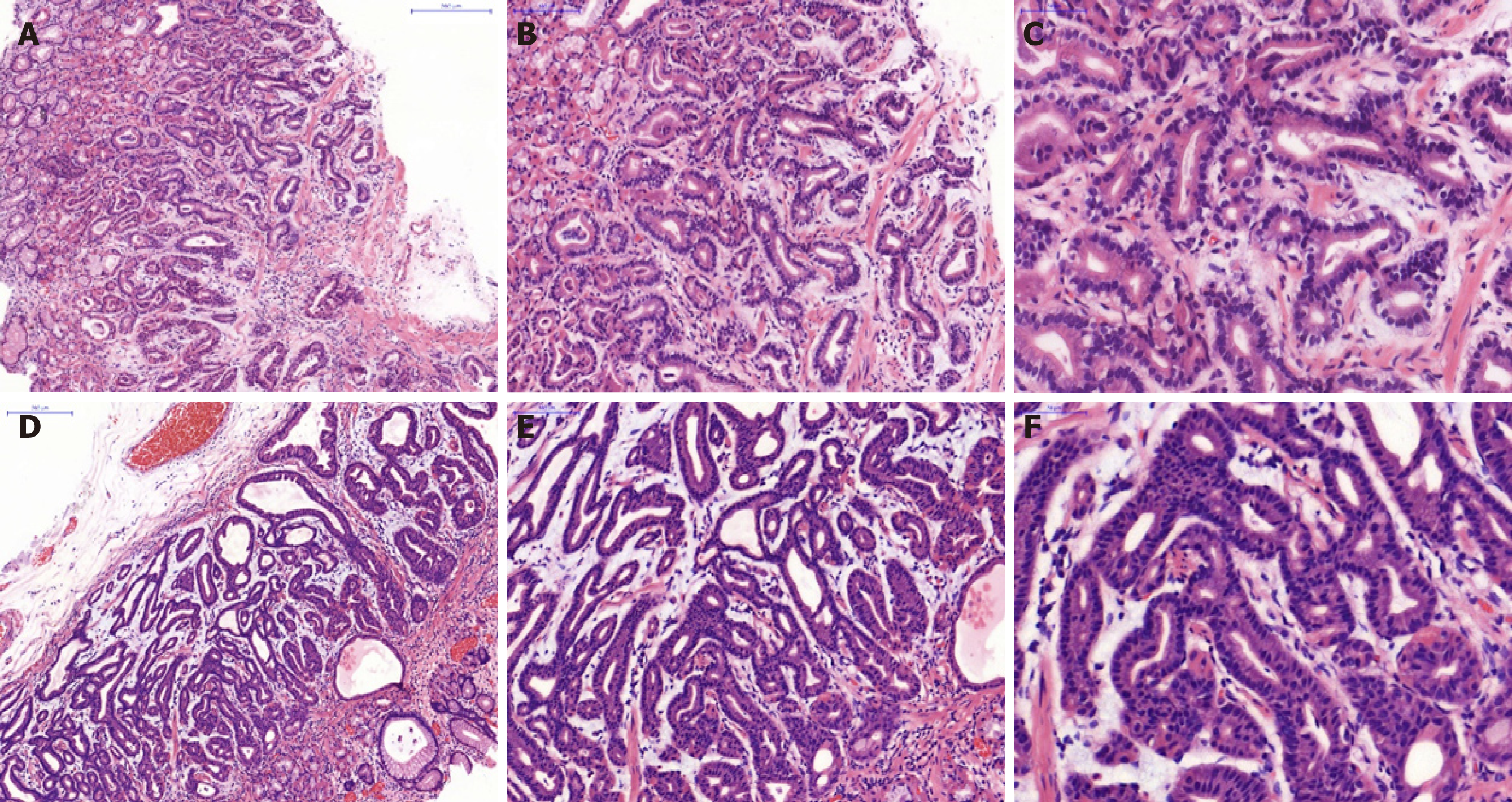
A biopsy of the SMT-like lesion was obtained, and histopathological findings showed numerous cells with basophilic cytoplasm and mildly atypical nuclei-like chief cells of the fundic gland, which were arranged in irregular branching and anastomosing tubules in a layer of the lamina propria mucosae (Figure 2A-C).
Based on endoscopic performance and histopathologic characteristics, GA-FG was diagnosed.
The patient underwent ESD subsequently. To observe the microstructure of the lesion, narrowband imaging with magnifying endoscopy was carried out. It showed an enlarged microvascular pattern and a mildly disordered microsurface pattern especially prominent in the upper part of the SMT-like elevated lesion, with an expected biopsy scar in the lower part (Figure 1C). On assessment of the ESD specimen, a slightly elevated tumor measuring 5 mm × 6 mm was identified in the submucosal layer (Figure 1D).
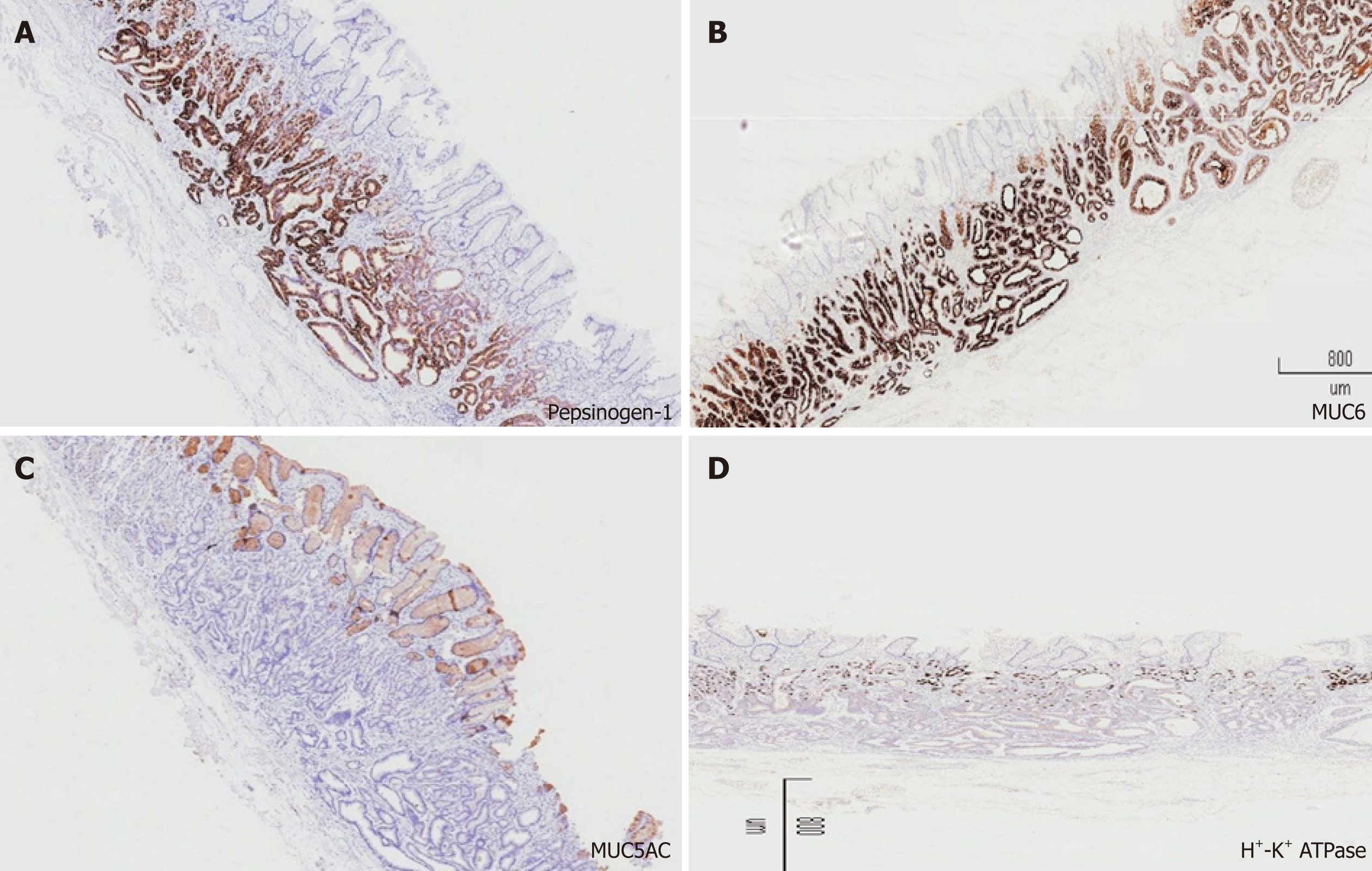
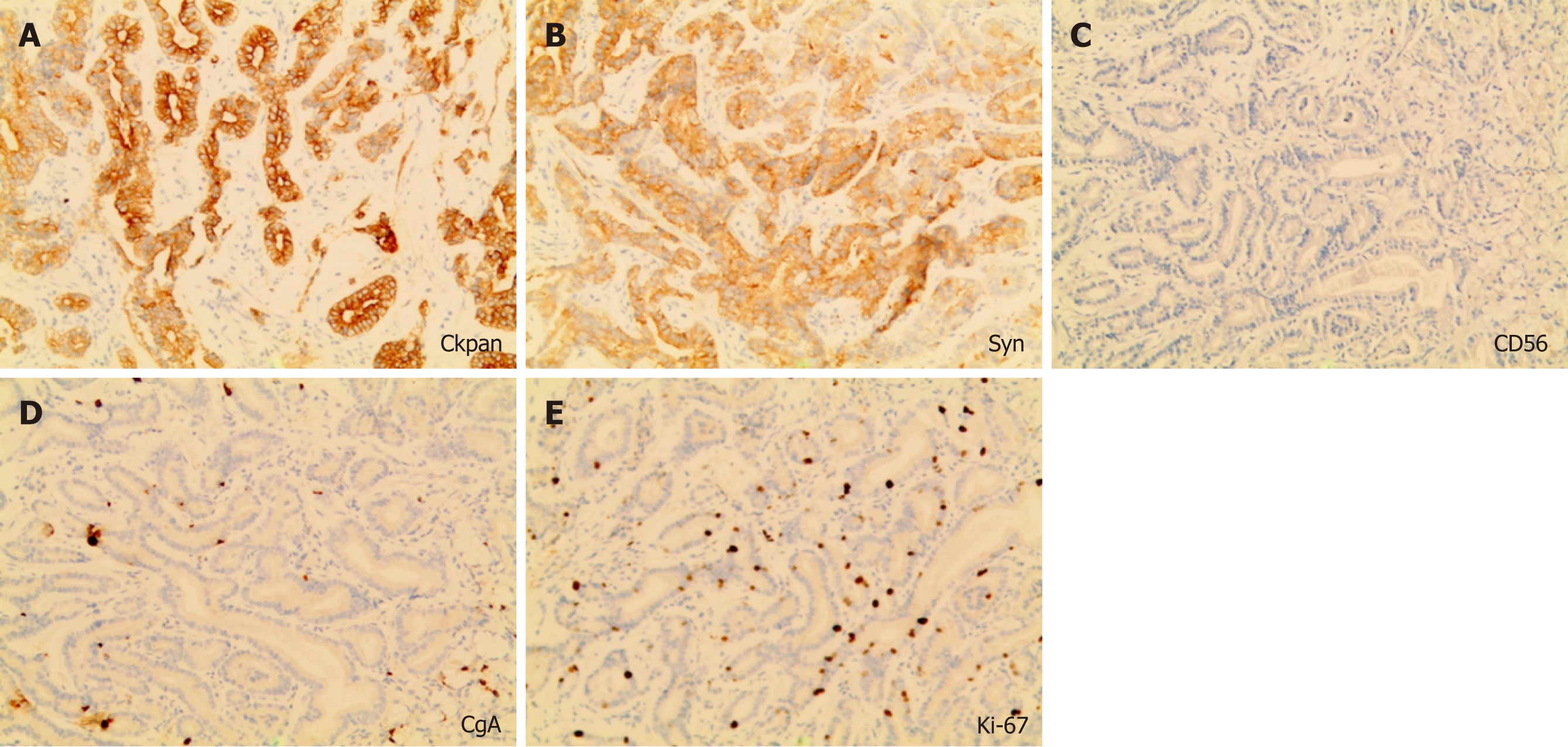
Similar to the histological characteristics, neoplastic glands infiltrating the submucosal layer were observed (Figure 2D-F). On immunohistochemical (IHC) examination, the tumor was strongly and diffusely reactive for pepsinogen-I and mucin 6, with scattered expression of H+/K+-ATPase but negative for MUC5AC (Figure 3), and the Ki-67 labeling index was 4% (Figure 4). The IHC staining results were consistent with the diagnosis of GA-FG with predominantly chief cell differentiation. Resection margin was safe without lymphatic and venous invasion, resulting in curative resection. No atrophic changes or intestinal metaplasia in the background mucosa of the ESD specimen were observed.
Complete and curable removal by ESD was performed. The patient has required regular clinic and gastroscopy follow up. She has remained stable.
GA-FG has been characterized as a new histological type of gastric cancer in recent years. Since Tsukamoto et al[3] first described a case of GA-FG in 2007, multiple cases of this disease have been reported. Most of these cases have been reported in Japan, with only a few reported in Western countries, suggesting that GA-FG is uncommon or there is a lack of awareness of this tumor. The carcinogenesis of GA-FG is still unclear. Benedict et al[6] reported 111 cases of GA-FG to highlight the key features and controversies associated with this uncommon neoplasm. The overwhelming majority of GA-FGs (approximately 80%) have occurred in the upper third of the stomach, followed by the middle third (18%), and only one tumor has been found in the lower third of the stomach (1%)[7-25]. In our patient, the GA-FG was localized in the lower part of the gastric body (i.e. the middle third of the stomach).
Of the 111 cases of GA-FG published, 40% cases were H. pylori positive from only 39% available data[6]. So, the presence or not of H. pylori does not seem to be relevant. Although GA-FG is generally thought related to non-atrophic mucosa and H. pylori negative, it has been reported to occur with a history of H. pylori eradication[9]. Our patient had a history of H. pylori eradication therapy twice, which supported this viewpoint. In addition, the background mucosa of GA-FG after eradication was endoscopically and pathologically free of atrophy or intestinal metaplasia. In a series of 12 cases reported, 7 of the 8 cases with available history of medication had used acid suppressive therapy (six PPI and one H2 blocker)[11]. However, our patient had no history of long-term medication of any PPIs and had no FGP in other sections of her stomach. It is unclear whether there was a relationship with PPIs use.
GA-FGs are often small, with an average size of 10 mm[7]. They can be elevated, flat, depressed, SMT-like or mimic a gastric FGP, and tend to be white or yellow in color, with branching dilated mucosal vessels in the non-atrophic adjacent mucosa[8,9,26]. The use of narrowband imaging enhances visualization of the irregular pattern of mucosal vessels, suggestive of the heterogeneity of the microvessels in GA-FG. Diagnosis is dependent on biopsy and pathology. Differential diagnosis is also important with other conditions such as neuroendocrine tumors, gastric SMT, and FGPs.
The histologic appearance of GA-FG is often a well-differentiated neoplasm, arising directly from the gastric mucosa, with resemblance to the fundic glands. The following three subcategories have been identified: Chief cell predominant pattern, parietal cell predominant pattern, or a mixture of both cell types. The lesion invariably shows the so-called “endless glands” pattern, which is the principal pathological feature of GA-FG. Most GA-FGs have mild cytologic atypia and slightly enlarged nuclei. The Ki-67 index is usually lower than 5%[8]. Typically, the adjacent mucosa is normal without atrophy or intestinal metaplasia[10]. When a pathologist is aware of this tumor, recognition of the so-called “endless glands” pattern in the well-differentiated mixed phenotypes is easy following hematoxylin and eosin staining. Some IHC markers can be helpful in identifying lineage differentiation, including pepsinogen-I, MIST1, H+/K+-ATPase, and mucin 6. To date, a lack of awareness of GA-FG in the pathology community and few available markers have resulted in diagnostic difficulties.
GA-FG is a less aggressive and slow-growing neoplasm with a favorable prognosis because of their low cell proliferation and cellular atypia. These tumors tend to invade the submucosa, lymphovascular invasion is infrequent, and follow a benign course. Tumors with superficial submucosal invasion can be treated with endoscopic mucosal resection, ESD, or limited gastric resection[6]. For deeply invasive tumors or suspected nodal metastasis, extended gastrectomy with lymph node dissection should be performed.
In our patient, the SMT-like lesion was located in the lower part of the gastric body. She was previously H. pylori positive and had received eradication therapy twice. In addition, she was followed up with annual gastroscopy and had not taken PPIs for 4 years. The background mucosa of the tumor was H. pylori negative without atrophy or intestinal metaplasia. As the tumor occurred after H. pylori eradication therapy, it is unclear whether there was a relationship with H. pylori eradication. Complete and curable removal by ESD was performed in this patient. According to the current understanding and a comprehensive literature analysis, the patient will be followed up with periodic gastroscopic observation.
GA-FG remains a rare disease despite a recent uptick in diagnoses owing to increased physician suspicion and improved endoscopic techniques. Here, we report a case of GA-FG with SMT-like appearance, located in the lower part of the gastric body. This case report discusses the probability-of-occurrence of GA-FG after H. pylori eradication therapy without long-term usage of PPIs. Treatment consisted of ESD only. Further analysis of similar cases will reveal the clinical behavior of GA-FG.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P-Reviewer: Koseoglu RD, Kopljar M, Lobo MDT, Park WS, Slomiany BL, Viola LA S-Editor: Ji FF L-Editor: Filipodia E-Editor: Xing YX
| 1. | Lauren P. The two histological main types of gastric carcinoma: diffuse and so-called intestinal-type carcinoma. an attempt at a histo-clinical classification. Acta Pathol Microbiol Scand. 1965;64:31-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4011] [Cited by in RCA: 4323] [Article Influence: 149.1] [Reference Citation Analysis (0)] |
| 2. | Kabashima A, Yao T, Sugimachi K, Tsuneyoshi M. Gastric or intestinal phenotypic expression in the carcinomas and background mucosa of multiple early gastric carcinomas. Histopathology. 2000;37:513-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Tsukamoto T, Yokoi T, Maruta S, Kitamura M, Yamamoto T, Ban H, Tatematsu M. Gastric adenocarcinoma with chief cell differentiation. Pathol Int. 2007;57:517-522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 67] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 4. | Ueyama H, Yao T, Nakashima Y, Hirakawa K, Oshiro Y, Hirahashi M, Iwashita A, Watanabe S. Gastric adenocarcinoma of fundic gland type (chief cell predominant type): proposal for a new entity of gastric adenocarcinoma. Am J Surg Pathol. 2010;34:609-619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 161] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 5. | Kimura K, Takemoto T. An endoscopic recognition of the atrophic border and its significance in chronic gastritis. Endoscopy. 1969;1:87-97. [RCA] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 743] [Article Influence: 43.7] [Reference Citation Analysis (3)] |
| 6. | Benedict MA, Lauwers GY, Jain D. Gastric Adenocarcinoma of the Fundic Gland Type: Update and Literature Review. Am J Clin Pathol. 2018;149:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 49] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 7. | Nomura R, Saito T, Mitomi H, Hidaka Y, Lee SY, Watanabe S, Yao T. GNAS mutation as an alternative mechanism of activation of the Wnt/β-catenin signaling pathway in gastric adenocarcinoma of the fundic gland type. Hum Pathol. 2014;45:2488-2496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 64] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 8. | Tohda G, Osawa T, Asada Y, Dochin M, Terahata S. Gastric adenocarcinoma of fundic gland type: Endoscopic and clinicopathological features. World J Gastrointest Endosc. 2016;8:244-251. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 17] [Cited by in RCA: 22] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Chiba T, Kato K, Masuda T, Ohara S, Iwama N, Shimada T, Shibuya D. Clinicopathological features of gastric adenocarcinoma of the fundic gland (chief cell predominant type) by retrospective and prospective analyses of endoscopic findings. Dig Endosc. 2016;28:722-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 36] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Ueyama H, Matsumoto K, Nagahara A, Hayashi T, Yao T, Watanabe S. Gastric adenocarcinoma of the fundic gland type (chief cell predominant type). Endoscopy. 2014;46:153-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 48] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 11. | Chan K, Brown IS, Kyle T, Lauwers GY, Kumarasinghe MP. Chief cell-predominant gastric polyps: a series of 12 cases with literature review. Histopathology. 2016;68:825-833. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Fukatsu H, Miyoshi H, Ishiki K, Tamura M, Yao T. Gastric adenocarcinoma of fundic gland type (chief cell predominant type) treated with endoscopic aspiration mucosectomy. Dig Endosc. 2011;23:244-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 22] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Sato Y, Fujino T, Kasagawa A, Morita R, Ozawa SI, Matsuo Y, Maehata T, Yasuda H, Takagi M, Itoh F. Twelve-year natural history of a gastric adenocarcinoma of fundic gland type. Clin J Gastroenterol. 2016;9:345-351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 14. | Takeda S, Mitoro A, Namisaki T, Yoshida M, Sawai M, Yamao J, Yoshiji H, Uejima M, Moriya K, Douhara A, Seki K, Ishida K, Morita K, Noguchi R, Kitade M, Kawaratani H, Okura Y, Takaya H, Fukui H. Gastric adenocarcinoma of fundic gland type (chief cell predominant type) with unique endoscopic appearance curatively treated by endoscopic submucosal resection. Acta Gastroenterol Belg. 2015;78:340-343. [PubMed] |
| 15. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of fundic gland type: Five cases treated with endoscopic resection. World J Gastroenterol. 2015;21:8208-8214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 30] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 16. | Kushima R, Sekine S, Matsubara A, Taniguchi H, Ikegami M, Tsuda H. Gastric adenocarcinoma of the fundic gland type shares common genetic and phenotypic features with pyloric gland adenoma. Pathol Int. 2013;63:318-325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 17. | Ueo T, Yonemasu H, Ishida T. Gastric adenocarcinoma of fundic gland type with unusual behavior. Dig Endosc. 2014;26:293-294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 18. | Terada T. Well differentiated adenocarcinoma of the stomach composed of chief cell-like cells and parietal cells (Gastric adenocarcinoma of fundic gland type). Int J Clin Exp Pathol. 2011;4:797-798. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 19. | Singhi AD, Lazenby AJ, Montgomery EA. Gastric adenocarcinoma with chief cell differentiation: a proposal for reclassification as oxyntic gland polyp/adenoma. Am J Surg Pathol. 2012;36:1030-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 76] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 20. | Parikh ND, Gibson J, Aslanian H. Gastric Fundic Gland Adenocarcinoma With Chief Cell Differentiation. Clin Gastroenterol Hepatol. 2015;13:A17-A18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 21. | Cha HJ, Kim K, Kim M, Choi H, Kim YM, Suh JH. Concurrent Gastric Adenocarcinoma of Fundic Gland Type and Carcinoma with Lymphoid Stroma: A Rare Case Report. Case Rep Gastroenterol. 2016;10:292-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Kato M, Uraoka T, Isobe Y, Abe K, Hirata T, Takada Y, Wada M, Takatori Y, Takabayashi K, Fujiyama Y, Sekiya K, Kawaguchi Y, Sukeda A, Shiraishi J. A case of gastric adenocarcinoma of fundic gland type resected by combination of laparoscopic and endoscopic approaches to neoplasia with non-exposure technique (CLEAN-NET). Clin J Gastroenterol. 2015;8:393-399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Abe T, Nagai T, Fukunaga J, Okawara H, Nakashima H, Syutou M, Kajimoto N, Wake R, Oyama T, Yao T. Long-term follow-up of gastric adenocarcinoma with chief cell differentiation using upper gastrointestinal tract endoscopy. Intern Med. 2013;52:1585-1588. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Chen WC, Rodriguez-Waitkus PM, Barroso A, Balsaver A, McKechnie JC. A Rare Case of Gastric Fundic Gland Adenocarcinoma (Chief Cell Predominant Type). J Gastrointest Cancer. 2012;43 Suppl 1:S262-S265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 25. | Fujisawa T, Takata M, Ouchi S, Ueyama S, Nakajima T, Mitsutsuji M, Kawamura T, Toyoda M, Okamura A. Intra-abdominal plexiform neurofibromatosis including periportal, mesentery, and gastrointestinal tract involvement in neurofibromatosis type 1: case report and review of the literature. Clin J Gastroenterol. 2011;4:292-297. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 26. | Miyazawa M, Matsuda M, Yano M, Hara Y, Arihara F, Horita Y, Matsuda K, Sakai A, Noda Y. Gastric adenocarcinoma of the fundic gland (chief cell-predominant type): A review of endoscopic and clinicopathological features. World J Gastroenterol. 2016;22:10523-10531. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |