Published online Jun 6, 2019. doi: 10.12998/wjcc.v7.i11.1344
Peer-review started: December 29, 2018
First decision: March 10, 2019
Revised: April 9, 2019
Accepted: May 1, 2019
Article in press: May 2, 2019
Published online: June 6, 2019
Processing time: 161 Days and 5.8 Hours
Pancreatic liposarcoma is a rare tumor. According to a literature review, the patient described in this study is the seventh case of pancreatic liposarcoma reported in the English literature and the third case of dedifferentiated liposarcoma. Furthermore, this case had the largest primary tumor volume, and a primary pancreatic liposarcoma was diagnosed based on sufficient evidence.
We here report a rare case of a 28-year-old female with a huge dedifferentiated liposarcoma in the pancreatic tail. In June 2015, the patient underwent distal pancreatectomy with splenectomy. During the operation, a huge liposarcoma of approximately 28.0 cm × 19.0 cm × 8.0 cm was found, which had a yellow and white fish-like incisal surface. Based on both pathology and MDM2 gene amplification, the tumor was diagnosed as a dedifferentiated liposarcoma. The patient was treated with surgery but declined postoperative chemotherapy. She was well at the 26-mo follow-up, and no relapse was observed.
Pancreatic liposarcoma has a low incidence. Chemotherapy should be included in the treatment regimens. Complete resection is the only effective treatment.
Core tip: Pancreatic liposarcoma is a rare tumor. We report a case of a 28-year-old female with a huge dedifferentiated liposarcoma in the pancreatic tail, 28.0 cm × 19.0 cm × 8.0 cm in size, with a yellow and white fish-like incisal surface. According to a literature review, this is the seventh case of pancreatic liposarcoma reported in the English literature and the third case of dedifferentiated liposarcoma. Furthermore, this case had the largest primary tumor volume, and a primary pancreatic liposarcoma was diagnosed based on sufficient evidence. The patient was treated with surgery but declined postoperative chemotherapy. She was well at the 26-mo follow-up, without relapse.
- Citation: Liu Z, Fan WF, Li GC, Long J, Xu YH, Ma G. Huge primary dedifferentiated pancreatic liposarcoma mimicking carcinosarcoma in a young female: A case report. World J Clin Cases 2019; 7(11): 1344-1350
- URL: https://www.wjgnet.com/2307-8960/full/v7/i11/1344.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i11.1344
Based on molecular morphology, liposarcoma can be divided into well-diffe-rentiated/dedifferentiated liposarcoma, myxoid/round cell liposarcoma, and pleomorphic liposarcoma[1-3]. For dedifferentiated liposarcoma, it is thought that a malignant non-fat sarcomatous region exists in the well-differentiated liposarcoma area at the same time. To date, six cases of pancreatic liposarcoma have been reported in the English literature[4-9]. In the present study, a patient with a huge dedifferentiated liposarcoma in the pancreatic tail is reported. Pathology after operation revealed coexisting areas of well-differentiated liposarcoma, dedifferentiated liposarcoma, and the tissue of pancreatic canal. MDM2 gene amplification by fluorescence in situ hybridization showed a dedifferentiated liposarcoma that did not originate from the retroperitoneum.
A 28-year-old female presented with left upper abdominal discomfort with nausea and vomiting for more than 2 mo and fever for 2 wk.
The patient developed left epigastric discomfort with postprandial nausea and vomiting 2 mo previously without obvious predilection, the vomit consisted of stomach contents and abdominal distension was relieved after vomiting. She attended a local hospital for gastroscopy and was diagnosed with chronic atrophic gastritis, but no significant improvement was achieved after symptomatic treatment including acid inhibition and gastric preservation. She also had fever and chills for 2 wk, and as her temperature reached 39 °C, she was sent to the People's Liberation Army 202 Hospital, where an abdominal mass was found during computed tomography (CT) examination. The patient was then transferred to our hospital for further diagnosis and treatment. She was mentally stable, with a poor diet and poor sleep, with no obvious defecation and urinary abnormalities, and her weight loss was approximately 8 kg.
None.
None.
The sclera showed no yellow staining, and no bleeding spots or petechiae were observed in the skin mucosa. No liver palm or spider mole was present, superficial lymph nodes were not palpable, the left epigastrium was slightly distended, gastrointestinal peristaltic wave was not observed, the abdomen was soft without tenderness, Murphy’s sign was negative, and the liver and spleen were not palpable under the rib. The mass was palpable in the left epigastric region and was approximately 15 cm in diameter. There was no muscle tension or rebound pain, no percussion pain in the liver and spleen area, shifting dullness was negative, and bowel sounds were 3-4 times/min, with no smell, air water sound, or high-pitched bowel sounds.
Carcinoembryonic antigen was 0.76 ng/mL, alpha-fetoprotein was 2.14 ng/mL, cancer antigen 12-5 was 45.40 U/mL, carbohydrate antigen 19-9 was 6.13 U/mL, total bilirubin was 8.80 µmol/L, and direct bilirubin was 4.70 µmol/L.
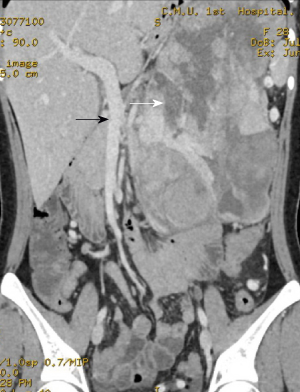
The enhanced pancreatic 3D-CT images suggested a huge mass in the left upper abdomen with low density and a maximum cross-sectional area of 14.0 cm × 18.0 cm. The plain CT value was 16-24 HU.
Multiple vascular shadows were observed in the arterial phase, and the enhanced CT value was 60-95 HU in the lag period. Compressed and tortuous superior and inferior mesenteric arteries and veins were observed. A pancreatic tumor was considered, and pancreatic carcinosarcoma was not excluded (Figure 1).
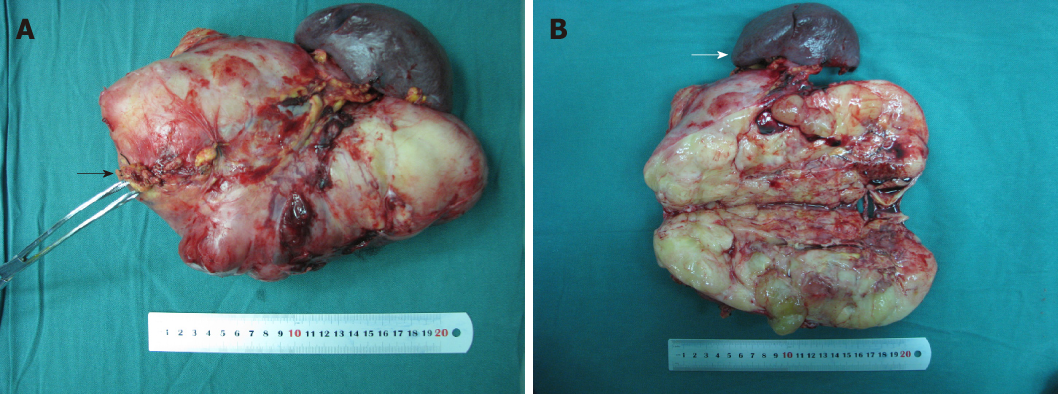
Preoperatively, pancreatic carcinosarcoma was suspected, and the patient underwent surgery. Intra-operatively, the transverse colon, stomach, and jejunum were compressed by the huge tumor, which had an irregular shape, an acceptable boundary, and a clear boundary with the retroperitoneum, suggesting that the tumor was originated from the pancreatic tail. Complete resection of the pancreatic tail was performed. A mass with a proximal margin of 1.0 cm of normal pancreatic tissue was removed (Figure 2A). The inside of the mass was fish-like in texture and yellow-white. The distal pancreatic tissue was normal. Morphology inside the tumor showed no normal tissue (Figure 2B). Thus, a pancreatic source was considered and not retroperitoneal encapsulated pancreas as suggested by visual observation.
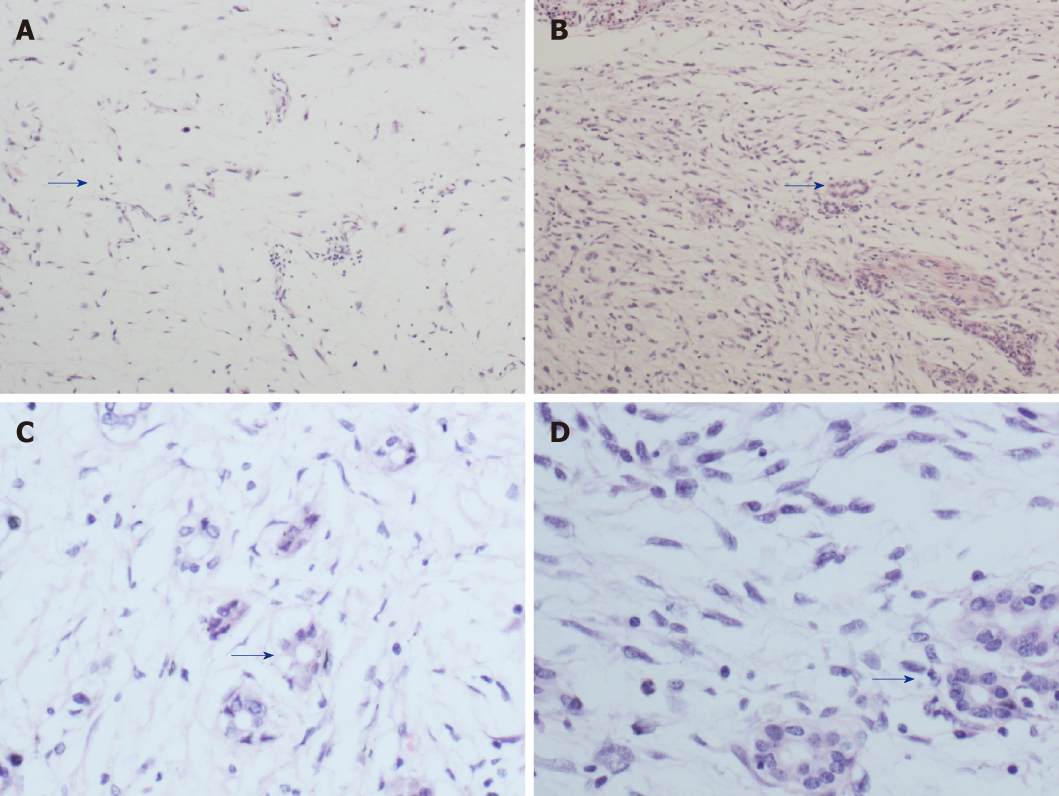
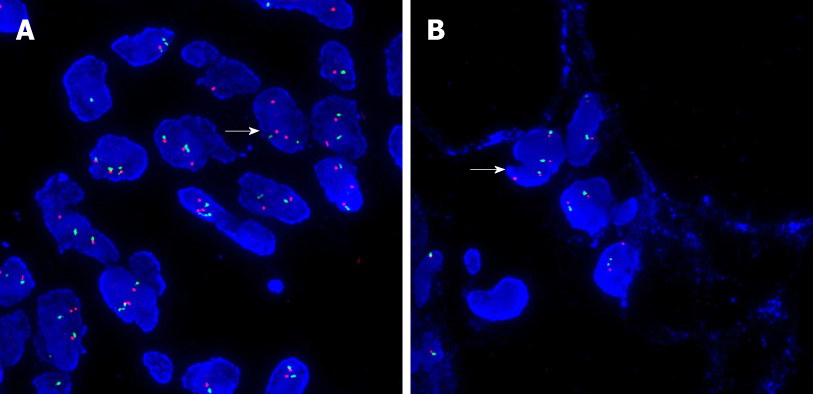
Postoperative pathology showed well-differentiated adipose tissue. Various cell sizes and shapes were observed with different trachychromatic atypia nuclei and a coexisting pancreatic canal (Figures 3A, C). Spindle cell hyperplasia was also observed when the coexisting area of undifferentiated liposarcoma and pancreatic canal were examined (Figures 3B, D). The pancreatic source was microscopically verified. Immunohistochemistry results showed the following: CK (-), vimentin (+), CD34 (+), CD117 (±), smooth muscle actin (-), S-100 (±), Dog-1 (±), CD68 (+), Desmin (-), MyoD1 (focus+), Bcl-2 (+), Beta-catenin (-), and Ki-67 (25%+). In order to verify whether the tumor was an undifferentiated liposarcoma, had a pancreatic source, and local infiltration and metastasis were present, MDM2 gene amplification of the mass and adjacent normal retroperitoneal tissue was performed. MDM2 gene amplification of the mass was positive (Figure 4A), while that of adjacent retroperitoneal tissue was negative (Figure 4B). This reconfirmed that the mass was pancreatic in origin and was a dedifferentiated liposarcoma.
Primary dedifferentiated pancreatic liposarcoma.
Surgery.
The patient declined chemotherapy, but was well at her 26-mo follow-up, without relapse.
Dedifferentiated liposarcoma is a frequent type of liposarcoma that can occur in any part of the body[10]. It mainly occurs in the head, neck, limbs, and retroperitoneum[11-14]. Studies have shown that it amplifies to the 12q13-15 area[15]. Genes such as MDM2 and CDK4 are included in this area, with MDM2 the most constantly expressed gene[16-19]. Therefore, high expression of MDM2 plays a vital role in diagnosing dedifferentiated liposarcoma. Although liposarcoma is a common tumor, pancreatic liposarcoma is rare. To date, there are only six cases of primary pancreatic liposarcoma reported in the English literature[4-9] (Table 1). According to a review of the existing literature, it is essential to confirm whether the tumor is a differentiated liposarcoma and to assess whether it originates from the pancreas. We suggest that this tumor can be diagnosed according to the following aspects: (1) Preoperative CT revealed pancreatic occupancy with an iconographic “interspace” in the retroperitoneum, stomach, colon, and small intestine; (2) A mass located in the pancreas or a pancreatic source was found during surgery, which could be completely cut out by blunt and sharp dissection; (3) Post-operative microscopic examination detected the co-existing area of the pancreatic canal, a well-differentiated liposarcoma, and undifferentiated liposarcoma; (4) MDM2 gene amplification by fluorescence in situ hybridization examination confirmed that the mass had a positive amplification, and the retroperitoneum or other control tissues had a negative amplification; and (5)There was no evidence of liposarcoma at the other sites.
| First author | Age/sex | Symptoms | Yr | Liposarcoma subtype | Liposarcoma size | Treatment | Outcome | Evidence of pancreatic origin |
| Elliott | 59/F | Abdominal distension | 1980 | Pleomorphic | 16 cm | DP | 6 yr | Surgery |
| Dodo | 76/M | Abdominal pain | 2005 | Well differentiated with area of de-differentiation | 9 cm | DP | 26 mo | Surgery |
| Kuramoto | 24/M | Abdominal distension | 2013 | Myxoid | 25 cm | MP | 44 mo | Surgery |
| Machado | 42/M | Abdominal pain | 2016 | Dedifferenti-ated with high grade components | 6.8 cm | DP | 5 yr | Intrapancreatic |
| Matthews | 65/F | None | 2016 | Well differentiated | 4 cm | DP | none | Intrapancreatic + adjacent retroperitoneal MDM2 FISH |
| Han | 29/F | None | 2017 | Dedifferenti-ated | 20 cm | DP | 1 yr | Surgery |
| Present case | 28/F | Abdominal pain | Dedifferenti-ated with high grade components | 28 cm | DP | 26 mo | Surgery + histology + adjacent retroperitoneal MDM2 FISH |
Most cases are diagnosed via surgery combined with pathology. One of the cases in the literature was diagnosed by positive amplification of MDM2. However, from the comprehensive review, the diagnosis lacked evidence, which is the key in diagnosing this disease. In addition, we did not exclude the pancreatic source according to preoperative CT, and the first histological report revealed a pancreatic liposarcoma. We revised the diagnosis following MDM2 detection and re-evaluated the microscopic hematoxylin and eosin staining. In all seven cases, it was found that the tumor may be derived from the pancreatic matrix, and evidence of a pancreatic source is not enough. This tumor has a poor prognosis when the onset site is the pancreatic tail. Therefore, post-operative chemotherapy was suggested for the patient in the present study with gemcitabine and cis-platinum, but it was declined. The patient was well at the 26-mo follow-up, without relapse. We conclude that this tumor has a low incidence. When a patient is diagnosed with liposarcoma, chemotherapy should be included in the treatment regimen. Complete resection is the only effective treatment.
Manuscript source: Invited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Dasgupta S, Neri V S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Mankin HJ, Mankin KP, Harmon DC. Liposarcoma: a soft tissue tumor with many presentations. Musculoskelet Surg. 2014;98:171-177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 4] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 2. | Dei Tos AP. Liposarcomas: diagnostic pitfalls and new insights. Histopathology. 2014;64:38-52. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 121] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 3. | Mullinax JE, Zager JS, Gonzalez RJ. Current diagnosis and management of retroperitoneal sarcoma. Cancer Control. 2011;18:177-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 4. | Matthews M, Nelson S, Hari D, French S. Well differentiated liposarcoma, sclerosing type, of the pancreas a case report. Exp Mol Pathol. 2016;101:320-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Elliott TE, Albertazzi VJ, Danto LA. Pancreatic liposarcoma: case report with review of retroperitoneal liposarcomas. Cancer. 1980;45:1720-1723. [PubMed] |
| 6. | Dodo IM, Adamthwaite JA, Jain P, Roy A, Guillou PJ, Menon KV. Successful outcome following resection of a pancreatic liposarcoma with solitary metastasis. World J Gastroenterol. 2005;11:7684-7685. [PubMed] |
| 7. | Kuramoto K, Hashimoto D, Abe S, Chikamoto A, Beppu T, Iyama K, Baba H. Education and imaging. Hepatobiliary and pancreatic: large pancreatic liposarcoma. J Gastroenterol Hepatol. 2013;28:1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Machado MC, Fonseca GM, de Meirelles LR, Zacchi FF, Bezerra RO. Primary liposarcoma of the pancreas: A review illustrated by findings from a recent case. Pancreatology. 2016;16:715-718. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Han T, Luan Y, Xu Y, Yang X, Li J, Liu R, Li Q, Zheng Z. Successful treatment of advanced pancreatic liposarcoma with apatinib: A case report and literature review. Cancer Biol Ther. 2017;18:635-639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 10. | Gyorki DE, Brennan MF. Management of recurrent retroperitoneal sarcoma. J Surg Oncol. 2014;109:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 11. | Zhu H, Sun J, Wei S, Wang D, Brandwein M. Well-Differentiated Laryngeal/Hypopharyngeal Liposarcoma in the MDM2 Era Report of Three Cases and Literature Review. Head Neck Pathol. 2017;11:146-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 12. | Arvinius C, Torrecilla E, Beano-Collado J, García-Coiradas J, García-Maroto R, Puerto-Vázquez M, Cebrián-Parra JL. A clinical review of 11 cases of large-sized well-differentiated liposarcomas. Eur J Orthop Surg Traumatol. 2017;27:837-841. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Moreau LC, Turcotte R, Ferguson P, Wunder J, Clarkson P, Masri B, Isler M, Dion N, Werier J, Ghert M, Deheshi B; Canadian Orthopaedic Oncology Society (CANOOS). Myxoid\round cell liposarcoma (MRCLS) revisited: an analysis of 418 primarily managed cases. Ann Surg Oncol. 2012;19:1081-1088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 14. | Gronchi A, Collini P, Miceli R, Valeri B, Renne SL, Dagrada G, Fiore M, Sanfilippo R, Barisella M, Colombo C, Morosi C, Stacchiotti S, Casali PG, Dei Tos AP, Pilotti S. Myogenic differentiation and histologic grading are major prognostic determinants in retroperitoneal liposarcoma. Am J Surg Pathol. 2015;39:383-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 86] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 15. | Ortega P, Suster D, Falconieri G, Zambrano E, Moran CA, Morrison C, Suster S. Liposarcomas of the posterior mediastinum: clinicopathologic study of 18 cases. Mod Pathol. 2015;28:721-731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 16. | Hostein I, Pelmus M, Aurias A, Pedeutour F, Mathoulin-Pélissier S, Coindre JM. Evaluation of MDM2 and CDK4 amplification by real-time PCR on paraffin wax-embedded material: a potential tool for the diagnosis of atypical lipomatous tumours/well-differentiated liposarcomas. J Pathol. 2004;202:95-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 81] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 17. | Tamborini E, Della Torre G, Lavarino C, Azzarelli A, Carpinelli P, Pierotti MA, Pilotti S. Analysis of the molecular species generated by MDM2 gene amplification in liposarcomas. Int J Cancer. 2001;92:790-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Coindre JM, Hostein I, Maire G, Derré J, Guillou L, Leroux A, Ghnassia JP, Collin F, Pedeutour F, Aurias A. Inflammatory malignant fibrous histiocytomas and dedifferentiated liposarcomas: histological review, genomic profile, and MDM2 and CDK4 status favour a single entity. J Pathol. 2004;203:822-830. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 92] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 19. | Coindre JM, Mariani O, Chibon F, Mairal A, De Saint Aubain Somerhausen N, Favre-Guillevin E, Bui NB, Stoeckle E, Hostein I, Aurias A. Most malignant fibrous histiocytomas developed in the retroperitoneum are dedifferentiated liposarcomas: a review of 25 cases initially diagnosed as malignant fibrous histiocytoma. Mod Pathol. 2003;16:256-262. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 159] [Article Influence: 7.2] [Reference Citation Analysis (0)] |