Published online Jun 6, 2019. doi: 10.12998/wjcc.v7.i11.1291
Peer-review started: March 4, 2019
First decision: April 18, 2019
Revised: April 26, 2019
Accepted: May 1, 2019
Article in press: May 2, 2019
Published online: June 6, 2019
Processing time: 99 Days and 9.5 Hours
Cardiogenic shock (CS) secondary to acute myocardial infarction (AMI) complicates management of the condition, and often leads to poor prognosis. Prompt and accurate monitoring of cardiovascular and accompanying hemodynamic changes is crucial in achieving adequate management of the condition. Advances in technology has availed procedures such as pulse index continuous cardiac output (PiCCO), which can offer precise monitoring of cardiovascular functions and hemodynamic parameters. In this study, PiCCO is evaluated for its potential utility in improving management and clinical outcomes among elderly patients with AMI complicated by CS.
To assess whether use of the PiCCO system can improve clinical outcomes in elderly patients with AMI complicated by CS.
Patients from emergency intensive care units (EICU) or coronary care units (CCU) were randomized to receive PiCCO monitoring or not. The APACHE II score, SOFA score, hs-TnI, NT-proBNP, PaO2/FiO2 ratio and lactate levels on day 1, 3 and 7 after treatment were compared. The infusion and urine volume at 0-24 h, 24-48 h and 48-72 h were recorded, as were the cardiac index (CI), extravascular lung water index (EVLWI), intrathoracic blood volume index (ITBVI) and global end diastolic volume index (GEDVI) at similar time intervals.
Sixty patients with AMI complicated by CS were included in the study. The PiCCO group had a significantly lower APACHE II score, SOFA score, hs-TnI and NT-proBNP levels on day 1, 3 and 7 after treatment. The infusion and urine volume during 0-24 h in the PiCCO group were significantly greater, and this group also showed significantly higher ADL scores. Furthermore, the PiCCO group spent lesser days on vasoactive agents, mechanical ventilation, and had a reduced length of stay in EICU/CCU. Additionally, the CI was significantly higher at 48 h and 72 h in the PiCCO group compared with that at 24 h, and the EVLWI, ITBVI and GEDVI were significantly decreased at 48 h and 72 h.
Applying the PiCCO system could improve the clinical outcomes of elderly patients with AMI complicated by CS.
Core tip: Previous studies investigating the usefulness of the pulse index continuous cardiac output (PiCCO) system have mainly focused on patients with septic shock, acute respiratory distress syndrome and necrotizing pancreatitis. There are few reported studies conducted in elderly patients with acute myocardial infarction (AMI) complicated by cardiogenic shock (CS). Therefore, the aim of the present randomized controlled trial was to assess whether the application of PiCCO could improve clinical outcomes for elderly patients with AMI complicated by CS.
- Citation: Zhang YB, Zhang ZZ, Li JX, Wang YH, Zhang WL, Tian XL, Han YF, Yang M, Liu Y. Application of pulse index continuous cardiac output system in elderly patients with acute myocardial infarction complicated by cardiogenic shock: A prospective randomized study. World J Clin Cases 2019; 7(11): 1291-1301
- URL: https://www.wjgnet.com/2307-8960/full/v7/i11/1291.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i11.1291
Acute myocardial infarction (AMI) is a leading cause of hospitalizations and mortality worldwide. The incidence of AMI increases progressively with age, and a larger proportion of patients presenting with AMI in the future is predicted to be older than 65 years. Classic symptoms of AMI include chest pain but, in the elderly, atypical presentations without chest pain are more common; this may lead to missed or delayed diagnosis[1]. Cardiogenic shock (CS) is a primary complication following AMI, and is the main cause of early death[2]. Therefore, AMI complicated by CS remains one of the most serious and challenging conditions to manage, with mortality rates approaching 50-80% according to data from the SHOCK registry[3,4]. Additionally, elderly patients, with a variety of disorders and multiple organ dysfunction have significantly higher mortality risks[5,6]. When it occurs, CS is an emergency requiring rapid diagnosis and effective clinical management. Hemodynamic monitoring and the associated fluid therapy are of critical importance in the management of these conditions. Prognosis of CS depends on the degree of hemodynamic abnormalities. In critical care settings, the management and optimization of fluid status remains a considerable challenge because inadequate circulatory volume can lead to insufficient tissue perfusion and oxygen delivery, whereas fluid overload can cause heart failure and pulmonary edema. Thus, inadequate fluid balance can increase the mortality associated with these conditions. For this reason, a monitoring method capable of reflecting the real status of the entire circulatory system is paramount for sufficient CS management.
Pulse index continuous cardiac output (PiCCO) is an efficient advanced procedure of continuously monitoring the hemodynamic status in clinical practice. The procedure is based on the use of a specific thermodilution arterial (femoral, brachial, or axillary) catheter and a central venous line[7]. The technique is minimally invasive, and can quantify different hemodynamic parameters reflecting the vascular tone, preload, and cardiac function. Cardiac function profiles that can be discerned from PiCCO include cardiac output, intrathoracic blood volume (ITBV), global end-diastolic volume (GEDV), extra vascular lung water (EVLW) and peripheral vascular resistance. Monitoring these parameters can enable a clinician to optimize volume status, myocardial contractility, and tissue perfusion[8]. Previous studies investigating the usefulness of the PiCCO system have mainly focused on patients with septic shock[9,10], acute respiratory distress syndrome[10,11] and necrotizing pancreatitis[12], while there are few available studies conducted in elderly patients with AMI complicated by CS. Therefore, the aim of the present randomized controlled trial was to assess whether the application of PiCCO can improve clinical outcomes for elderly patients with AMI complicated by CS.
This prospective study was conducted from January 2015 to January 2017 in the Department of Emergency Medicine, PLA Army General Hospital, Beijing, China. The study was approved and monitored by the Institutional Review Board of the PLA General Hospital, Beijing, China. The registration identification number for the study is ChiCTR-IOC-16009923. Patients admitted to the emergency intensive care unit (EICU) or coronary care unit (CCU) were recruited if they were over 65 years of age and had CS consequent to AMI. The diagnosis of AMI was confirmed as follows: (1) An increase in cardiac troponin I (cTnI) or creatine kinase-MB (CK-MB) levels above the 99th percentile of a healthy; (2) Characteristic electrocardiogram (ECG) changes- either ST segment elevation, new ST segment depression or T wave flattening, inversion; and (3) At least one of the following signs or symptoms: (a) Persistent ischemic chest pain; (b) Echocardiographic findings/segmental wall motion abnormalities; and (c) Abnormal coronary angiography findings. CS was diagnosed on the basis of the following criteria: (1) Objective evidence of cardiac dysfunction; (2) Systolic blood pressure < 90 mmHg for at least 30 min, or vasopressors required to maintain the systolic blood pressure ≥ 90 mmHg; (3) A reduced cardiac index (CI) (< 2.2 L/min/m2); and (4) Clinical signs of tissue hypoperfusion (cold, clammy skin, altered mental status, oliguria or peripheral vasoconstriction). Exclusion criteria included the following: viral myocarditis, pericardial tamponade, CS due to chronic heart failure and congenital heart disease, malignant tumor, septic shock and multiple organ dysfunction syndrome, moribund, or informed consent cannot be obtained; contraindications to catheter insertion, including overlying infection and arterial grafting; conditions likely to render PiCCO measurements inaccurate, including intracardiac shunts, significant tricuspid regurgitation, and cooling or rewarming. A written informed consent form was provided by family members of the patients, and the study was approved by the Ethics Committee of PLA Army General Hospital.
This study aimed to explore the clinical efficacy of PiCCO versus control group in the treatment of elderly patients with AMI complicated by CS. The patients were randomized into the two groups. The APACHE II score was used as the main measuring index. Considering the outcomes based on the work by Zhang and colleagues[13], a minimum sample size of 20 patients in each group (control and PiCCO) was determined to be enough to provide statistical differences with α = 0.05 and 80% power, assuming a 20% failure rate.
Patients that met the inclusion criteria were randomly assigned to either PiCCO or control groups based on the random number table method. The control group received conventional treatment, such as ECG monitoring, oxygen inhalation, establishing intravenous access and inserting a central venous catheter for the measurement of central venous pressure (CVP). Anticoagulants, antiplatelet agents and antiarrhythmic therapy were offered. Emergency revascularization with direct percutaneous coronary intervention (PCI) or emergency coronary artery bypass graft (CABG) was required. If patients were not suitable for receiving revascularization therapy, intravenous thrombolysis was given. In the control group, blood pressure, heart rate and CVP were used to guide fluid resuscitation therapy, including the administration of intravenous fluid, vasoactive agents, diuretics or inotropic drugs to maintain appropriate cardiac output.
For the patients enrolled in the PiCCO group, either they themselves or their legal representatives signed the consent forms for arterial catheterization. The PiCCO procedure was conducted within 2 h of patient enrollment. A central venous catheter was placed into a central vein (right subclavian or jugular vein), and a thermistor-tipped arterial catheter was inserted into the femoral artery; both were then connected to the PiCCO system for detecting hemodynamics[14]. An infusion via central venous catheter was stopped for at least 30 s before the infusion of saline. Then, 10-15 mL of normal saline at a temperature of 0-8 °C was injected into the central vein, and various hemodynamic parameters were obtained via the analysis of variations in blood temperature taken by the temperature sensor of the arterial catheter[15]. At least three cold boluses were considered necessary for each calibration to obtain readings with acceptable precision. The measurements of hemodynamic parameters representing an average of three readings were recorded at least every 8 h[16]. These hemodynamic parameters were used to guide the application of vasoactive drugs, fluids and diuretics according to the protocol of our institution on the basis of conventional treatment. If CI < 3 L/min/m2, global end-diastolic volume index (GEDI) < 680 mL/m2 and extravascular lung water index (EVLWI) < 3 mL/kg, intravenous fluid was applied. Vasoactive drugs (inotropes and vasopressors) were used to improve cardiac contractility when CI < 3 L/min/m2, 800 mL/m2 > GEDI > 680 mL/m2 and EVLWI < 3 mL/kg. If CI < 3 L/min/m2, GEDI > 800 mL/m2 and EVLWI > 3 mL/kg, both vasoactive drugs and diuretics were applied. If CI > 3 L/min/m2, GEDI < 680 mL/m2 and EVLWI < 3 mL/kg, intravenous fluid was administrated. If CI > 3 L/min/m2, 800 mL/m2 > GEDI > 680 mL/m2 and EVLWI < 3 mL/kg, there was no need to give intravenous fluid, and real-time monitoring was performed based on the changes of patient conditions. Diuretics were used to reduce fluid overload when CI > 3 L/min/m2, GEDI > 800 mL/m2 and EVLWI > 3 mL/kg. The target was to maintain 5 L/min/m2 > CI > 3 L/min/m2, 800 mL/m2 > GEDI > 680 mL/m2 and EVLWI < 3 mL/kg. The PiCCO procedure was discontinued and removed if the patient was clinically stable for 48 h, as determined by the attending physicians. The system was maintained for a maximum of 10 d. If catheter-related bloodstream infection was suspected, the central venous catheter was removed and sent for microbiological study, and the catheter was exchanged for a new one.
The following data were recorded: age, sex, body mass index, comorbidities, acute physiology and chronic health evaluation II (APACHE II) score, sequential organ failure assessment (SOFA) score, high-sensitive Troponin I (hs-TnI), N-terminal pro-brain natriuretic peptide (NT-proBNP), PaO2/FiO2 ratio, lactate levels, urine output, infusion volume, activities of daily living (ADL) scale, days on vasoactive agents, days on mechanical ventilation, duration of mechanical ventilation, EICU/CCU length of stay and incidence of pulmonary edema. The CI, EVLWI, ITBVI, and GEDVI were recorded at various time points: 0-24 h, 24-48 h and 48-72 h of the PICCO group.
Statistical analyses were completed using SPSS 17.0 software. Data are reported as the mean (standard deviation) or number (%). Outcomes were compared between two groups with two-sided t-tests for continuous variables and chi-square tests for categorical variables; ANOVA was used to calculate multi-parametric significance. All tests were two-sided, and P < 0.05 was considered to be statistically significant.
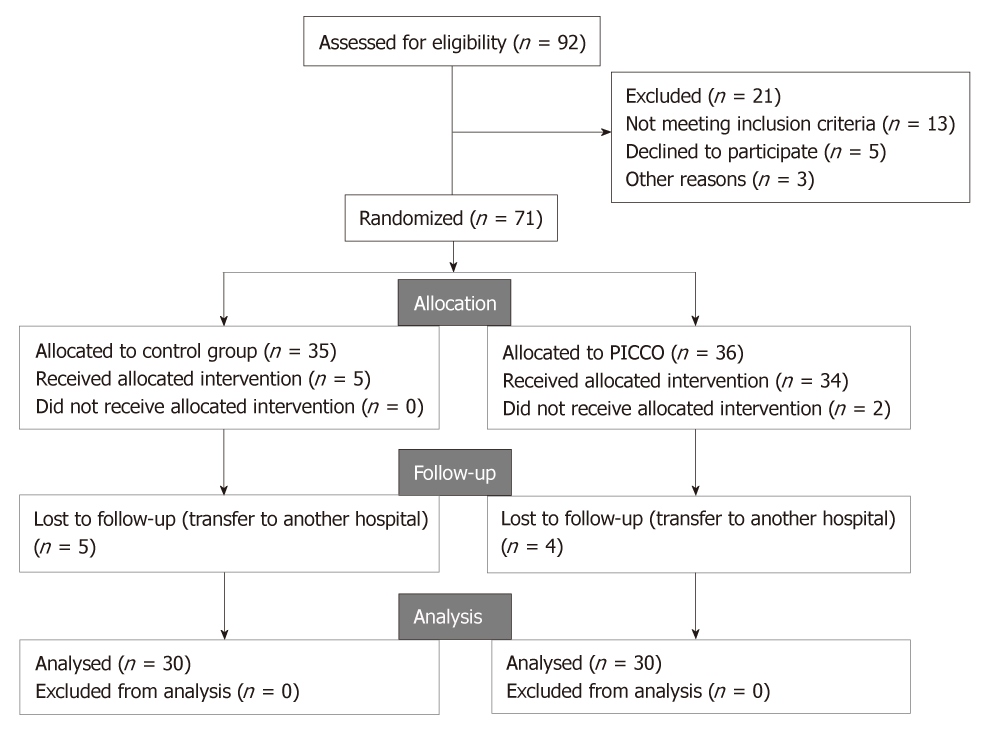
The patient screening process is as outlined in Figure 1. A total of 92 patients were assessed for eligibility to participate in the study. Out of this, 21 patients were excluded for: not meeting inclusion criteria (n = 13), declined to participate (n = 5), and other reasons (n = 3). The remaining 71 patients were randomized to control group (n = 35) and PICCO group (n = 36). However, in the control group, 5 patients in the control group were lost to follow-up, leaving 30 patients who completed the study. On the other hand, in the PICCO group, 34 patients received the procedure, 2 patients did not receive the procedure due to relative’s refusal, and 4 patients were lost to follow-up. Therefore 30 patients from this group were analyzed at the end of the study. Consequently, a total of 60 patients, comprising 34 men and 26 women, with AMI complicated by CS were included in the present study. The age range was 66-87 years. Baseline characteristics of patients in the two groups are presented in Table 1. No statistically significant differences regarding baseline characteristics of patients between the two groups were observed (all P > 0.05).
| PiCCO group, n = 30 | Control group, n = 30 | P value | |
| Age in yr | 76.37 ± 6.67 | 75.93 ± 6.55 | 0.801 |
| Male | 18 (60.0%) | 16 (53.3%) | 0.602 |
| BMI | 24.89 ± 2.90 | 24.50 ± 3.52 | 0.752 |
| Comorbidities | |||
| Hypertension | 18 (60.0%) | 15 (50.0%) | 0.436 |
| Hyperlipidemia | 8 (26.7%) | 7 (23.3%) | 0.766 |
| Diabetes | 8 (26.7%) | 7 (23.3%) | 0.766 |
| Pneumonia | 8 (26.7%) | 6 (20.0%) | 0.542 |
| Respiratory failure | 3 (10.0%) | 4 (13.3%) | 0.688 |
| APACHE II score | 24.07 ± 6.54 | 26.03 ± 7.00 | 0.266 |
| SOFA score | 10.07 ± 3.34 | 10.09 ± 3.23 | 0.330 |
| Hs-TnI in ng/mL | 0.39 ± 0.32 | 0.48 ± 0.32 | 0.304 |
| NT-proBNP in pg/mL | 10459.47 ± 4784.84 | 12871.13 ± 8681.39 | 0.189 |
| PaO2/FiO2 in mmHg | 239.18 ± 96.67 | 252.50 ± 109.64 | 0.619 |
| Lac in mmol/L | 2.32 ± 1.13 | 2.40 ± 1.35 | 0.820 |
A comparison was made of the APACHE II score, SOFA score, hs-TnI, NT-proBNP, lactate levels and oxygenation index on day 1, 3 and 7 after treatment between the groups (Table 2). In the PiCCO group, APACHE II score, SOFA score, hs-TnI and NT-proBNP levels gradually decreased after treatment, and these indicators were significantly lower (P < 0.05 or P < 0.01) in comparison to the control group. When comparing oxygenation index and lactate levels, no significant difference on day 1 and 3 after treatment was seen between two groups (P > 0.05). However, on day 7 after treatment, the PiCCO group showed greater oxygenation indices and lower lactate levels (P < 0.05).
| Group | No. | Treatment time | APACHE II score | SOFA score | Hs-TnI (ng/mL) | NT-proBNP (pg/ml) | PaO2/FiO2 (mmHg) | Lac (mmol/L) |
| Control group | 30 | 1 d | 25.03 ± 7.35 | 11.31 ± 3.57 | 0.54 ± 0.33 | 13781.31 ± 9508.70 | 260.32 ± 111.50 | 2.41 ± 1.17 |
| 3 d | 22.00 ± 5.61 | 9.00 ± 3.39 | 0.40 ± 0.35 | 11537.69 ± 9701.62 | 294.94 ± 102.80 | 2.30 ± 1.03 | ||
| 7 d | 17.57 ± 4.89 | 7.09 ± 3.34 | 0.33 ± 0.28 | 9083.04 ± 7702.01 | 341.10 ± 98.05 | 1.99 ± 0.70 | ||
| PiCCO Group | 30 | 1 d | 21.10 ± 5.95a | 8.37 ± 3.44b | 0.34 ± 0.25a | 8947.00 ± 5739.86a | 284.05 ± 127.06 | 2.15 ± 1.13 |
| 3 d | 17.52 ± 4.88b | 6.38 ± 3.05b | 0.17 ± 0.24b | 7294.83 ± 3638.23a | 346.96 ± 108.39 | 1.80 ± 0.95 | ||
| 7 d | 11.89 ± 3.38b | 4.07 ± 2.02b | 0.11 ± 0.14b | 5939.14 ± 2396.84a | 395.36 ± 88.20a | 1.52 ± 0.74a |
The infusion and urine volume at various time frames are presented in Table 3. The infusion (P < 0.05) and urine volume (P < 0.01) during 0-24 h in the PiCCO group was significantly greater than the control group. Notably, there were no differences between the two groups in the infusion and urine volume between 24-48 h and 48-72 h.
| Group | No. | Time frame | Infusion volume in mL | Urine volume in mL |
| Control group | 30 | 0-1 d | 2673.52 ± 945.22 | 1895.28 ± 717.58 |
| 1-2 d | 2806.61 ± 724.07 | 2111.75 ± 684.02 | ||
| 2-3 d | 2643.42 ± 674.59 | 2199.85 ± 666.83 | ||
| PiCCO group | 30 | 0-24 h | 3201.07 ± 967.64a | 2492.67 ± 868.05b |
| 24-48 h | 3162.48 ± 770.95 | 2363.10 ± 755.36 | ||
| 48-72 h | 2842.76 ± 765.30 | 2502.76 ± 728.34 |
A comparison was made of the primary outcomes of ADL score, and secondary outcomes such as the days on vasoactive agents, days on mechanical ventilation, duration of mechanical ventilation, EICU/CCU length of stay and occurrence of pulmonary edema between the two groups (Table 4). The PiCCO group showed significantly higher ADL scores (P = 0.000) compared to control group. Days on vasoactive agents (P = 0.013), duration of mechanical ventilation (P = 0.000), days on mechanical ventilation (P = 0.011) and EICU/CCU length of stay (P = 0.005) were significantly lower in the PiCCO group than in the control group. However, no significant difference was observed in the incidence of pulmonary edema between the two groups (P = 0.589).
| PiCCO group, n = 30 | Control group, n = 30 | P value | |
| Primary outcome | |||
| ADL score | 66.83 ± 14.65 | 11.33 ± 5.71 | 0.000 |
| Secondary outcomes | |||
| Days on vasoactive agents | 10.04 ± 2.52 | 12.09 ± 3.16 | 0.013 |
| Duration of mechanical ventilation in d | 8.13 ± 1.51 | 10.81 ± 2.10 | 0.000 |
| Days on MV | 9.21 ± 4.40 | 12.39 ± 4.14 | 0.011 |
| EICU/CCU length of stay | 12.57 ± 2.78 | 14.83 ± 2.59 | 0.005 |
| Pulmonary edema | 18 (60%) | 21 (70%) | 0.589 |
The parameters CI, EVLWI, ITBVI and GEDVI were assessed at various time points, and the results are summarized in Table 5. The results show that the levels of EVLWI (P = 0.000), ITBVI (P = 0.000) and GEDVI (P = 0.000) were significantly lower at 48 h and 72 h than those at 24 h. On the other hand, the value of CI (P = 0.001) was significantly higher at 48 h and 72 h than that at 24 h.
| Index | 24 h | 48 h | 72 h | Integral analysis | Multiple comparison | ||||
| Adjustment coefficients | F | P | P1 | P2 | P3 | ||||
| CI in L/min/m2 | 2.16 ± 0.43 | 2.62 ± 0.39 | 2.88 ± 0.91 | 0.835 | 13.723 | 0.001 | 0.000 | 0.000 | 0.104 |
| EVLWI in mL/kg | 8.95 ± 1.85 | 7.59 ± 1.45 | 6.84 ± 0.82 | 1.024 | 21.669 | 0.000 | 0.001 | 0.000 | 0.018 |
| ITBVI in mL/m2 | 972.49 ± 104.28 | 753.91 ± 85.28 | 583.18 ± 65.61 | 0.799 | 199.825 | 0.000 | 0.000 | 0.000 | 0.000 |
| GEDVI in mL/m2 | 783.85 ± 88.36 | 604.28 ± 94.11 | 452.29 ± 67.89 | 0.948 | 133.476 | 0.000 | 0.000 | 0.000 | 0.000 |
AMI is a serious cardiovascular emergency, and CS, characterized by inadequate tissue perfusion resulting from cardiac dysfunction, is a dreadful complication of AMI, occurring in around 10% of patients with AMI[17]. Elderly patients with AMI are more likely to have severe coronary artery disease and large MI size, and most of them often suffer from diabetes mellitus, hypertension and other chronic diseases. The clinical features and prognosis associated with AMI in the elderly warrant special consideration. The elderly are considered to be at high risk for AMI complicated by CS[18]. Abnormal distribution of systemic blood flow upon CS leads to absolute or relative lack of effective circulating blood volume, which also makes CS refractory. To improve prognosis, in addition to the need for an early and accurate diagnosis, fast coronary artery revascularization including PCI and CABG is performed to improve perfusion. Then, medications such as vasoactive drugs, diuretics and positive inotropic agents are a key component of treatment strategies for hemodynamic stabilization and shock reversal. For these critically ill patients, effective mechanical circulatory support is also required.
Presently, there are several different monitoring systems available for patients with circulatory failure to evaluate cardiac output, cardiac function and preload. However, none of these methods are ideal, as they do not meet all the criteria in being non-invasive, continuous, safe, reproducible and having a fast response time. Pulmonary artery catheterization, also known as Swan-Ganz catheter, has been accepted as a gold standard for the clinical measurement of cardiac output for more than 20 years, and it is an important hemodynamic monitoring tool for critically ill patients to guide diagnosis and treatment. However, its use has progressively declined due to difficulties in data interpretation and the potential development of serious com-plications[19,20]. Doppler echocardiography has become a standard imaging modality for the assessment of cardiac pumping function, and can provides a number of non-invasive hemodynamic measurements. Unfortunately, its clinical utility for hemodynamic monitoring is limited by the fact that echocardiography cannot be used as a continuous monitoring procedure at the patient’s bedside[21,22]. Intra-aortic balloon pump counter-pulsation (IABP) is a widely used mechanical assist device for hemodynamic support in the clinical treatment for patients with CS due to AMI[23]. Unfortunately, in the largest randomized controlled clinical trial (IABP-SHOCK II) where 600 patients with CS in 37 centers in Germany were enrolled, results showed that IABP support was not associated with reduced 30-d mortality compared with control[24]. As a result, there are an increasing number of alternative methods for hemodynamic monitoring. The PiCCO system is one such alternative in clinical practice, integrating a wide array of both static and dynamic hemodynamic data through a combination of the lung heat dilution method and pulse contour analysis technique. Readings obtained from PiCCO procedures such as CVP, GEDVI and ITBVI provide more precise estimations of the cardiac preload, and are able to more accurately predict a patient’s response to fluid administration[25,26]. In addition, for the elderly with CS, the elasticity of peripheral vessels decreases and central venous access is often required in the care of critically ill patients. During PiCCO, only a small arterial thermodilution catheter is inserted into the femoral artery, then an accurate assessment and monitoring of cardiac function can be performed. For this reason, use of PiCCO is very suitable among these elderly patients.
Severity assessment is necessary for the management of patients, including decision-making for treatment choices and patient disposition. The results of the present study showed that in comparison to the control group, the PiCCO group had a significantly lower APACHE II score and SOFA score on day 1, 3 and 7 after treatment (P < 0.05 or P < 0.01). APACHE II, a severity-of-disease classification system, has been widely used to measure the illness severity among critically ill patients admitted to the intensive care unit (ICU). It has been demonstrated that the APACHE II is a very useful tool to prognosticate hospital mortality of ICU patients[27]. On the other hand, the SOFA score is commonly used to quantify the degree of organ dysfunction/failure and the prognosis of severely ill patients[28]. A previous study showed that the SOFA score can provide potentially valuable prognostic information for predicting long-term mortality in AMI patients[29]. Lower APACHE II and SOFA scores indicate a decrease in disease severity. Cardiac troponin I (cTnI) is a highly sensitive, specific marker for myocardial cell injury, and is recommended for the management of patients presenting with AMI. However, Hs-TnI has been shown to be more sensitive in detecting AMI than cTnI[30]. On the other hand, NT-proBNP is synthesized by myocytes and fibroblasts principally in the ventricles in response to left ventricular filling pressure and wall stress[31]. In a study, blood NT-proBNP levels showed a positive correlation with GEDVI and CI, suggesting that it can be used as a good indicator of cardiac preload in hemodynamically unstable patients[32]. Lower levels of both hs-TnT and NT-proBNP in the PiCCO group were associated with sufficient coronary perfusion and improved heart function. The PaO2/FiO2 ratio is often used as an indicator of lung function in critically ill patients. Our study found that there were significant differences in the PaO2/FiO2 ratio and lactate levels on day 7 after treatment between the two groups (P < 0.05). This reveals that tissue oxygen supply and metabolic status of patients in the PiCCO group improved over the course of treatment. In terms of fluid management, although the infusion (P < 0.05) and urine volume (P < 0.01) during 0-24 h in the PiCCO group was significantly greater than the control group, there was no significant difference in the incidence of pulmonary edema (P > 0.05). Adequate fluid resuscitation is one of the most important components of early management following CS, which can effectively maintain tissue perfusion and increase aerobic metabolism.
This randomized trial showed that days on vasoactive agents, days on mechanical ventilation and EICU/CCU length of stay (all P < 0.05) in the PiCCO group were significantly lower than in the control group. This indicates that for the elderly patients with AMI complicated by CS, the use of PiCCO technology with the conventional treatment can guide accurate adjustments in the use of vasoactive drugs and optimize mechanical ventilation therapy, thereby improving rehabilitation and survival of patients. The patients in the PiCCO group had higher ADL scores reflective of improved functional status. ADL scoring is an instrument widely used for functional status assessment, and has been effective as a significant predictor of prognosis in elderly patients with AMI[33].
Guided by the results of the study, we thus conclude that the clinical outcomes of elderly patients with AMI complicated by CS are improved under the monitoring and guidance of the PiCCO system. This randomized controlled trial, therefore, provides support for the use of the PiCCO technique in fluid management in critical care settings.
Acute myocardial infarction (AMI) continues to cause morbidity and mortality, with the outcomes worsened by the development of cardiogenic shock (CS). Classical management of AMI in the setting of CS is based on hemodynamic monitoring and the use of vasopressor, as well as inotropic agents. To effectively monitor and control hemodynamic changes, techniques such as Pulse index Continuous Cardiac output (PiCCO) can be applied. In PiCCO, a central venous pressure (CVP) catheter and a thermodilution arterial line are used to monitor pressure changes thereby enabling precise evaluation of cardiovascular functions. The technique has been applied in hemodynamic assessments of conditions such as septic shock and acute respiratory syndrome. However, there is scanty utilization of the technique reported in the management of AMI complicated by CS, more so among elderly patients.
During AMI, compromised cardiovascular functions leading to CS often occur, and this can be fatal in the absence of timely intervention. Hemodynamic changes that manifest in the setting of CS can cause heart failure and inadequate tissue perfusion. Accurate and precise measurement of hemodynamic parameters that reflect the changes experienced during CS is critical for adequate management of the condition. There is clinical need to adopt methods and techniques that can provide the clinician with accurate hemodynamic changes for appropriate measures to be instituted to correct the condition. When this is achieved, better prognosis following CS after AMI can be attained.
The main objective of the study was to explore the usefulness of PiCCO in the management of elderly patients who have suffered from AMI and developed CS. Accordingly, the study aimed to evaluate and compare various hemodynamic parameters reflective of vascular tone and myocardial contractility among patients who received the PiCCO services and the control group, which was not assigned to receive PiCCO. A further objective was to compare the clinical outcomes and functional status, as described by daily activity life scores, in addition to the duration of hospitalization between the two groups.
This was a prospective clinical trial study involving patients who satisfied predetermined inclusion criteria, which included being over 65-years-old and having suffered from AMI, together with encountering CS. All participants or their legal representatives provided written informed consent to participate in the study, which received ethical approval from the Review Board of the PLA General Hospital, Beijing, China. Diagnosis of AMI was confirmed using classic clinical techniques such as ECG readings, echocardiogram findings and determination of cardiac troponin I and creatine kinase-MB levels. On the other hand, CS was established by clinical observation of features consistent with hypoperfusion, and measurements of blood pressure changes during the cardiac cycle. The PiCCO procedure was conducted by insertion of a CVP catheter and a thermodilution arterial line. These allowed for the measurement of cardiac output functions and hemodynamic parameters. Other information gathered during the study included patients’ biodata and history of prevailing comorbidities, as well as biomarkers of AMI. Additionally, details regarding the use of vasoactive agents, mechanical ventilation and length of hospitalization in the emergency and critical care units were gathered. Differences between the groups were analyzed using the SPSS 17.0 software with two-sided t-tests and chi-square tests used for continuous and categorical variables, respectively. Multiparametric analysis was performed using ANOVA and, in all cases, a P < 0.05 was considered statistically significant.
This study provides promising outcomes in the use of PiCCO among elderly patients being managed for AMI with accompanying CS. Compared to the control group, patients who received PiCCO services displayed statistically significant lower APACHE II and SOFA scores, as well as lower levels of hs-TnI and NT-proBNP. Similarly, there were generally lower lactate levels, and a diminished oxygenation index among patients in the PiCCO group on day 7 after treatment. Infusion and urine volumes were evidently higher (P < 0.01) in the PiCCO group in the first day after treatment; thereafter, no differences in these parameters were discernible on subsequent days between the two groups. There was an appreciable increase in the functional health status of patients in the PiCCO, as demonstrated by the greater ADL scores (P < 0.001). Moreover, patients in this group needed less critical care support, use of mechanical ventilation, and blood pressure modifying drugs compared to the control group (all P < 0.05). The difference in the incidence of pulmonary edema, although significantly higher among the control group, did not reach the threshold for statistical significance (P = 0.589). Considering indicators of cardiac function and vascular competence, the levels of EVLWI, ITBVI and GEDVI were all significantly lower at 48 h and 72 h as compared to 24 h after initiation of the PiCCO procedure (P < 0.001).
Our study provides clinical data that supports the need to consider applying PiCCO in managing elderly patients who have AMI that has been further confounded by CS. Improved precision in the monitoring of cardiovascular and hemodynamic changes empowers the clinician to implement appropriate and timely interventions to maintain systemic functions. Importantly, there is undisputable benefits with regards to reduced length and, by inference, cost of hospitalizations when the PiCCO technique is used. Additional positive outcomes of using PiCCO concerns the improved prognosis as manifested in better ADL scores. Based on the study findings and resources permitting, we argue for the consideration to employ the PiCCO procedure when attending to elderly patients with AMI who have also developed CS. More studies involving this technique and incorporating more, and diverse patient groups are needed to provide threshold clinical evidence that can influence future practice in managing these conditions.
Arising from the present study, it is evident that the application of PiCCO can go beyond its traditional use in septic shock and respiratory distress syndrome. Improved clinical outcomes observed among patients who received the PiCCO procedure call for conscious efforts to explore this technique more routinely among related groups of patients. For greater application, more robust data involving clinical trials in other population segments and geographical settings need to be generated to contribute to the pool of evidence in support of the utility of this method in managing AMI confounded by CS.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Wong KL S-Editor: Dou Y L-Editor: Filipodia E-Editor: Xing YX
| 1. | Chien DK, Huang MY, Huang CH, Shih SC, Chang WH. Do elderly females have a higher risk of acute myocardial infarction? A retrospective analysis of 329 cases at an emergency department. Taiwan J Obstet Gynecol. 2016;55:563-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 2. | Khalid L, Dhakam SH. A review of cardiogenic shock in acute myocardial infarction. Curr Cardiol Rev. 2008;4:34-40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Hochman JS, Sleeper LA, Webb JG, Sanborn TA, White HD, Talley JD, Buller CE, Jacobs AK, Slater JN, Col J, McKinlay SM, LeJemtel TH. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999;341:625-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1988] [Cited by in RCA: 2046] [Article Influence: 78.7] [Reference Citation Analysis (0)] |
| 4. | Hochman JS, Sleeper LA, White HD, Dzavik V, Wong SC, Menon V, Webb JG, Steingart R, Picard MH, Menegus MA, Boland J, Sanborn T, Buller CE, Modur S, Forman R, Desvigne-Nickens P, Jacobs AK, Slater JN, LeJemtel TH; SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. One-year survival following early revascularization for cardiogenic shock. JAMA. 2001;285:190-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 468] [Cited by in RCA: 412] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 5. | Batchelor WB, Anstrom KJ, Muhlbaier LH, Grosswald R, Weintraub WS, O'Neill WW, Peterson ED. Contemporary outcome trends in the elderly undergoing percutaneous coronary interventions: results in 7,472 octogenarians. National Cardiovascular Network Collaboration. J Am Coll Cardiol. 2000;36:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 271] [Cited by in RCA: 273] [Article Influence: 10.9] [Reference Citation Analysis (0)] |
| 6. | DeGeare VS, Stone GW, Grines L, Brodie BR, Cox DA, Garcia E, Wharton TP, Boura JA, O'Neill WW, Grines CL. Angiographic and clinical characteristics associated with increased in-hospital mortality in elderly patients with acute myocardial infarction undergoing percutaneous intervention (a pooled analysis of the primary angioplasty in myocardial infarction trials). Am J Cardiol. 2000;86:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 112] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Perny J, Kimmoun A, Perez P, Levy B. Evaluation of cardiac function index as measured by transpulmonary thermodilution as an indicator of left ventricular ejection fraction in cardiogenic shock. Biomed Res Int. 2014;2014:598029. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Cottis R, Magee N, Higgins DJ. Haemodynamic monitoring with pulse-induced contour cardiac output (PiCCO) in critical care. Intensive Crit Care Nurs. 2003;19:301-307. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 9. | Liu X, Ji W, Wang J, Pan T. Application strategy of PiCCO in septic shock patients. Exp Ther Med. 2016;11:1335-1339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 10. | Zhang Z, Xu X, Yao M, Chen H, Ni H, Fan H. Use of the PiCCO system in critically ill patients with septic shock and acute respiratory distress syndrome: a study protocol for a randomized controlled trial. Trials. 2013;14:32. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 11. | Muller L, Candela D, Nyonzyma L, Mattatia L, Suehs C, Fabbro-Peray P, Louart G, de La Coussaye JE, Jaber S, Leone M, Lefrant JY; AzuRéa group. Disagreement between pulse contour analysis and transpulmonary thermodilution for cardiac output monitoring after routine therapeutic interventions in ICU patients with acute circulatory failure. Eur J Anaesthesiol. 2011;28:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Huber W, Umgelter A, Reindl W, Franzen M, Schmidt C, von Delius S, Geisler F, Eckel F, Fritsch R, Siveke J, Henschel B, Schmid RM. Volume assessment in patients with necrotizing pancreatitis: a comparison of intrathoracic blood volume index, central venous pressure, and hematocrit, and their correlation to cardiac index and extravascular lung water index. Crit Care Med. 2008;36:2348-2354. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 85] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 13. | Zhang YB, Han JQ, Guo K, Yang M, Jin JR, Chang H, Chen DM, Wang YH, Zhou RB, He YB. Role of PiCCO in monitoring elderly acute myocardial infarction patients with cardiac shock. Zhonghua Laonian Xinnaoxueguanbing Zazhi. 2017;19:708-711. [DOI] [Full Text] |
| 14. | Segal E, Katzenelson R, Berkenstadt H, Perel A. Transpulmonary thermodilution cardiac output measurement using the axillary artery in critically ill patients. J Clin Anesth. 2002;14:210-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Monnet X, Persichini R, Ktari M, Jozwiak M, Richard C, Teboul JL. Precision of the transpulmonary thermodilution measurements. Crit Care. 2011;15:R204. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 137] [Cited by in RCA: 147] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 16. | Bendjelid K. When to recalibrate the PiCCO? From a physiological point of view, the answer is simple. Acta Anaesthesiol Scand. 2009;53:689-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Kolte D, Khera S, Aronow WS, Mujib M, Palaniswamy C, Sule S, Jain D, Gotsis W, Ahmed A, Frishman WH, Fonarow GC. Trends in incidence, management, and outcomes of cardiogenic shock complicating ST-elevation myocardial infarction in the United States. J Am Heart Assoc. 2014;3:e000590. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 463] [Cited by in RCA: 458] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 18. | Yoo YP, Kang KW, Yoon HS, Myung JC, Choi YJ, Kim WH, Park SH, Jung KT, Jeong MH; Korean Acute Myocardial Infarction Registry Investigators. One-year clinical outcomes in invasive treatment strategies for acute ST-elevation myocardial infarction complicated by cardiogenic shock in elderly patients. J Geriatr Cardiol. 2013;10:235-241. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 19. | Chatterjee K. The Swan-Ganz catheters: past, present, and future. A viewpoint. Circulation. 2009;119:147-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 146] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 20. | Rossello X, Vila M, Rivas-Lasarte M, Ferrero-Gregori A, Sans-Roselló J, Duran-Cambra A, Sionis A. Impact of Pulmonary Artery Catheter Use on Short- and Long-Term Mortality in Patients with Cardiogenic Shock. Cardiology. 2017;136:61-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 56] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 21. | Steiner HA, Hasin Y. Echo is the preferred modality for hemodynamic monitoring in the cardiac intensive care unit. World J Cardiovasc Dis. 2012;2:165-167. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 22. | Khan SS, Rich JD. Novel technologies and devices for monitoring and treating pulmonary arterial hypertension. Can J Cardiol. 2015;31:478-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 23. | Prondzinsky R, Lemm H, Swyter M, Wegener N, Unverzagt S, Carter JM, Russ M, Schlitt A, Buerke U, Christoph A, Schmidt H, Winkler M, Thiery J, Werdan K, Buerke M. Intra-aortic balloon counterpulsation in patients with acute myocardial infarction complicated by cardiogenic shock: the prospective, randomized IABP SHOCK Trial for attenuation of multiorgan dysfunction syndrome. Crit Care Med. 2010;38:152-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 205] [Cited by in RCA: 234] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 24. | Thiele H, Schuler G, Neumann FJ, Hausleiter J, Olbrich HG, Schwarz B, Hennersdorf M, Empen K, Fuernau G, Desch S, de Waha S, Eitel I, Hambrecht R, Böhm M, Kurowski V, Lauer B, Minden HH, Figulla HR, Braun-Dullaeus RC, Strasser RH, Rochor K, Maier SK, Möllmann H, Schneider S, Ebelt H, Werdan K, Zeymer U. Intraaortic balloon counterpulsation in acute myocardial infarction complicated by cardiogenic shock: design and rationale of the Intraaortic Balloon Pump in Cardiogenic Shock II (IABP-SHOCK II) trial. Am Heart J. 2012;163:938-945. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 25. | Marik PE, Monnet X, Teboul JL. Hemodynamic parameters to guide fluid therapy. Annals of Intensive Care. 2011;11:1. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 389] [Cited by in RCA: 391] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 26. | Saugel B, Huber W, Nierhaus A, Kluge S, Reuter DA, Wagner JY. Advanced Hemodynamic Management in Patients with Septic Shock. Biomed Res Int. 2016;2016:8268569. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 27. | Zhou XY, Ben SQ, Chen HL, Ni SS. A comparison of APACHE II and CPIS scores for the prediction of 30-day mortality in patients with ventilator-associated pneumonia. Int J Infect Dis. 2015;30:144-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 28. | Arts DG, de Keizer NF, Vroom MB, de Jonge E. Reliability and accuracy of Sequential Organ Failure Assessment (SOFA) scoring. Crit Care Med. 2005;33:1988-1993. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 94] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 29. | Huang SS, Chen YH, Lu TM, Chen LC, Chen JW, Lin SJ. Application of the Sequential Organ Failure Assessment score for predicting mortality in patients with acute myocardial infarction. Resuscitation. 2012;83:591-595. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Lim SH, Lin ZW. Update on the use of cardiac markers in the diagnosis of acute coronary syndrome. J Acute Med. 2013;3:125-131. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Möllmann H, Weber M, Elsässer A, Nef H, Dill T, Rixe J, Schmitt J, Sperzel J, Hamm CW. NT-ProBNP predicts rhythm stability after cardioversion of lone atrial fibrillation. Circ J. 2008;72:921-925. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Yamanouchi S, Kudo D, Endo T, Kitano Y, Shinozawa Y. Blood N-terminal proBNP as a potential indicator of cardiac preload in patients with high volume load. Tohoku J Exp Med. 2010;221:175-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 33. | Nakajima H, Yoshioka J, Totsuka N, Miyazawa I, Usui T, Urasawa N, Kobayashi T, Mochidome T. Activities of daily living as an additional predictor of complications and outcomes in elderly patients with acute myocardial infarction. Clin Interv Aging. 2016;11:1141-1147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.3] [Reference Citation Analysis (0)] |