Published online May 26, 2019. doi: 10.12998/wjcc.v7.i10.1206
Peer-review started: January 17, 2019
First decision: March 10, 2019
Revised: March 25, 2019
Accepted: April 9, 2019
Article in press: April 9, 2019
Published online: May 26, 2019
Processing time: 130 Days and 19.2 Hours
Twenty percent of patients infected with hepatitis B virus (HBV) develop extrahepatic manifestations with HBV detected in the lymph nodes, spleen, bone marrow, kidneys, and skin. HBV infection has been associated with some autoimmune disorders. Dermatomyositis (DM) is an idiopathic inflammatory myopathy, which involves a viral infection, and DM has been identified in patients infected with HBV, but there is no direct histological evidence for an association between HBV and DM.
We describe a familial HBV-infected patient admitted with liver function abnormality, rashes, a movement disorder, and an elevated level of creatine kinase (CK). A computed tomography scan of the lung showed pulmonary fibrosis, and a liver biopsy identified nodular cirrhosis. An electromyogram revealed myogenic damage, and a muscle biopsy showed nuclear migration in local sarcolemma and infiltration of chronic inflammatory cells. Immunohistochemical staining showed negative results for HBsAg and HBcAg. Fluorescence in situ hybridization showed a negative result for HBV DNA. The patient was diagnosed with HBV-related liver cirrhosis complicated with DM and was treated with methylprednisolone, mycophenolate mofetil, and lamivudine. Eight months later, the patient was readmitted for anorexia and fatigue. The blood examination showed elevated levels of aminotransferases and HBV DNA, however, the CK level was within the normal range. The patient developed a virological breakthrough and lamivudine was replaced with tenofovir.
DM in chronic HBV-infected patients does not always associate with HBV. Antiviral and immunosuppressive drugs should be taken into consideration.
Core tip: We report a patient diagnosed with hepatitis B virus (HBV)-related liver cirrhosis complicated with dermatomyositis (DM). However, HBV was not detected in his muscle sample, thus we concluded that his DM did not associate with his HBV infection. Diagnosis of DM on the basis of HBV infection is relatively uncommon. Diagnosis and treatment are difficult due to the complex relationship between these diseases and their conflicting treatment strategies. By providing our experience in diagnosing and treating DM with HBV, we hope to assist with similar cases and to stimulate further research on the relationship between DM and HBV.
- Citation: Zhang J, Wen XY, Gao RP. Hepatitis B virus-related liver cirrhosis complicated with dermatomyositis: A case report. World J Clin Cases 2019; 7(10): 1206-1212
- URL: https://www.wjgnet.com/2307-8960/full/v7/i10/1206.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i10.1206
Dermatomyositis (DM) is an idiopathic inflammatory myopathy with typical rashes yet little is known about the etiology of DM. DM is accepted widely as an auto-immune disease induced by infectious and noninfectious environmental factors in genetically susceptible individuals[1-4]. Hepatitis B virus (HBV) infection is a global public health problem with up to two billion people with pre-existing and current HBV infections[5], HBV was detected in the lymph nodes, spleen, bone marrow, kidneys, and skin[6] and 20% of patients infected with HBV develop extrahepatic manifestations, such as polyarteritis nodosa, polymyositis, Sjogren’s syndrome, and glomerulonephritis[7,8]. One DM case as the consequence of HBV infection has been reported[9], but DM does not always associate with hepatitis. Here, we report a case of HBV-related liver cirrhosis complicated with DM.
A 46-year-old man presented with rashes and a movement disorder.
The patient has been HBsAg-positive for 15 years and has had moderate fatigue and elevated aminotransferases for four years, he was not treated. Facial and peripheral rashes were present for four months with no definitive cause. The patient suffered from simultaneous muscle soreness for half a month, which did not draw his attention until he could not move his extremities.
The patient reported no known systemic illness.
The patient’s mother, brother, and grandfather had chronic HBV infections.
On physical examination, the patient’s pharynx was congested. Purple-red edematous maculae were on the patient’s forehead and the malar areas around on his orbits. The peripheral skin was also involved sporadically, and telangiectasia was observed on the anterior chest wall. The abdominal examination was normal. A neurologic examination indicated muscle strength of 3/5 and limb muscle pain was positive.
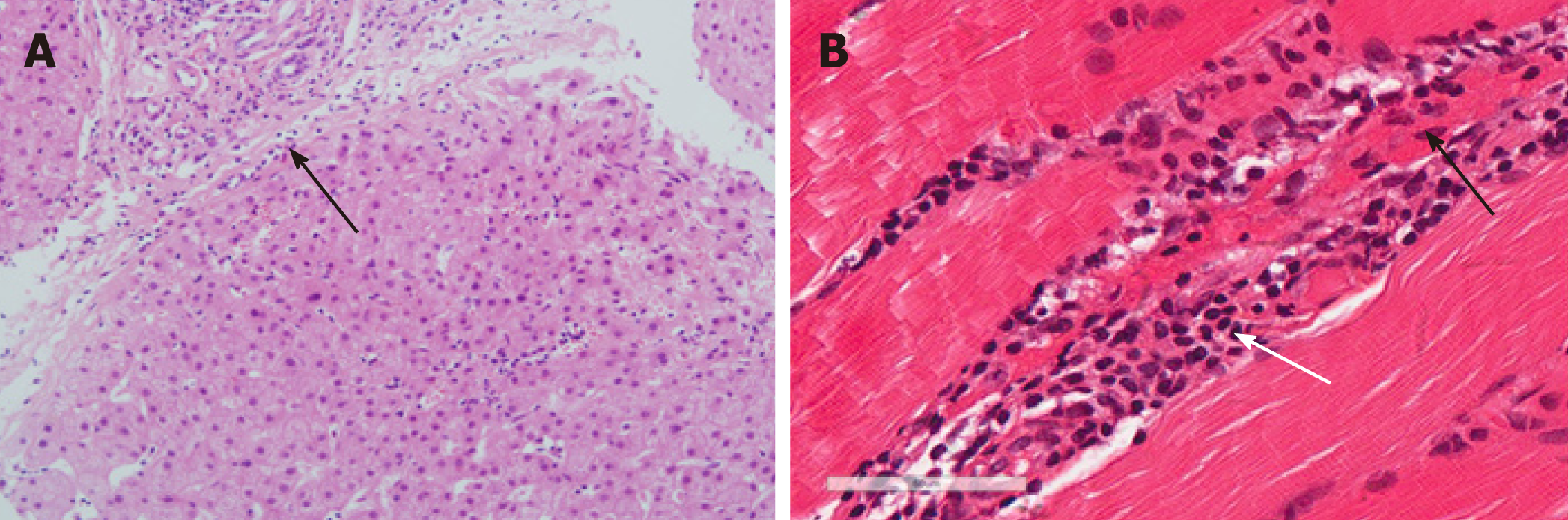
The blood examination showed increased levels of creatine kinase (CK, 11889 U/L), C-reactive protein (32.4 mg/L), aspartate aminotransferase (AST, 280 U/L), and alanine aminotransferase (ALT, 242 U/L). The tests for HBsAg, HBeAb, and HBcAb were positive, and the tests for anti-HIV, anti-HCV, anti-EBV IgM, anti-cytomegalovirus IgM and HCV RNA were negative. HBV DNA was undetectable (< 50 IU/mL). Alpha fetoprotein (AFP) and Carbohydrate antigen 19-9 (CA19-9) were within normal range. Tests for the autoantibodies anti-PM-Scl and anti-Jo-1 were negative, and the test for anti-Ro-52 was positive. With regards to antinuclear antibodies, the membranous pattern was 1:1000 and the particle type cytoplasm was 1:100. The test for anti-M2 was negative. The indocyanine green retention rate at 15 min was 11.7% and a FibroScan revealed a stiffness value of 21.3 kPa. A liver biopsy showed nodular cirrhosis with extensive lymphocytes and few plasmocytes infiltrating the fibrous septa (Figure 1A).
Magnetic resonance imaging (MRI) of the abdomen revealed hepatic cirrhosis and mild splenomegaly. A computed tomography (CT) scan of the lung showed streaky opacities in the right middle lobe, right lower lobe, and ligula.
The patient was diagnosed with hepatitis B cirrhosis, liver function compensation, and preliminary pneumonia or pulmonary fibrosis. Upon admission, the patient accepted antibiotic (ceftezole), hepatoprotective, and supportive treatment. Nine days after admission, the patient developed dysphagia, dyspnea, and incontinence, and an electromyogram (EMG) revealed myogenic damage. A muscle biopsy showed nuclear migration in local sarcolemma and infiltration of chronic inflammatory cells (Figure 1B). Immunohistochemical staining showed negative results for HBsAg and HBcAg. Fluorescence in situ hybridization showed a negative result for HBV DNA. The streaky opacities in the lungs were more severe. The patient was finally diagnosed with HBV-related liver cirrhosis complicated with DM and pulmonary fibrosis.
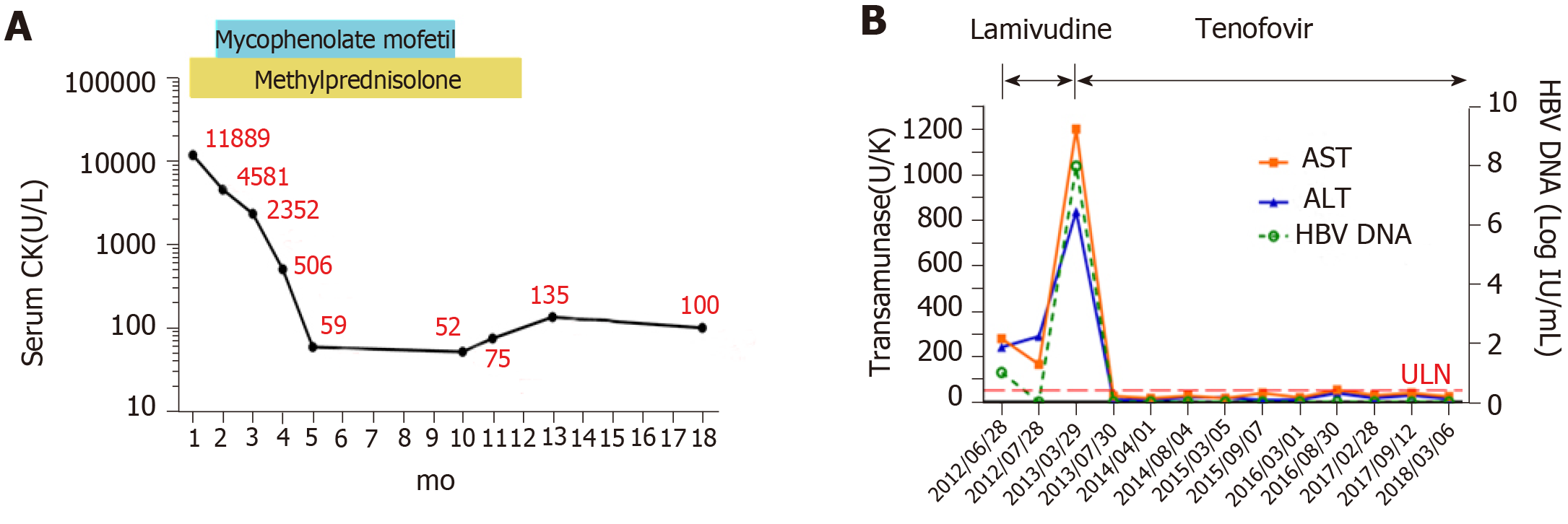
The patient was treated with 80 mg/d of methylprednisolone instead of antibiotics. Lamivudine was given at a dose of 100 mg/d to prevent virus replication. Additional treatments were supportive and symptomatic. After seven days of steroid treatment, the dysphagia, dyspnea, and incontinence improved and the CK level was 4791 U/L. After seventeen days of steroid treatment, the CK level was 5710 U/L leading to administration of 750 mg of mycophenolate mofetil twice a day. After a week, the patient’s eating and sleeping improved, micturition and defecation were normal, and the patient’s facial and peripheral rashes faded. The patient’s AST level was 166 U/L, ALT level was 290 U/L, and CK level was 4581 U/L. The streaky opacities did not change. The patient was prescribed 40 mg of methylprednisolone, 1500 mg of mycophenolate mofetil, and 100 mg of lamivudine per day for another three months after discharge.
Upon discharge, the patient did not feel uncomfortable. The patient’s amino-transferase and CK levels were in the normal range during the three months after discharge, and there were no more streaky opacities in the lungs. The mycophenolate mofetil treatment was stopped after another six months, but one week after stopping this immunosuppressive drug, the patient was readmitted for anorexia and fatigue. The blood examination showed increased levels of HBV DNA (5.05 × 105 IU/L) and AST (840 U/L), but the CK level was normal (53 U/L) (Figure 2A, B). The frequency of the L180M mutation in HBV is 51.8%, and the frequency of the M204V mutation in HBV is 78.2%. In this patient, HBV reactivation followed lamivudine drug resistance that arose due to long-term lamivudine treatment along with long-term immunosuppressive therapy. To reduce HBV replication, the patient was given 300 mg of tenofovir per day. One month later, the patient’s aminotransferase levels returned to normal and his HBV DNA levels fell to a level below detection (Figure 2B). The patient was prescribed tenofovir as a long-term antiviral therapy, his aminotransferase and CK levels remained normal at the subsequent five-year follow-up (Figure 2A, B), and he was able to perform moderate-intensity physical activities.
The presentations of DM are proximal weakness, elevated CK, myopathic EMG, inflammatory pathology, and typical rashes. The characteristic rashes include periorbital violaceous erythema, Gottron’s sign, periungual telangiectasia, mechanic’s hands, and other mucocutaneous lesions. In addition, DM can affect the lung (interstitial pneumonia, pulmonary fibrosis, and pleurisy) and the alimentary tract (the pharynx and the striated muscle at the lower end of the esophagus)[10]. The patient in this study fully met the diagnostic criteria for DM[1]. The patient’s lung and pharyngeal muscle were affected.
The etiology and pathogenesis of DM are uncertain, and DM is generally recognized as an autoimmune disorder induced by environmental factors in genetically susceptible individuals[1]. Tests for autoantibodies in DM patients are usually partial positive. Tests for anti-aminoacyl-tRNA synthetases, such as anti-Jo1, and anti-Mi autoantibodies, which associate with the hepatitis C virus (HCV), may be positive in DM patients. HCV may cross-react with the host to produce specific autoantibodies that result in DM[11]. However, we can exclude the possibility of HCV-related DM in this patient based on the lack of anti-HCV and HCV RNA in his serum.
Distinct from the mechanism of HCV-related DM, approximately 20% of HBV-infected patients develop extrahepatic disorders, including glomerulonephritis, polyarteritis nodosa, arthritis, and polymyositis through HBV-related immune complex circulation and deposition. In 2005, Mason A reported a case of HBV-related polymyositis in which HBV immune complex deposition and HBV DNA replication were detected in the interstitial vascular endothelium of diseased muscle tissues[8]. We summarized ten hepatitis virus infection and DM cases since 2000 (Table 1, Table 2), and found that HBV production was absent in the skeletal muscle samples of these patients. Most of these patients were diagnosed with DM associated with hepatoce-llular carcinoma (HCC). In these patients with HCC, paraneoplastic syndrome, a compromised immune system, common carcinogenic environmental factors, and cross-reactive immune reactions against the tumor may have played a role in disease development. Cross-reactions with cutaneous and muscular antigens may have led to the autoimmune syndrome[12-14]. DM improved after cancer resection but recurred upon relapse of the cancer demonstrating that paraneoplastic syndrome plays an important role in HCC-related DM[14]. However, the effect of HBV infection on HCC-related DM has not yet been proven. Recently, Han et al[9] reported a case of DM associated with HCC in which steroid treatment had limited effect, but antiviral therapy improved muscle strength, thus Han hypothesized that DM developed as a consequence of HBV infection. Muscle biopsy evidence remains to be obtained to confirm the association between HBV and DM.
| Ref. | Published yr | Age / Sex | Virus infection | Associated disorders | Autoantibody profile | Treatment | Outcome |
| Nakamura et al[18] | 2000 | 60/F | HCV | Left ventricular dysfunction | ANA(+), Anti-Jo-1(-) | Interferon-α, Steroid | ND |
| Germany et al[19] | 2002 | 40/F | HCV | Collagenous colitis | ANA(+), RF(-). | Azathioprine | Improved |
| Altman et al[20] | 2008 | 6/F | HBV vaccine | - | ND | ND | ND |
| Our case | 46/M | HBV | Pulmonary fibrosis | ANA(+), Anti-Jo-1(-), Anti-Mi2(-) | Steroid, Mycophenolate mofetil, Tenofovir | Stable |
| Ref. | Published yr | Age / Sex | Virus infection | Associated disorders | Autoantibody profile | Treatment | Outcome |
| Cheng et al[21] | 2002 | 50/F | HBV | Erythrocytosis | ND | Steroid, TACE, Hepatectomy | Died |
| Inuzuka et al[22] | 2001 | 51/M | HCV | Acquired ichthyosis | ANA(+), Anti-Jo-1(-) | Steroid | Died |
| Kee et al[11] | 2004 | 71/M | HCV | - | ANA(+), Anti-Jo-1(-) | Steroid | ND |
| Toshikuni et al[23] | 2006 | 79/F | HCV | - | ANA(+), RF(-), Anti-Jo-1 (-) | Steroid, TACE | Died |
| Kee et al[13] | 2009 | 58/M | HBV | - | ANA(+), RF(-), Anti-Jo-1 (-) | Steroid, IVIG, TACE | Died |
| Yang et al[14] | 2014 | 55/M | HBV | - | ANA(+), Anti-Jo-1(-) | Lamivudine, Steroid | Died |
| Han et al[9] | 2018 | 62/M | HBV | - | ANA(+), Anti-Jo-1(-), Anti-Mi2(-) | Steroid, Entecavir, Radio- frequency ablation | Died |
Upon initial admission, our patient presented AFP within normal range in serum and MRI without mass lesion in liver, we excluded the possibility of HCC-associated DM in this study. The level of HBV DNA in our patient was below detection, and HBsAg, HBcAg, and HBV DNA were not found in diseased muscle tissues suggesting no HBV immune complex deposition or HBV DNA replication. The patient’s tests for anti-Ro-52 and anti-antinuclear antibodies were positive. The patient responded well to treatment with methylprednisolone and mycophenolate mofetil. Nevertheless, the immunosuppressive therapy reactivated the HBV and HBV-related liver cirrhosis symptoms recurred, but the DM symptoms did not recur. The patient did not meet the diagnosis criteria for HBV-related DM as described above. In this case, the DM occurred independently of the HBV infection.
Although patients have been reported that their symptoms improved after tumor controlling, antiviral, and immunosuppressive therapies, long-term follow-up information is lacking. In this case, lamivudine was not able to prevent HBV reactivation caused by immunosuppressive agents, which contributed to lamivudine resistance and recurrence of HBV-related liver cirrhosis. The patient was stabilized after changing the treatment from lamivudine to tenofovir. Lamivudine is the first nucleoside analogue used to treat chronic hepatitis B virus infections and it is widely used because of its low price and minimal side effects, but high rates of resistance with long-term lamivudine monotherapy have been observed. The incidence of HBV resistance to lamivudine in nucleoside-naive patients with chronic hepatitis B is 24% after one year of treatment and 70% after five years of treatment[15]. Thirty-three percent of patients who were treated with lamivudine for five years and who had a virological response showed virological breakthrough after 15 mo of treatment with a median dose. Lamivudine resistant mutations occurred in 97.4% of the breakthrough patients[16]. According to recent expert recommendations for antiviral therapy based on the risk of HBV recurrence, in this HBV-related cirrhosis patient, an HBsAg-positive result, and methylprednisolone combined with mycophenolate mofetil treatment are both high-risk factors that contribute to HBV recurrence. Thus, efficacious treatment with an antiviral with a low resistance rate, such as tenofovir or entecavir, should be considered[17].
Not all DM cases based on chronic HBV infections are associated with HBV. Therefore, when HBV coexists with DM, it is necessary to define HBV-related DM, strictly evaluate the risk factors for HBV recurrence, and use antiviral drugs and immunosuppressive agents reasonably to control DM and HBV.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, Research and Experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Asaad AM, Abushady EA S-Editor: Dou Y L-Editor: A E-Editor: Wu YXJ
| 1. | Sasaki H, Kohsaka H. Current diagnosis and treatment of polymyositis and dermatomyositis. Mod Rheumatol. 2018;28:913-921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 2. | Pandya JM, Loell I, Hossain MS, Zong M, Alexanderson H, Raghavan S, Lundberg IE, Malmström V. Effects of conventional immunosuppressive treatment on CD244+ (CD28null) and FOXP3+ T cells in the inflamed muscle of patients with polymyositis and dermatomyositis. Arthritis Res Ther. 2016;18:80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 3. | Rajadhyaksha A, Baheti TG, Mehra S, Sonawale AS, Jain N. Dermatomyositis: a rare presentation of HIV seroconversion illness. J Clin Rheumatol. 2012;18:298-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 4. | Yamamoto SP, Kaida A, Naito T, Hosaka T, Miyazato Y, Sumimoto S, Kohdera U, Ono A, Kubo H, Iritani N. Human parechovirus infections and child myositis cases associated with genotype 3 in Osaka City, Japan, 2014. J Med Microbiol. 2015;64:1415-1424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Schweitzer A, Horn J, Mikolajczyk RT, Krause G, Ott JJ. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386:1546-1555. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1806] [Cited by in RCA: 2000] [Article Influence: 200.0] [Reference Citation Analysis (4)] |
| 6. | Mason A, Wick M, White H, Perrillo R. Hepatitis B virus replication in diverse cell types during chronic hepatitis B virus infection. Hepatology. 1993;18:781-789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 139] [Cited by in RCA: 147] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Cacoub P, Terrier B. Hepatitis B-related autoimmune manifestations. Rheum Dis Clin North Am. 2009;35:125-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 67] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 8. | Mason A, Theal J, Bain V, Adams E, Perrillo R. Hepatitis B virus replication in damaged endothelial tissues of patients with extrahepatic disease. Am J Gastroenterol. 2005;100:972-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 44] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 9. | Han J, Wang S, Kwong TNY, Liu J. Dermatomyositis as an extrahepatic manifestation of hepatitis B virus-related hepatocellular carcinoma: A case report and literature review. Medicine (Baltimore). 2018;97:e11586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Shu XM, Ma L, Lu X, Xie Y, Wang GC. [Myositis disease activity tool in Chinese patients with polymyositis/dermatomyositis]. Zhonghua Yi Xue Za Zhi. 2011;91:1328-1330. [PubMed] |
| 11. | Kee KM, Wang JH, Lee CM, Changchien CS, Eng HL. Chronic hepatitis C virus infection associated with dermatomyositis and hepatocellular carcinoma. Chang Gung Med J. 2004;27:834-839. [PubMed] |
| 12. | András C, Ponyi A, Constantin T, Csiki Z, Szekanecz E, Szodoray P, Dankó K. Dermatomyositis and polymyositis associated with malignancy: a 21-year retrospective study. J Rheumatol. 2008;35:438-444. [PubMed] |
| 13. | Kee SJ, Kim TJ, Lee SJ, Cho YN, Park SC, Kim JS, Kim JC, Kang HS, Lee SS, Park YW. Dermatomyositis associated with hepatitis B virus-related hepatocellular carcinoma. Rheumatol Int. 2009;29:595-599. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Yang SY, Cha BK, Kim G, Lee HW, Kim JG, Chang SK, Kim HJ. Dermatomyositis associated with hepatitis B virus-related hepatocellular carcinoma. Korean J Intern Med. 2014;29:231-235. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | European Association For The Study Of The Liver. EASL clinical practice guidelines: Management of chronic hepatitis B virus infection. J Hepatol. 2012;57:167-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2323] [Cited by in RCA: 2401] [Article Influence: 184.7] [Reference Citation Analysis (0)] |
| 16. | Fasano M, Lampertico P, Marzano A, Di Marco V, Niro GA, Brancaccio G, Marengo A, Scotto G, Brunetto MR, Gaeta GB, Rizzetto M, Angarano G, Santantonio T. HBV DNA suppression and HBsAg clearance in HBeAg negative chronic hepatitis B patients on lamivudine therapy for over 5 years. J Hepatol. 2012;56:1254-1258. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Aygen B, Demir AM, Gümüş M, Karabay O, Kaymakoğlu S, Köksal AŞ, Köksal İ, Örmeci N, Tabak F. Immunosuppressive therapy and the risk of hepatitis B reactivation: Consensus report. Turk J Gastroenterol. 2018;29:259-269. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Nakamura K, Matsumori A, Kusano KF, Banba K, Taniyama M, Nakamura Y, Morita H, Matsubara H, Yamanari H, Ohe T. Hepatitis C virus infection in a patient with dermatomyositis and left ventricular dysfunction. Jpn Circ J. 2000;64:617-618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Germany RE, Cohen SM. Hepatitis C, collagenous colitis, and dermatomyositis occurring in the same patient. Am J Gastroenterol. 2002;97:1848-1849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 20. | Altman A, Szyper-Kravitz M, Shoenfeld Y. HBV vaccine and dermatomyositis: is there an association? Rheumatol Int. 2008;28:609-612. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 21. | Cheng TI, Tsou MH, Yang PS, Sung SM, Chuang VP, Sung JL. Dermatomyositis and erythrocytosis associated with hepatocellular carcinoma. J Gastroenterol Hepatol. 2002;17:1239-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 16] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | Inuzuka M, Tomita K, Tokura Y, Takigawa M. Acquired ichthyosis associated with dermatomyositis in a patient with hepatocellular carcinoma. Br J Dermatol. 2001;144:416-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 33] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Toshikuni N, Torigoe R, Mitsunaga M, Omoto A, Nakashima K. Dermatomyositis associated with hepatocellular carcinoma in an elderly female patient with hepatitis C virus-related liver cirrhosis. World J Gastroenterol. 2006;12:1641-1644. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 18] [Cited by in RCA: 20] [Article Influence: 1.1] [Reference Citation Analysis (0)] |