Published online May 26, 2019. doi: 10.12998/wjcc.v7.i10.1184
Peer-review started: January 13, 2019
First decision: January 26, 2019
Revised: January 29, 2019
Accepted: March 8, 2019
Article in press: March 9, 2019
Published online: May 26, 2019
Processing time: 135 Days and 1.9 Hours
Thyroid storm is resistant to conventional treatments including antithyroid drugs and 131I therapeutic means. Plasma exchange (PE) and double plasma molecular absorption system (DPMAS) can be used as an effective treatment for thyroid storm with severe liver injury.
A 52-year-old woman presented with a 10-day history of nausea and vomiting accompanied by yellowing of the skin and mucosa. Further, her free T3 (FT3) and FT4 levels were significantly elevated, whereas her thyrotropin level was reduced. After admission, her condition continued to deteriorate, and she presented with continued high fever, vomiting, palpitation, and shortness of breath. After being diagnosed with thyroid storm, the patient was immediately treated with PE combined with DPMAS. Her symptoms improved immediately. After three PE + DPMAS treatments, and she was discharged from the hospital. She was treated with methylprednisolone and methylthimidazole. After six months, the patient spontaneously discontinued methylthimidazole treatment. Her previous clinical manifestations and liver dysfunction reoccurred. The patient was treated with PE + DPMAS two times, and her condition rapidly improved. Liver histopathology indicated immunological liver injury.
Our experience suggests that PE combined with DPMAS can effectively relieve the development of thyroid storm.
Core tip: Plasma exchange (PE) and double plasma molecular absorption system (DPMAS) can be used as an effective treatment for thyroid storm with severe liver injury. A 52-year-old woman presented with a 10-d history of nausea and vomiting accompanied by yellowing of the skin and mucosa. After being diagnosed with thyroid storm accompanied by severe liver injury, the patient was immediately treated with PE combined with DPMAS for three times and discharged from the hospital. After six months, her previous clinical manifestations and liver dysfunction reoccurred and treated with PE + DPMAS again, her condition rapidly improved and liver histopathology indicated immunological liver injury.
- Citation: Tan YW, Sun L, Zhang K, Zhu L. Therapeutic plasma exchange and a double plasma molecular absorption system in the treatment of thyroid storm with severe liver injury: A case report. World J Clin Cases 2019; 7(10): 1184-1190
- URL: https://www.wjgnet.com/2307-8960/full/v7/i10/1184.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i10.1184
Thyroid storm is a syndrome of acute hyperthyroidism exacerbation[1] that can lead to high fever, heart failure, shock, coma, and other life-threatening manifestations. Thyroid storm accounts for about 1%-2% of hyperthyroidism patients, the incidence is about 0.002%, and the mortality rate after acute onset is high, above 20%[2]. Thyroid storm is resistant to conventional treatments including antithyroid drugs and 131I therapeutic means. Drug therapy increases the risk of progressing to liver failure in hyperthyroidism storm patients with severe liver injury[3]. Plasma exchange (PE) is a method to purify blood by replacing abnormal components of plasma with fresh normal human plasma or plasma substitutes[4]. The whole plasma containing pathogenic factors (drugs, poisons and toxins) that cannot be controlled or expelled by drugs are discarded by blood purification technology in vitro and then resume load fresh plasma. As the most commonly used treatment in artificial liver support system, PE has been widely used in the treatment of liver failure in China and is considered to have a good therapeutic effect[5]. Severe liver injury in thyroid storm is rare[3,6]. We report a case of hyperthyroidism with thyroid storm accompanied by severe liver damage that was successfully treated with PE and double plasma molecular absorption system (DPMAS).
A 52-year-old woman presented with a 10-d history of nausea and vomiting accompanied by yellowing of the skin and mucosa and reoccurred after six months.
On the evening of admission, April 4, 2018, the patient began to vomit her stomach content frequently. The patient's condition gradually worsened, and irritability and heart rate gradually increased. The patient had no history of hyperthyroidism, but often had palpitation, sweating, agitation, and other symptoms.
No viral hepatitis (A–E) history, no nonalcoholic fatty liver disease history and no cholestatic liver diseases include primary biliary cirrhosis, primary sclerosing cholangitis, and secondary sclerosing cholangitis.
No alcohol abuse and no other drug and herbal used. No additional family history.
Her highest heart rate was 180 beats per minute, body temperature 39.8 °C, highest breathing rate was 25 breaths per minute, blood pressure 147/86 mmHg. PE + DPMAS were conducted once every other day, and three times in all. The patient’s symptoms improved obviously and rapidly. Nausea and vomiting stopped, and body temperature, heart rate, and respiration gradually returned to normal the next day.
On April 4, 2018, her liver function test showed significantly increased transaminase and bilirubin levels [aspartate aminotransferase (AST) 1364 U/L, alanine aminotransferase (ALT) 521 U/L, alkaline phosphatase 153 U/L, glutamete transpeptidase 68 U/L, total bilirubin (TBIL) 223.3 μmol/L, direct bilirubin 164.4 μmol/L)]. Her prothrombin time (PT) was 16 s, PT activity was 67%, and international ratio was 1.54. Her thyrotropin (TSH) level was decreased, and free T3 (FT3) and her free T4 (FT4) levels were significantly increased (TSH < 0.005 mIU/L, FT3 28.6 pmol/L, FT4 81.273 pmol/L). The antinuclear antibody (ANA) test was positive (1:320), and the anti-smooth muscle antibody, anti-liver/kidney microsome antibody type 1, anti-nuclear glycoprotein antibody, anti-soluble acid nucleoprotein antibody, soluble acidic nucleoprotein antibody, anti-hepatocyte cytoplasmic antigen type 1 antibody, anti-soluble liver antigen/hepatopancreatic antigen antibody, and other tests were negative. The immunoglobulin G (IgG) level was 19.1 g/L (< 17.1 g/L). Liver function returned to normal (TBIL 15.2 μmol/L, ALT 26 U/L, AST 31 U/L), and the hyperthyroidism index was restored (FT3 6.1 pmol/L, FT4 20.4 pmol/L, TSH 0.007 µU/mL) at first discharge. Liver and thyroid function were normal during follow-ups on June 16 and August 28, 2018. By November, methylthimidazole administration was discontinued by the patient without a doctor’s advice. About 40 d later, symptoms including fatigue, loss of appetite, and urinary yellowing reoccurred. Liver function (TBIL 115.6 µmol/L, ALT 473 U/L, AST 664 U/L) and thyroid index (TSH < 0.005 mIU/L, FT3 13.6 pmol/L) abnormalities were checked for, and the patient was re-admitted. Changes in liver function and thyroid index are shown in Table 1. Epstein-Barr virus, and cytomegalovirus infections were ruled out. No pathogenic bacteria were found in blood culture
| PE + DPMAS | T (36.2-37.2 °C) | P (60-100 beats/ min) | TBIL (1.71-17.1 μmol/L) | DBIL (0-6.8 μmol/L) | ALT (10-40 U/L) | AST (10-40 U/L) | ALP (40-120 U/L) | GGT (10-40U/L) | FT3 (3.1-6.8 pmol/L) | FT4 (9.2-22.8 pmol/L) | TSH (0.27-4.2 µU/mL) | TG (5-40 μg/L) | |
| First attack | |||||||||||||
| 1st | Preoperative | 39.8 | 180 | 223.3 | 164.4 | 521 | 1364 | 153 | 68 | 28.6 | 81.3 | < 0.005 | 364.6 |
| Postoperative | 37.5 | 86 | 102.4 | 64.3 | 253 | 643 | 124 | 47 | 15.3 | 36.8 | 0.008 | 204.4 | |
| 2nd | Preoperative | 38.4 | 102 | 116.4 | 74.3 | 226 | 1054 | 115 | 42 | 17.4 | 46.6 | < 0.005 | 286.4 |
| Postoperative | 36.7 | 76 | 63.4 | 37.5 | 123 | 436 | 57 | 37 | 10.4 | 25.4 | 0.006 | 126.5 | |
| 3rd | Preoperative | 37.2 | 74 | 79.4 | 42.8 | 78 | 332 | 64 | 41 | 16.4 | 34.2 | < 0.005 | 171.7 |
| Postoperative | 37.1 | 73 | 53.7 | 26.7 | 64 | 117 | 47 | 37 | 8.4 | 21.6 | 0.006 | 97.5 | |
| Second attack | |||||||||||||
| 1st | Preoperative | 39.1 | 142 | 115.6 | 64.2 | 473 | 664 | 116 | 57 | 13.4 | 49.3 | < 0.005 | 263.5 |
| Postoperative | 38.3 | 112 | 63.2 | 32.4 | 216 | 312 | 78 | 42 | 6.3 | 25.8 | 0.007 | 167.4 | |
| 2nd | Preoperative | 36.8 | 85 | 46.4 | 31.8 | 163 | 226 | 83 | 41 | 8.4. | 33.5 | < 0.005 | 183.4 |
| Postoperative | 36.6 | 76 | 32.7 | 16.4 | 64 | 75 | 54 | 36 | 4.4 | 16.4 | 0.008 | 74.3 | |
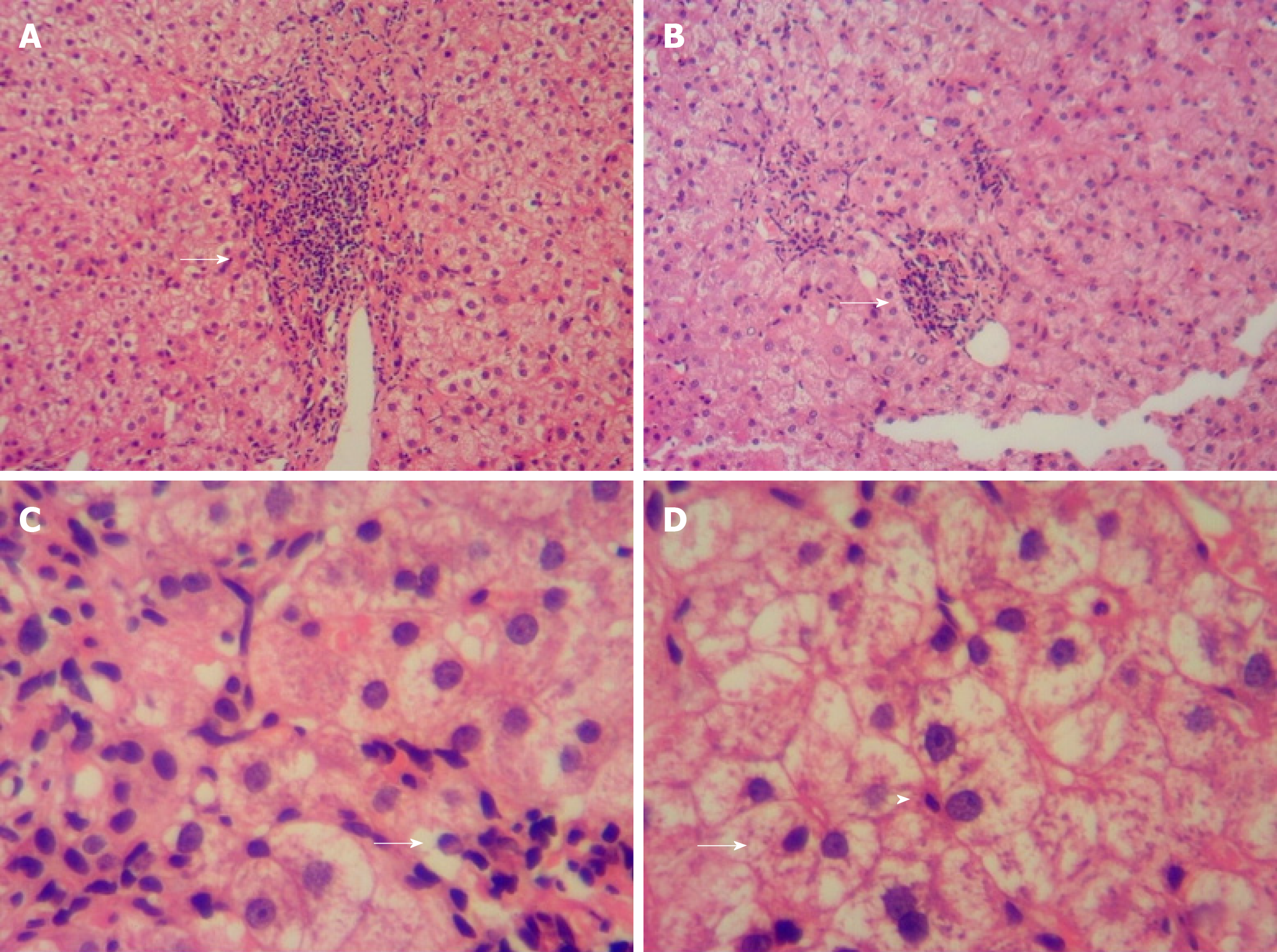
Thyroid B-ultrasonography showed diffuse thyroid lesions and abdominal B-ultrasonography showed no hepatic space-occupying lesions, echoes dense and uniform in the liver, and no abnormalities in other organs. Liver biopsy was performed one week later at second hospitalization, and showed the pathological features of AIH including moderate interfacial inflammation, lymphocyte-plasma cell infiltration, rosette-like hepatocyte infiltration, and hepatocyte lymphocyte penetration (Figure 1).
Thyroid storm with severe liver injury.
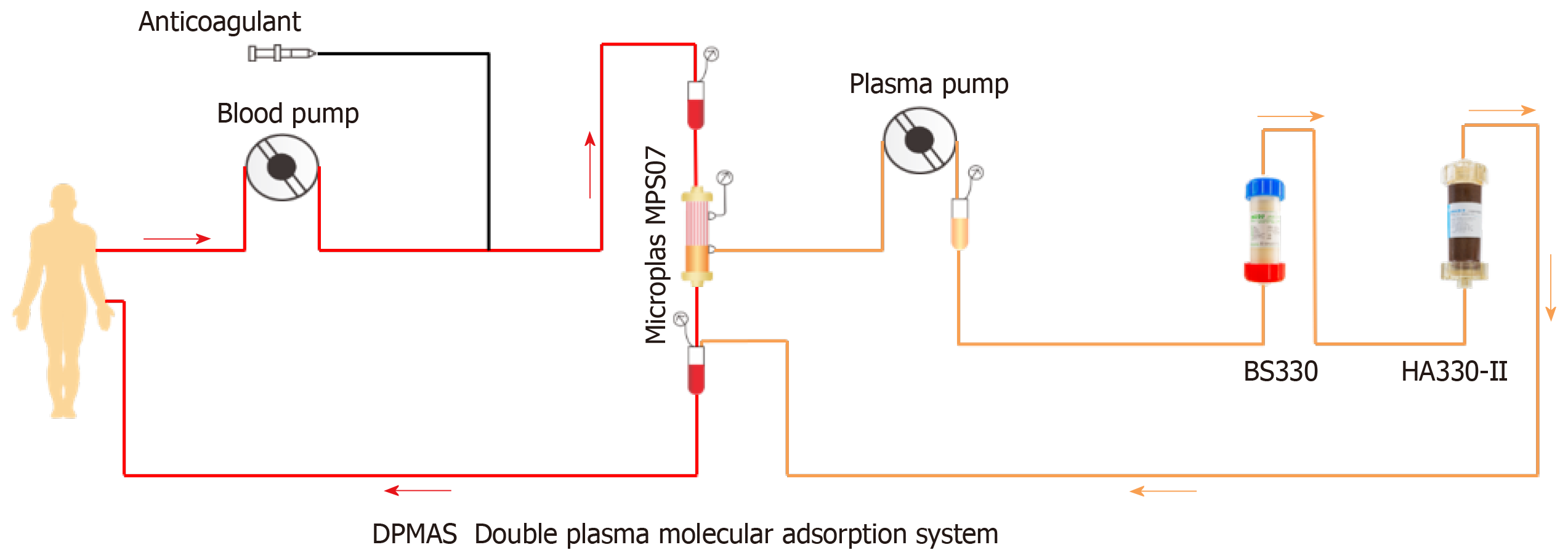
Forty milligram methylprednisolone, 50 mg methimazole, and 20 mg propranolol were given orally each day, and the patient was treated immediately with PE + DPMAS. The velocity of PE was 25-30 mL/min, PE volume was 1500 mL, and DPMAS was performed after PE. DPMAS consists of blood and plasma circuits and is shown in Figure 2. First, whole blood was collected from the femoral vein and pumped into the plasma separator. Plasma was then adsorbed by a bilirubin adsorber (BS330, Zhuhai Health Sails Biotechnology Co., LTD) and a macroporous neutral resin (HA330, Zhuhai Health Sails Biotechnology Co., LTD) in turn. Later, plasma was merged with the separated blood cells and reintroduced into the body (Figure 1). PE + DPMAS were conducted once every other day, and three times in all performed at first addmited. After 22 d, it was suggested that the patient continue with 8 mg/d methylprednisolone, 10 mg/d methylthimidazole, and 10 mg/d propranolol and attend regular follow-up appointments. However, methylthimidazole administration was discontinued by the patient without a doctor's advice. About 40 d later, the patient was re-admitted presented similar symptoms and liver function and thyroid index abnormalities. Methylprednisolone (32 mg) and PE + DPMAS were administered for twice.
Liver function and hyperthyroidism index returned to normal and the patient was discharged two weeks later. Long-term oral administration of 10 mg methyl-prednisolone and 10 mg methylthimidazole was prescribed. The discharging doctor suggested long-term treatment with 8 mg oral methylprednisolone and 10 mg oral methylthimidazole and regular follow-up appointments.
The etiology of hyperthyroidism-induced liver injury includes Graves’ disease hyperthyroidism, antithyroid drugs, and basic liver diseases[7]. Its pathogenesis may be related to the following factors[8]: (1) The direct toxic effect of thyroid hormone on the liver; (2) Hyperthyroidism making the liver relatively hypoxic, causing free radical production and hepatocyte damage; (3) Hyperthyroidism leading to Kupffer cell proliferation and serum AST elevation; (4) Thyroid hormone affecting intrahepatic enzyme activity to alter bilirubin metabolism and binding; and (5) Immune damage. According to clinical manifestation and laboratory examination, hyperthyroidism-induced liver injury can be divided into hepatocyte, cholestasis, and mixed types.
Thyroid storm is a pathological condition characterized by high elevated thyroid hormone levels leading to multiple organ failure[9]. Thyroid storms often occur in untreated or inadequately treated patients with hyperthyroidism and are often triggered by infection, high fever, and so on. Common manifestations include severe heart, lung, liver, and gastrointestinal damage and neurological dysfunction. Thyroid storm has a very high mortality rate of 10%-30%[10]. Traditional thyroid storm therapies include beta blockers, antithyroid drugs, iodine, and glucocorticoids.
AIH is a hepatic parenchymal inflammation mediated by an autoimmune response to hepatocytes. It is characterized by positive serum autoantibodies, hyperim-munoglobulinemia, and/or γ-globulinemia and histologically demonstrates interface hepatitis. AIH is often associated with other organ or systemic autoimmune diseases such as Hashimoto's disease. Thyroiditis (10%-23%), diabetes mellitus (7%-9%), inflammatory bowel disease (2%-8%), rheumatoid arthritis (2%-5%), Sjogren’s syndrome (1%-4%), psoriasis (3%), and systemic lupus erythematosus (1%-2%) are common associations[11]. On the other hand, one mechanism of hyperthyroidism-induced liver injury is an immune-mediated liver disorder[12].
Is AIH complicated by thyroid storm or liver immune injury caused by hyperthyroidism? According to the simplified criteria for the diagnosis of AIH by the International Autoimmune Hepatitis Group[13], AIH can be diagnosed by a score of 7 points (ANA 1:320, +2; IgG > 1.1 ULN, +2; pathological characteristics of liver, +1; excluding viral hepatitis, +2). In our patient, however, immune liver injury was more likely to be caused by hyperthyroidism storm rather than AIH complicated by thyroid storm. Although the patient had severe liver dysfunction, pathological characteristics, namely, inflammation and necrosis, were not serious, and pathological features and clinical and biochemical manifestations are separated. Furthermore, the second relapse was caused by anti-hyperthyroidism drug discontinuation and not corticosteroid discontinuation.
PE, which is called an intermediate artificial liver, can not only remove toxins, but also supplement coagulation factors, albumin, complements, and other beneficial substances lacking in liver failure. It has become the most important artificial liver treatment mode in the past decade, and is widely used. Since the 1970s, there have been many reports on the treatment of hyperthyroidism storm[14-19]. PE can quickly remove antibodies, thyroid hormones, and a large number of inflammatory factors from the blood and achieves good clinical results.
However, for a hyperbilirubinemia caused by liver failure, it may take more than 5 L plasma to replace 50% of the serum hyperbilirubin levels. Similarly, for a thyroid strom, it may take 30-50 L plasma to dilute FT3 to normal levels. It is obviously difficult to achieve adequate blood purification by PE alone. In recent years, decreased plasma collection has greatly limited the clinical application of PE. Blood adsorption technology is a blood purification method that uses different adsorbents to non-selectively absorb and remove endogenous or exogenous poisons in the blood. DPMAS is a combination of BS330 bilirubin adsorption and HA330-II hemoperfusion. The resin in a BS330 bilirubin adsorber is specific for bilirubin. It adsorbs bilirubin and bile acid by electrostatic force and lipophilic binding properties. Resin in a HA330-II hemoperfusion device is a relatively broad-spectrum adsorbent with a macroporous structure and large surface area. It can adsorb macromolecular toxins (such as inflammatory mediators IL-6 and IL-10) by Van der Waals force and a skeleton molecular sieve[20]. The combination of the two adsorbents can quickly remove harmful substances such as bilirubin, antibodies, thyroid hormones, and inflammatory mediators, alleviate inflammation, and improve the immune response. A DPMAS system can purify and absorb more than 6000 mL of plasma at a time, which can compensate for the decrease in clearance efficiency caused by insufficient PE.
We report a case of severe liver injury in a patient with severe hyperthyroidism and thyroid storm. The liver histopathology suggests that the immunological liver injury was caused by hyperthyroidism. This patient was treated with PE combined with DPMAS and achieved a good curative effect. This novel therapeutic combination may be worth popularizing.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Gabriel S, Loustaud-Ratti V, Milovanovic T S-Editor: Ji FF L-Editor: A E-Editor: Wu YXJ
| 1. | Galindo RJ, Hurtado CR, Pasquel FJ, García Tome R, Peng L, Umpierrez GE. National Trends in Incidence, Mortality, and Clinical Outcomes of Patients Hospitalized for Thyrotoxicosis With and Without Thyroid Storm in the United States, 2004-2013. Thyroid. 2019;29:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 69] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 2. | Thumma S, Manchala V, Mattana J. Radiocontrast-Induced Thyroid Storm. Am J Ther. 2018;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 3. | Choudhary AM, Roberts I. Thyroid storm presenting with liver failure. J Clin Gastroenterol. 1999;29:318-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 44] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Riveiro-Barciela M, Muñoz-Couselo E, Fernandez-Sojo J, Diaz-Mejia N, Parra-López R, Buti M. Acute liver failure due to immune-mediated hepatitis successfully managed with plasma exchange: New settings call for new treatment strategies? J Hepatol. 2018;pii:S0168-8278(18)32504-2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 47] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 5. | Chen JJ, Huang JR, Yang Q, Xu XW, Liu XL, Hao SR, Wang HF, Han T, Zhang J, Gan JH, Gao ZL, Wang YM, Lin SM, Xie Q, Pan C, Li LJ. Plasma exchange-centered artificial liver support system in hepatitis B virus-related acute-on-chronic liver failure: a nationwide prospective multicenter study in China. Hepatobiliary Pancreat Dis Int. 2016;15:275-281. [PubMed] |
| 6. | Oguntolu V. Severe thyrotoxicosis (thyroid storm) with liver failure. Acute Med. 2007;6:30-32. [PubMed] |
| 7. | Khemichian S, Fong TL. Hepatic dysfunction in hyperthyroidism. Gastroenterol Hepatol (NY). 2011;7:337-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 8. | Bhuyan AK, Sarma D, Kaimal Saikia U, Choudhury BK. Grave's Disease with Severe Hepatic Dysfunction: A Diagnostic and Therapeutic Challenge. Case Rep Med. 2014;2014:790458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 9. | Soleimanpour SA. Fulminant liver failure associated with delayed identification of thyroid storm due to heterophile antibodies. Clin Diabetes Endocrinol. 2015;1:pii: 12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Kanbay M, Sengul A, Güvener N. Trauma induced thyroid storm complicated by multiple organ failure. Chin Med J (Engl). 2005;118:963-965. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | Liberal R, Grant CR, Mieli-Vergani G, Vergani D. Autoimmune hepatitis: a comprehensive review. J Autoimmun. 2013;41:126-139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 155] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 12. | Yamada M, Shibata H, Masugi Y, Ishi T, Kameyama K, Ebinuma H, Hasegawa T. Histological Changes in Autoimmune Hepatitis with Graves' Disease: A Child Case Report. Intern Med. 2017;56:2139-2143. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 13. | Hennes EM, Zeniya M, Czaja AJ, Parés A, Dalekos GN, Krawitt EL, Bittencourt PL, Porta G, Boberg KM, Hofer H, Bianchi FB, Shibata M, Schramm C, Eisenmann de Torres B, Galle PR, McFarlane I, Dienes HP, Lohse AW; International Autoimmune Hepatitis Group. Simplified criteria for the diagnosis of autoimmune hepatitis. Hepatology. 2008;48:169-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1205] [Cited by in RCA: 1253] [Article Influence: 73.7] [Reference Citation Analysis (0)] |
| 14. | Schlienger JL, Faradji A, Demangeat C, Sapin R, Chabrier G, Simon C, Imler M. [Quantitative evaluation of hormonal extraction performed by continuous plasma exchange in euthyroid patients. Application to the treatment of severe hyperthyroidism]. Presse Med. 1983;12:499-502. [PubMed] [DOI] [Full Text] |
| 15. | Schlienger JL, Faradji A, Sapin R, Blickle JF, Chabrier G, Simon C, Imler M. [Treatment of severe hyperthyroidism by plasma exchange. Clinical and biological efficacy. 8 cases]. Presse Med. 1985;14:1271-1274. [PubMed] |
| 16. | Pinsard D, Chadenas D, Pierre D, Walle T, Aumaitre J. [Plasma exchange and hyperthyroidism. Current indications]. Ann Endocrinol (Paris). 1985;46:89-98. [PubMed] |
| 17. | Miljić D, Stojanović M, Ješić R, Bogadnović G, Popović V. Role of plasma exchange in autoimmune hyperthyroidism complicated by severe tiamazol-induced cholestatic jaundice. Transfus Apher Sci. 2013;49:354-356. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 18. | Keklik M, Kaynar L, Yilmaz M, Sivgin S, Solmaz M, Pala C, Aribas S, Akyol G, Unluhizarci K, Cetin M, Eser B, Unal A. The results of therapeutic plasma exchange in patients with severe hyperthyroidism: a retrospective multicenter study. Transfus Apher Sci. 2013;48:327-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 19. | Garla V, Kovvuru K, Ahuja S, Palabindala V, Malhotra B, Abdul Salim S. Severe Hyperthyroidism Complicated by Agranulocytosis Treated with Therapeutic Plasma Exchange: Case Report and Review of the Literature. Case Rep Endocrinol. 2018;2018:4135940. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 20. | Wan YM, Li YH, Xu ZY, Yang J, Yang LH, Xu Y, Yang JH. Therapeutic plasma exchange versus double plasma molecular absorption system in hepatitis B virus-infected acute-on-chronic liver failure treated by entercavir: A prospective study. J Clin Apher. 2017;32:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 41] [Article Influence: 5.1] [Reference Citation Analysis (0)] |