Published online May 26, 2019. doi: 10.12998/wjcc.v7.i10.1093
Peer-review started: February 6, 2019
First decision: March 5, 2019
Revised: March 24, 2019
Accepted: May 1, 2019
Article in press: May 1, 2019
Published online: May 26, 2019
Processing time: 113 Days and 2.1 Hours
Perioperative allogeneic blood transfusion is associated with poorer outcomes.
To identify the factors that were associated with perioperative transfusion and to examine the impact of perioperative transfusion in patients undergoing resection of colorectal cancer (CRC) liver metastases.
The United States National Inpatient Sample (NIS) database was searched for patients with CRC who received surgery for liver metastasis. Linear and logistic regression analyses were performed.
A total of 2018 patients were included, and 480 had a perioperative transfusion. Emergency admission (adjusted odds ratio [aOR] = 1.42; 95%CI: 1.07-1.87), hepatic lobectomy (aOR = 1.76; 95%CI: 1.42-2.19), and chronic anemia (aOR = 2.62; 95%CI: 2.04-3.35) were associated with increased chances of receiving a transfusion, but receiving surgery at a teaching hospital (aOR = 0.75; 95%CI: 0.58-0.98) was associated with a decreased chance of receiving a transfusion. Receiving a perioperative transfusion was significantly associated with increased in-hospital mortality (aOR = 3.38; 95%CI: 1.57-7.25), and increased overall postoperative complications (aOR = 1.67; 95%CI: 1.31-2.13), as well as longer length of hospital stay
Patients with an emergency admission, hepatic lobectomy, chronic anemia, and who have surgery at a non-teaching hospital are more likely to receive a perioperative transfusion. Patients with CRC undergoing surgery for hepatic metastases who receive a perioperative transfusion are at a higher risk of in-hospital mortality, postoperative complications, and longer length of hospital stay.
Core tip: Among patients undergoing resection of colorectal cancer liver metastases, those with an emergency admission, hepatic lobectomy, chronic anemia, and undergoing surgery at a non-teaching hospital are more likely to receive a perioperative transfusion. Furthermore, patients who receive a perioperative transfusion are at a higher risk of in-hospital mortality, postoperative complications, and longer length of hospital stay.
- Citation: Long B, Xiao ZN, Shang LH, Pan BY, Chai J. Impact of perioperative transfusion in patients undergoing resection of colorectal cancer liver metastases: A population-based study. World J Clin Cases 2019; 7(10): 1093-1102
- URL: https://www.wjgnet.com/2307-8960/full/v7/i10/1093.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i10.1093
The use of allogeneic blood transfusion during surgery has improved outcomes and saved countless lives. However, blood transfusion is not without risk. Risks of a blood transfusion include allergic reactions, infectious diseases, acute or delayed hemolytic reaction, and transfusion-related immune modulation (TRIM)[1]. Furthermore, studies have linked perioperative allogeneic blood transfusions with poorer outcomes such as increased postoperative complications and mortality[2,3].
Patients with malignancies undergoing surgery are frequently anemic or the anticipated blood loss during surgery may be large, and as such the transfusion threshold is relatively low. However, a number of studies have shown that a peri-operative transfusion is associated with poorer oncological outcomes, such as cancer recurrence[4-8]. While the pathophysiological mechanism is not entirely clear, it is thought to be related to an altered immune response as a result of TRIM which changes the body’ surveillance for malignant cells.[3]
Colorectal cancer (CRC) is potentially curative by surgery. Patients with a metastatic disease confined to the liver also have the potential for a curative surgery such as hepatic lobectomy or hepatic metastectomy[6]. However, these patients are at an increased risk for a perioperative transfusion, and thus the effect of a transfusion on surgical and oncological outcomes is an area of study. A number of studies have indicated that patients with CRC undergoing colon resection and/or surgical treatment of liver metastasis who receive a perioperative allogeneic transfusion have increased mortality, reduced overall survival, reduced disease free survival, more postoperative complications, and longer length of hospital stay[4,9-12]. Studies, however, have not been consistent in all of their findings.
Thus, the purpose of this study was to use a United States nationwide population-based database to identify factors associated with transfusing blood, and to examine the impact of perioperative transfusion in patients undergoing resection of CRC liver metastases. Analysis of the impact of perioperative transfusion in this population may help to assess the significance of appropriate blood management measures and transfusion avoidance strategies.
Data for this population-based study were extracted from the United States Nationwide Inpatient Sample (NIS) database. The NIS was developed as part of the United States Healthcare Cost and Utilization Project (HCUP), which is sponsored by the Agency for Healthcare Research and Quality. The NIS represents a 20% sample of inpatient admissions from the 45 states that participate in the program. The NIS database contains over 100 clinical and nonclinical data elements from approximately 8 million hospital stays each year. Data available include primary and secondary diagnoses, primary and secondary procedures, admission and discharge status, patient demographic information, expected payment source, length of hospital stay, and hospital characteristics. The present study did not require either Institutional Review Board approval or informed consent by the study subjects because the NIS data are de-identified. We obtained permission to access the research data files of the HCUP program (certificate number, HCUP- 1M44EVV39), and the data use agreement for the NIS from the HCUP Project was followed.
We first identified patients in the 2005 to 2014 NIS database who had a partial hepatectomy (ICD-9: 50.22) and/or hepatic lobectomy (ICD-9: 50.3). From this population, we identified patients with liver metastases (ICD-9: 197) from CRC (ICD-9: 153; 154.1, 154.8).
Patients with a history of blood transfusion (V58.2) or coagulopathy (CM_COAG) were excluded. Finally, the eligible patients were divided into two groups: those who received a perioperative transfusion (ICD-9: 99.0) and those who did not.
Length of hospital stay (LOS) was defined using (LOS_X), and in-hospital mortality was defined using (DIED). In addition, patients with one of the following postoperative complications were recognized as positive with respect to overall postoperative complications: infection (ICD-9: 998.59); intra-abdominal abscess (ICD-9: 567.22); infected post-operative seroma (ICD-9: 998.51); wound dehiscence (ICD-9: 998.31, 998.32); urinary tract infection (UTI) (ICD-9: 595.0, 996.64, 997.5); pulmonary complications, including pulmonary embolus and pneumonia (ICD-9: 415.11, 997.31, 997.32, 997.39); deep vein thrombosis (DVT) (ICD-9: 451.11, 451.19, 451.2, 451.81, 451.82, 451.83, 451.84, 451.89, 451.9, 453.4, 453.41, 453.42, 997.2); postoperative myocardial infarction (ICD-9: 997.1, 410.0-410.9, 998.0); sepsis (ICD-9: 995.91); ascites (ICD-9: 789.5); acute liver failure (ICD-9: 570); and other digestive system complications (ICD-9: 997.49).
Patient demographic characteristics (age, sex, and race) and data of income status, type of admission, insurance status, hospital bed size, hospital location, hospital teaching status, and hospital region were collected from the NIS database. The following potential confounding variables were also examined with data extracted for the NIS database: hepatic precondition, primary tumor site, extent of liver resection, laparoscopic procedure, robotic-assisted procedure, and comorbidities (chronic anemia, congestive heart failure, chronic pulmonary disease, diabetes, hypertension, obesity, and renal failure). The definition of comorbidities used in this study was based on the NIS database. Variables CM_ANEMDEF and CM_BLDLOSS were used for chronic anemia. Variables CM_CHF and CM_CHRNLUNG were used for congestive heart failure and chronic pulmonary disease, respectively. Variables CM_DM and CM_DMCX were used for diabetes. Variable CM_HTN_C was used for hypertension. CM_OBESE and CM_RENLFAIL were used for obesity and renal failure, respectively.
Statistical review of the present study was performed by a biomedical statistician. Descriptive statistics were used to present patient demographic and clinical characteristics and hospital data, and are presented as unweighted counts and weighted percentages. Data were weighted according to recommendations from the Healthcare Cost and Utilization Project (HCUP), using three variables for weights (DISCWT), stratum (NIS_STRATUM), and cluster (HOSPID) to produce national estimates. Chi-square tests were performed to examine associations between characteristics and perioperative transfusion. To illustrate that patients who received a perioperative transfusion would have a greater risk of mortality and postoperative complications, and longer length of stay as reported in other studies[10], we estimated the association between perioperative transfusion and mortality, postoperative complications, and length of stay by linear regression and logistic regression. Significant findings in baseline demographic, clinical, and hospital characteristics were selected and input into multivariate models. Finally, univariate and multivariate logistic regressions were performed to examine associations between perioperative transfusion and clinical outcomes and comorbidities with adjustment for demographic characteristics and hospital characteristics. Two-sided P-values < 0.05 were considered statistically significant. All statistical analyses were performed with SAS version 9.4 (Windows NT version, SAS Institute, Inc., Cary, NC, United States).
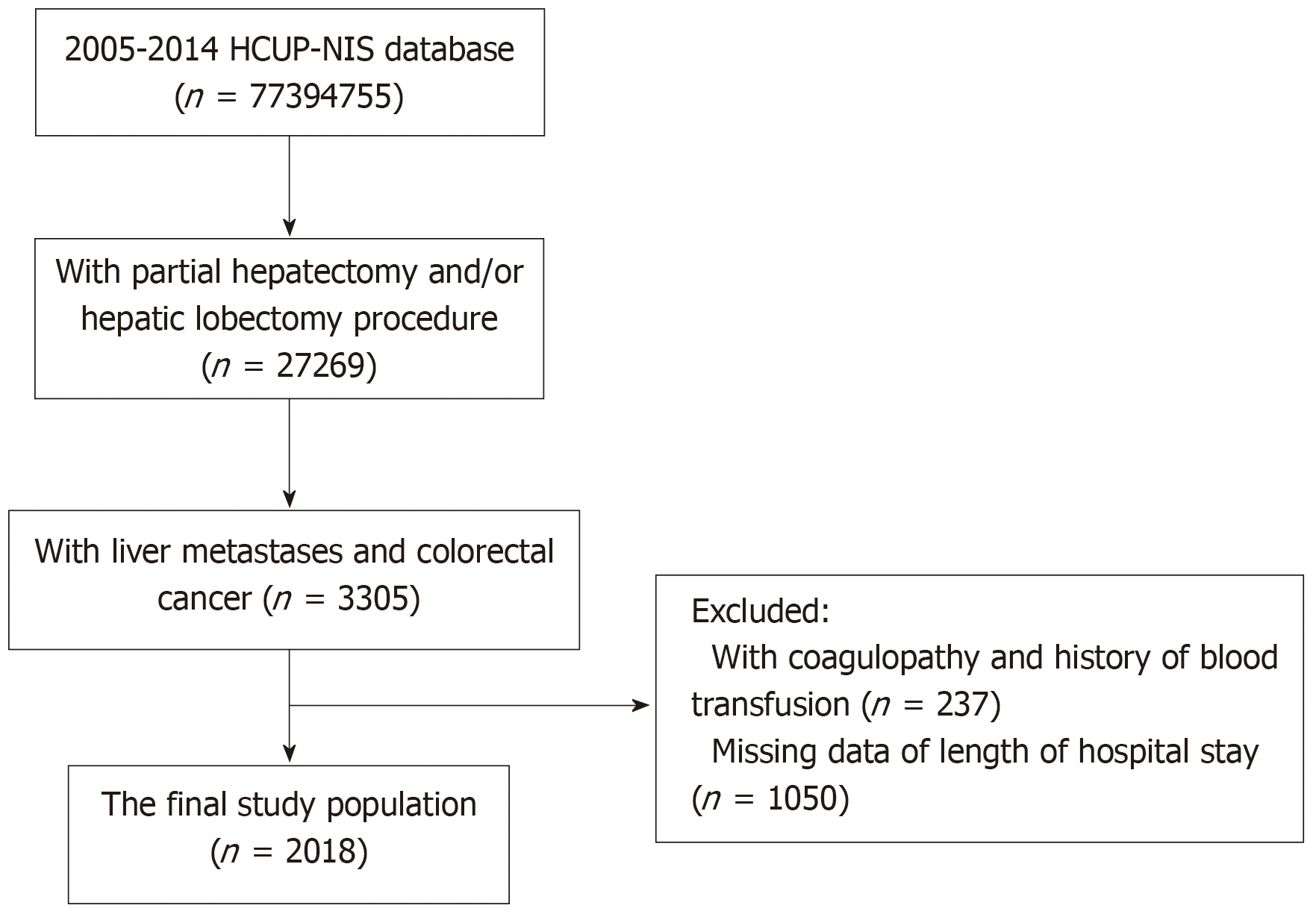
A total of 27269 patients who had a partial hepatectomy and/or hepatic lobectomy were initially identified in the 2005-2014 HCUP-NIS database. Of them, 3305 patients had liver metastases and CRC. After excluding patients with coagulopathy and a history of blood transfusion (n = 237), and patients who lacked data of length of hospital stay (n = 1050), 2018 patients were included in the study analysis (Figure 1). G*Power was used to calculate the number of samples required, which revealed that the present study required at least 150 subjects, so the sample size of this study is sufficient. In addition, after applying weights, the analytic sample size was equivalent to a population-based sample size of 9991 individuals.
The mean patient age was 59.75 years, with 1532 patients below the age of 70 (Table 1). Of the 2018 patients, 480 had a perioperative transfusion, while 1538 did not. The non-transfusion group and the perioperative transfusion group were significantly different in age, sex, type of admission, insurance status, primary tumor site, extent of liver resection, chronic anemia, congestive heart failure, hypertension, bed size of hospital, and teaching status of hospital. A higher proportion of perioperative transfusion patients also had their primary tumor site in the colon (vs rectum) (83.59%), compared with patients who did not have a transfusion (77.64%). More patients with transfusions had hepatic lobectomies (29.81%), when compared to patients who did not have transfusions (21.60%). Comorbidities were also more prevalent in patients who had a perioperative transfusion, especially for the comorbidities of chronic anemia (36.37% vs 15.87%), congestive heart failure (5.02% vs 2.31%), and hypertension (46.32% vs 40.43%). Patients who had a transfusion were more likely to be in medium sized hospitals (21.12% vs 15.15%) and non-teaching hospitals (27.64% vs 18.46%).
| Total | No transfusion | Perioperative transfusion | P-value1 | |
| n = 2018 | n = 1538 | n = 480 | ||
| Demographic data | ||||
| Age | ||||
| <70 yr | 1532 (76.04) | 1195 (77.88) | 337 (70.12) | 0.0004a |
| ≥70 yr | 486 (23.96) | 343 (22.12) | 143 (29.88) | |
| Sex | ||||
| Male | 1112 (55.10) | 870 (56.59) | 242 (50.32) | 0.01a |
| Female | 903 (44.90) | 665 (43.41) | 238 (49.68) | |
| Race | ||||
| White | 1251 (76.29) | 955 (76.94) | 296 (74.22) | 0.10 |
| Black | 175 (10.53) | 121 (9.63) | 54 (13.32) | |
| Hispanic | 115 (7.07) | 86 (7.04) | 29 (7.16) | |
| Asian/Pacific | 55 (3.32) | 40 (3.21) | 15 (3.62) | |
| Others | 46 (2.80) | 39 (3.16) | 7 (1.68) | |
| Household income | ||||
| Q1 | 484 (24.34) | 355 (23.59) | 129 (26.90) | 0.14 |
| Q2 | 473 (23.79) | 369 (24.34) | 104 (22.06) | |
| Q3 | 471 (23.93) | 372 (24.89) | 99 (20.87) | |
| Q4 | 541 (27.93) | 400 (27.22) | 141 (30.16) | |
| Type of admission | ||||
| Elective | 1676 (83.35) | 1311 (85.59) | 365 (76.12) | <0.0001a |
| Emergency | 338 (16.65) | 224 (14.41) | 114 (23.88) | |
| Insurance status | ||||
| Medicare/Medicaid | 877 (43.31) | 628 (40.62) | 249 (51.97) | <0.0001a |
| Private including HMO | 1027 (51.05) | 816 (53.25) | 211 (43.95) | |
| Self-pay/no charge/other | 114 (5.65) | 94 (6.13) | 20 (4.07) | |
| Clinical data | ||||
| Hepatic precondition | ||||
| No | 1967 (97.41) | 1494 (97.07) | 473 (98.52) | 0.24 |
| Steatosis/fibrosis | 44 (2.23) | 38 (2.52) | 6 (1.29) | |
| Cirrhosis | 7 (0.36) | 6 (0.41) | 1 (0.19) | |
| Primary tumor site | ||||
| Colon | 1595 (79.04) | 1194 (77.64) | 401 (83.59) | 0.003a |
| Rectum | 423 (20.95) | 344 (22.36) | 79 (16.40) | |
| Extent of liver resection | ||||
| Partial hepatectomy | 1541 (76.46) | 1204 (78.39) | 337 (70.19) | <0.0001a |
| Hepatic lobectomy | 477 (23.54) | 334 (21.60) | 143 (29.81) | |
| Laparoscopic procedure | ||||
| No | 1971 (97.64) | 1498 (9737.) | 473 (98.52) | 0.15 |
| Yes | 47 (2.36) | 40 (2.63) | 7 (1.48) | |
| Robotic-assisted | ||||
| No | 2010 (99..59) | 14532 (99.59) | 478 (99.56) | 0.91 |
| Yes | 8 (0.41) | 6 (0.40) | 2 (0.43) | |
| Comorbidity | ||||
| Chronic anemia | 422 (20.71) | 247 (15.87) | 175 (36.37) | <0.0001a |
| Congestive heart failure | 59 (2.95) | 35 (2.31) | 24 (5.02) | 0.002a |
| Chronic pulmonary disease | 188 (9.26) | 135 (8.72) | 53 (11.02) | 0.12 |
| Diabetes | 293 (14.32) | 221 (14.16) | 72 (14.84) | 0.70 |
| Hypertension | 848 (41.82) | 625 (40.43) | 223 (46.32) | 0.02a |
| Obesity | 118 (5.85) | 89 (5.80) | 29 (6.02) | 0.86 |
| Renal failure | 39 (1.89) | 30 (1.936) | 9 (1.79) | 0.86 |
| Hospital data | ||||
| Hospital bed size | ||||
| Small | 212 (10.21) | 162 (10.28) | 50 (9.97) | 0.007a |
| Medium | 327 (16.58) | 227 (15.15) | 100 (21.12) | |
| Large | 1453 (73.22) | 1125 (74.56) | 328 (68.91) | |
| Location of hospital | ||||
| Rural | 77 (3.71) | 62 (3.89) | 15 (3.14) | 0.34 |
| Urban | 1915 (96.29) | 1452 (96.10) | 463 (96.87) | |
| Teaching status of hospital | ||||
| Non-teaching | 419 (20.65) | 284 (18.46) | 135 (27.64) | <0.0001a |
| Teaching | 1573 (79.35) | 1230 (81.54) | 343 (72.36) | |
| Region of hospital | ||||
| Northeast | 437 (22.65) | 351 (23.79) | 86 (18.97) | 0.11 |
| Midwest | 480 (23.71) | 360 (23.27) | 120 (25.11) | |
| South | 737 (35.65) | 566 (35.99) | 171 (34.53) | |
| West | 364 (17.98) | 261 (16.94) | 103 (21.39) |
In order to identify the factors that were associated with perioperative transfusion, logistic regression models were performed (Table 2). Univariate logistic regression analysis found that perioperative transfusion was significantly associated with age, sex, type of admission, insurance status, primary tumor site, extent of liver resection, comorbidities, and teaching status of the hospital. Patients who were 70 years of age and above had a 1.5-times higher risk of getting a perioperative transfusion as compared to patients who were younger than 70 (95%CI: 1.19-1.88). Female patients had a 1.31-times higher risk of getting a perioperative transfusion as opposed to male patients (95%CI: 1.08-1.58). Patients who had an emergency admission had a 1.9-times higher risk of getting a perioperative transfusion than patients who had an elective admission (95%CI: 1.45-2.50). Patients who had a hepatic lobectomy had increased odds for perioperative transfusion as compared to patients who had a partial hepatectomy (OR = 1.54, 95%CI: 1.24-1.91). Furthermore, patients with chronic anemia, congestive heart failure, and hypertension had 2.98, 2.20, and 1.27 higher risks, respectively, of receiving a perioperative transfusion as compared to patients who did not have these conditions.
| OR (95%CI) | aOR (95%CI) | |
| Age | ||
| <70 yr | 1 | 1 |
| ≥70 yr | 1.50 (1.19, 1.88) | 1.10 (0.83, 1.47) |
| Sex | ||
| Male | 1 | 1 |
| Female | 1.31 (1.08, 1.58) | 1.19 (0.97, 1.45) |
| Type of admission | ||
| Elective | 1 | 1 |
| Emergency | 1.90 (1.45, 2.50) | 1.42 (1.07, 1.87) |
| Insurance status | ||
| Medicare/Medicaid | 1 | 1 |
| Private including HMO | 0.65 (0.53, 0.80) | 0.78 (0.60, 1.01) |
| Self-pay/no charge/other | 0.49 (0.29, 0.85) | 0.56 (0.31, 1.00) |
| Primary tumor site | ||
| Colon | 1 | 1 |
| Rectum | 0.67 (0.52, 0.87) | 0.86 (0.66, 1.14) |
| Extent of liver resection | ||
| Partial hepatectomy | 1 | 1 |
| Hepatic lobectomy | 1.54 (1.24, 1.91) | 1.76 (1.42, 2.19) |
| Comorbidity | ||
| Chronic anemia | 2.98 (2.34, 3.80) | 2.62 (2.04, 3.35) |
| Congestive heart failure | 2.20 (1.32, 3.69) | 1.57 (0.91, 2.68) |
| Hypertension | 1.27 (1.03, 1.57) | 1.10 (0.88, 1.37) |
| Bed size of hospital | ||
| Small | 1 | 1 |
| Medium | 1.43 (1.00, 2.04) | 1.26 (0.85, 1.86) |
| Large | 0.95 (0.69, 1.30) | 0.92 (0.65, 1.30) |
| Teaching status of hospital | ||
| Non-teaching | 1 | 1 |
| Teaching | 0.59 (0.46, 0.76) | 0.75 (0.58, 0.98) |
In contrast, compared to users of Medicare/Medicaid, patients who had private insurance (OR = 0.65; 95%CI: 0.53-0.80) or were self-paid (OR = 0.49; 95%CI: 0.29-0.85) had a lower chance of receiving a perioperative transfusion. Patients whose primary tumor site was located in the rectum had a lower risk of having a perioperative transfusion compared to patients with a primary colon tumor (OR = 0.67; 95%CI: 0.52-0.87). Patients at teaching hospitals had a lower risk of receiving a perioperative transfusion than those at non-teaching hospitals (OR = 0.59; 95%CI: 0.46-0.76).
Multivariate logistic regression analysis found that only four factors retained their significant association with perioperative transfusion: emergency admission (aOR = 1.42; 95%CI: 1.07-1.87), hepatic lobectomy (aOR = 1.76; 95%CI: 1.42-2.19), chronic anemia (aOR = 2.62; 95%CI: 2.04-3.35), and teaching hospital (aOR = 0.75; 95%CI: 0.58-0.98).
The frequencies of postoperative complications in the non-transfusion and perioperative transfusion group are shown in Table 3. Of the 2018 included patients, 338 had postoperative complications, with approximately one-third of infections (n = 108). The results of association analysis between perioperative transfusion and clinical outcomes are shown in Table 4. Both univariate and multivariate linear regression models revealed significant associations between perioperative transfusion and length of hospital stay (univariate: β = 2.01, standard error [SE] = 0.37; multivariate: β = 1.51, SE = 0.36). These results indicate that perioperative transfusion was associated with an increased length of hospital stay after adjustment for sex, type of admission, chronic anemia, congestive heart failure, hypertension, and hospital size.
| Total | No transfusion | Perioperative transfusion | |
| n = 2018 | n = 1538 | n = 480 | |
| Overall postoperative complications | 338 (16.67) | 229 (11.31) | 109 (5.36) |
| Infection | 108 (5.36) | 67 (3.31) | 41 (2.05) |
| Intra-abdominal abscess | 45 (2.19) | 27 (1.30) | 18 (0.89) |
| Infected post-operative seroma | 5 (0.26) | 4 (0.22) | 1 (0.05) |
| Wound dehiscence | 27 (1.31) | 21 (1.02) | 6 (0.29) |
| UTI | 32 (1.59) | 26 (1.29) | 6 (0.30) |
| Pulmonary complications | 41 (2.05) | 21 (1.04) | 20 (1.00) |
| DVT | 26 (1.29) | 18 (0.90) | 8 (0.39) |
| Postoperative myocardial infarction | 58 (2.88) | 42 (2.10) | 16 (0.78) |
| Sepsis | 30 (1.44) | 23 (1.09) | 7 (0.34) |
| Ascites | 65 (3.20) | 43 (2.13) | 22 (1.07) |
| Acute liver failure | 14 (0.70) | 10 (0.49) | 4 (0.21) |
| Other digestive system complications | 17 (0.81) | 14 (0.67) | 3 (0.14) |
The univariate logistic regression model showed significant associations between perioperative transfusion and in-hospital mortality (OR = 4.42; 95%CI: 2.05-9.51), and overall postoperative complications (OR = 1.69; 95%CI: 1.33-2.15). After adjustment for age, type of admission, extent of liver resection, and hypertension, multivariate analysis showed a significant association between perioperative transfusion and in-hospital mortality (aOR = 3.38; 95%CI: 1.57-7.25). After adjustment for congestive heart failure and hypertension, multivariate analysis indicated a significant association between perioperative transfusion and overall postoperative comp-lications (aOR = 1.67; 95%CI: 1.31-2.13).
The results of this study using data from the United States NIS showed that significant risk factors for receiving a perioperative transfusion were emergency admission, hepatic lobectomy, and chronic anemia, while having surgery at a teaching hospital was associated with a lower risk of receiving a transfusion. In addition, for patients with CRC undergoing surgery for hepatic metastasis, perioperative transfusion was significantly associated with greater in-hospital mortality, a higher rate of postoperative complications, and longer length of hospital stay.
The results of this study have confirmed the results of other studies of CRC, and are consistent with studies that have shown perioperative transfusions are associated with poorer outcomes in patients with lung cancer undergoing resection[8], and patients with gastric cancer undergoing curative surgeries[7]. Importantly, this study identified factors associated with receiving a transfusion, which may assist in identifying patients in whom more intensive management may help to avoid a transfusion and thus obtain a better outcome.
It has become clear that perioperative allogeneic blood transfusions are associated with worse outcomes in patients with CRC who receive surgery. A recent systematic review and meta-analysis by Acheson et al[4] included approximately 21000 CRC patients, of whom 58% received a transfusion. The analysis revealed that allogeneic blood transfusion was significantly associated with increased all-cause mortality, cancer-related mortality, combined recurrence-metastasis-death, postoperative infection, surgical re-intervention, and longer length of hospital stay. Studies of patients who undergo surgery for CRC liver metastases have provided similar results.
A recent meta-analysis by Lyu et al[9] examined the outcomes of patients who received a hepatectomy for CRC metastasis. The analysis included 10621 patients, and the authors found that transfused patients had higher overall morbidity (OR = 1.98), higher mortality (OR = 4.13), longer hospital stays (OR = 4.43), reduced overall survival (RR = 1.24), and reduced disease-free survival (RR = 1.38). Another recent meta-analysis by Bennet et al[2] also reported that a transfusion in patients undergoing liver resection was associated with postoperative complications and decreased cancer survival.
The American College of Surgeons National Surgical Quality Improvement Program reviewed approximately 27000 cases of CRC surgery, of which 14% had blood transfusions[6]. They found that transfusions were associated with increased mortality (OR = 1.78), morbidity (OR = 2.38), length of hospital stay (mean difference 3.5 d), pneumonia (OR = 2.70), and surgical site infection (OR = 1.45). Interestingly, they found that the effect was dose dependent; patients who received a greater amount of transfused blood were more likely to have adverse events. Another study also reported that in patients undergoing hepatic resection for CRC metastasis, the operative mortality rate was 2.5% for patients who received one or two units of blood, 11.1% for those who received more than two units, and only 1.2% for those who did not receive a transfusion[10].
Other studies have also examined factors associated with receiving a transfusion. The aforementioned American College of Surgeons study found that predictors of a blood transfusion were hematocrit < 38%, open surgery, proctectomy, low platelet count, American Society of Anesthesiologists class IV or V, total colectomy, metastatic cancer, emergency surgery, ascites, and infection[6]. Schiergens et al[11] also examined factors associated with having a transfusion and found that female sex, preoperative anemia, major intraoperative blood loss, and major postoperative complications were independently associated with the necessity of a transfusion. The authors also reported that a perioperative transfusion was independently associated with earlier disease recurrence. An earlier study that examined 480 consecutive patients who underwent hepatic resection reported that a preoperative hemoglobin level below 12.5 g/dL, largest tumor more than 4 cm, exposure of the vena cava, an associated procedure, and cirrhosis were associated with the need for a transfusion[13]. The authors used these data to develop a transfusion risk score (TRS), and in a validation set, the area under the receiver operating characteristic curve was 0.89. A still earlier study demonstrated that prothrombin rate and the size of the liver resection were independently correlated with receiving a blood transfusion in patients with liver tumors undergoing resection[14].
Although it appears clear that perioperative blood transfusions are associated with worse surgical and oncological outcomes, the exact reasons remain to be determined. Current evidence suggests that TRIM, a complex immunological condition that results in transient immunosuppression, is involved[1,3].
In addition to identifying factors associated with receiving a perioperative transfusion, efforts have been made to reduce the need for allogenic blood transfusions. Bui et al[15] examined “blood saving strategies” such as administration of aprotinin, low CVP anesthesia, use of the Pringle maneuver, and ultrasonic dissection in patients undergoing hepatic resection and reported that blood saving strategies decreased the estimated blood loss from a mean of 4500 mL to 1000 mL. Another study showed that preoperative autologous blood donation reduced the need for transfusion of homologous blood, as well as reducing the overall complication rate[16].
There are both strengths and limitations to this study. An important strength of the study is that it included a relatively large representative sample of patients from the United States, despite the relative rarity of the procedure. However, diagnoses were identified based on ICD-9 codes only, and coding errors and misclassifications might exist. In addition, the severities of the comorbidities were not known, as that information is not available in the database, and thus may confound the results. As a retrospective observational study, only associations can be demonstrated; causation cannot be determined. The primary aim of this study was to determine factors associated with a perioperative blood transfusion, and the findings may help surgeons and anesthetists anticipate the need for blood products during surgery. Examination of the long-term clinical and oncological outcomes was beyond the scope of this study.
In summary, patients with an emergency admission, hepatic lobectomy, chronic anemia, and who have surgery at a teaching hospital are more likely to receive a perioperative transfusion. Patients with CRC undergoing surgery for hepatic metastases who receive a perioperative transfusion are at a higher risk of in-hospital mortality, postoperative complications, and longer length of hospital stay.
Since perioperative allogeneic blood transfusion is associated with poorer outcomes, the risk of blood transfusion is high, including allergic reactions, infectious diseases, acute or delayed hemolytic reaction, and transfusion-related immune modulation. Previous studies have shown that perioperative allogeneic blood transfusion was associated with poor outcomes, such as increased postoperative complications and mortality. Patients with malignant tumors undergoing surgery often showed anemia. The amount of blood loss during surgery may be large, so the blood transfusion threshold is relatively low.
The use of allogeneic blood transfusion during surgery can improve outcomes and save countless lives. However, blood transfusions have higher risks, such as allergic reactions, infectious diseases, acute or delayed hemolysis, and transfusion-related immune regulation.
Based on above motivation, the study was designed to determine factors associated with perioperative blood transfusions and to examine the effects of perioperative blood transfusions on patients with colorectal cancer (CRC) metastasis undergoing liver resection.
A total of 2018 patients were included from The United States National Inpatient Sample database, of whom 480 had a perioperative transfusion. Comorbidities such as chronic anemia, congestive heart failure, chronic pulmonary disease, diabetes, hypertension, obesity, and renal failure were used.
Emergency admission, hepatectomy, and chronic anemia were significantly positively associated with the chance of receiving a blood transfusion, but there was a significant negative correlation between the chances of undergoing surgery and receiving blood transfusions at teaching hospitals. Perioperative blood transfusions were significantly associated with increased in-hospital mortality, overall increase in postoperative complications, and prolonged hospital stay.
The results of this study demonstrated that in addition to hepatic lobectomy, emergency admission, chronic anemia, and surgery at a non-teaching hospital are more likely to receive a perioperative transfusion. In addition, the study provides an initial hit that patients with liver metastases who undergo perioperative transfusion are at a higher risk of hospital mortality, postoperative complications, and longer hospital stays.
Based on this study, patients with liver metastasis who undergo tumor resection have a higher chance of receiving a blood transfusion, and a higher risk of hospital mortality. In future studies, it is worthwhile to continue to study the impact of liver resection area and extent on mortality in CRC patients.
Manuscript source: Invited manuscript
Specialty type: Peripheral Vascular Disease
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Bang YJ, Saner F S-Editor: Ma YJ L-Editor: Wang TQ E-Editor: Wu YXJ
| 1. | Cata JP, Wang H, Gottumukkala V, Reuben J, Sessler DI. Inflammatory response, immunosuppression, and cancer recurrence after perioperative blood transfusions. Br J Anaesth. 2013;110:690-701. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 291] [Cited by in RCA: 335] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 2. | Bennett S, Baker LK, Martel G, Shorr R, Pawlik TM, Tinmouth A, McIsaac DI, Hébert PC, Karanicolas PJ, McIntyre L, Turgeon AF, Barkun J, Fergusson D. The impact of perioperative red blood cell transfusions in patients undergoing liver resection: a systematic review. HPB (Oxford). 2017;19:321-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 69] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 3. | Velásquez JF, Cata JP. Transfusions of blood products and cancer outcomes. Rev Esp Anestesiol Reanim. 2015;62:461-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 4. | Acheson AG, Brookes MJ, Spahn DR. Effects of allogeneic red blood cell transfusions on clinical outcomes in patients undergoing colorectal cancer surgery: a systematic review and meta-analysis. Ann Surg. 2012;256:235-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 305] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 5. | Kaneko M, Sasaki S, Ishimaru K, Terai E, Nakayama H, Watanabe T. The impact of perioperative allogeneic blood transfusion on survival in elderly patients with colorectal cancer. Anticancer Res. 2015;35:3553-3558. [PubMed] |
| 6. | Halabi WJ, Jafari MD, Nguyen VQ, Carmichael JC, Mills S, Pigazzi A, Stamos MJ. Blood transfusions in colorectal cancer surgery: incidence, outcomes, and predictive factors: an American College of Surgeons National Surgical Quality Improvement Program analysis. Am J Surg. 2013;206:1024-32; discussion 1032-3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 7. | Sun C, Wang Y, Yao HS, Hu ZQ. Allogeneic blood transfusion and the prognosis of gastric cancer patients: systematic review and meta-analysis. Int J Surg. 2015;13:102-110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 71] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 8. | Luan H, Ye F, Wu L, Zhou Y, Jiang J. Perioperative blood transfusion adversely affects prognosis after resection of lung cancer: a systematic review and a meta-analysis. BMC Surg. 2014;14:34. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 9. | Lyu X, Qiao W, Li D, Leng Y. Impact of perioperative blood transfusion on clinical outcomes in patients with colorectal liver metastasis after hepatectomy: a meta-analysis. Oncotarget. 2017;8:41740-41748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 10. | Kooby DA, Stockman J, Ben-Porat L, Gonen M, Jarnagin WR, Dematteo RP, Tuorto S, Wuest D, Blumgart LH, Fong Y. Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg. 2003;237:860-9; discussion 869-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 301] [Cited by in RCA: 368] [Article Influence: 16.7] [Reference Citation Analysis (0)] |
| 11. | Schiergens TS, Rentsch M, Kasparek MS, Frenes K, Jauch KW, Thasler WE. Impact of perioperative allogeneic red blood cell transfusion on recurrence and overall survival after resection of colorectal liver metastases. Dis Colon Rectum. 2015;58:74-82. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 97] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 12. | Gruttadauria S, Saint Georges Chaumet M, Pagano D, Marsh JW, Bartoccelli C, Cintorino D, Arcadipane A, Vizzini G, Spada M, Gridelli B. Impact of blood transfusion on early outcome of liver resection for colorectal hepatic metastases. J Surg Oncol. 2011;103:140-147. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 37] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 13. | Pulitanò C, Arru M, Bellio L, Rossini S, Ferla G, Aldrighetti L. A risk score for predicting perioperative blood transfusion in liver surgery. Br J Surg. 2007;94:860-865. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Mariette D, Smadja C, Naveau S, Borgonovo G, Vons C, Franco D. Preoperative predictors of blood transfusion in liver resection for tumor. Am J Surg. 1997;173:275-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 15. | Bui LL, Smith AJ, Bercovici M, Szalai JP, Hanna SS. Minimising blood loss and transfusion requirements in hepatic resection. HPB (Oxford). 2002;4:5-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 22] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Nagino M, Kamiya J, Arai T, Nishio H, Ebata T, Nimura Y. One hundred consecutive hepatobiliary resections for biliary hilar malignancy: preoperative blood donation, blood loss, transfusion, and outcome. Surgery. 2005;137:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 4.6] [Reference Citation Analysis (0)] |