Published online Jan 6, 2019. doi: 10.12998/wjcc.v7.i1.102
Peer-review started: September 27, 2018
First decision: November 2, 2018
Revised: December 6, 2018
Accepted: December 7, 2018
Article in press: December 8, 2018
Published online: January 6, 2019
Processing time: 99 Days and 3.6 Hours
Intraductal papillary neoplasm of the bile duct (IPNB) is pathologically similar to intraductal papillary mucinous neoplasm (IPMN). However, there are several significant differences between them. The rate of IPMN associated with extrapancreatic malignancies has been reported to range from 10%-40%, and it may occasionally be complicated with the presence of fistulas. IPMN associated with malignant IPNB is extremely rare and only nine cases have been reported in the literature.
We report a 52-year-old man who presented with recurrent cholangitis for nine months. Computed tomography and magnetic resonance cholangiopancreatography showed the common bile duct stricture with dilated pancreatobiliary duct without other abnormal findings. The underlying pathogenesis could not be identified based on the radiologic images. Endoscopic retrograde cholangiopancreatography revealed a pancreatobiliary fistula with dilated main pancreatic duct, biliary stricture with dilated biliary tree, and mucus discharge from the enlarged orifice of the major papilla. The patient underwent SpyGlass cholangiopancreatoscopy due to a suspected mucin-producing biliary neoplasm and indeterminate main pancreatic duct dilatation. Multiple papillary growing neoplasms with vascular images, with the extent of lesions spreading in the biliopancreatic ductal lumens, were identified by SpyGlass. In addition, the presence of a pancreatobiliary fistula was also identified. The patient was diagnosed as having benign IPMN and malignant IPNB with focal invasion by postoperative pathology. Furthermore, varying histological subtypes were present in both IPMN and IPNB. Pylorus-preserving pancreaticoduodenectomy was performed on the patient with excellent results during the 52 month follow-up period.
We deemed that pancreatography and SpyGlass allowed for an efficient diagnosis of IPMN with pancreatobiliary fistula, whereas the etiology could not be identified by radiologic imaging.
Core tip: We report a patient with an extremely rare co-occurrence of intraductal papillary mucinous neoplasm (IPMN) and malignant intraductal papillary neoplasm of the bile duct (IPNB) accompanied with a pancreatobiliary fistula. The etiology of biliopancreatic duct dilatations could not be identified by radiologic imaging. After the patient underwent endoscopic retrograde cholangiopancreatography, the presence of a pancreatobiliary fistula was revealed by pancreatography. SpyGlass cholangiopancreatoscopy showed multiple papillary neoplasms in the pancreaticobiliary duct. In addition, SpyGlass was able to identify a pancreatobiliary fistula. The patient was diagnosed with benign IPMN and malignant IPNB. Different histological subtypes for both IPMN and IPNB were also identified. Radical resection on the patient achieved excellent results.
- Citation: Ren X, Zhu CL, Qin XF, Jiang H, Xia T, Qu YP. Co-occurrence of IPMN and malignant IPNB complicated by a pancreatobiliary fistula: A case report and review of the literature. World J Clin Cases 2019; 7(1): 102-108
- URL: https://www.wjgnet.com/2307-8960/full/v7/i1/102.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v7.i1.102
Intraductal papillary mucinous neoplasm (IPMN) is characterized by intraductal papillary growth with excessive mucin secretion leading to cystic dilatation of the main duct and/or the branch duct of the pancreas. Intraductal papillary neoplasm of the bile duct (IPNB) has similar pathological features as IPMN and, resemble characteristics of slow progression and high potential for malignancy. However, several significant differences distinguish them[1]. Approximately 10%-40% of IPMN are associated with extrapancreatic malignancies[2,3], and the most common extrapancreatic neoplasms are colorectal, stomach, lung, and breast cancers[2]. IPMN associated with malignant IPNB are extremely rare[4,5]. Furthermore, IPMN can occasionally develop fistulas in adjacent organs, such as the duodenum, stomach, and common bile duct. The majority of pancreatobiliary fistulas with IPMN develop from malignant IPMN due to direct tumor invasion into the bile duct. Pancreatobiliary fistulas with IPMN can be detected by radiologic imaging[6], and diagnosed using cholangiography and intraductal ultrasonography (IDUS) during surgery or via direct peroral cholangiopancreatoscopy using an ultra-slim videoendoscope[7,8]. We report a patient with a co-occurrence of benign IPMN and malignant IPNB, accompanied by a pancreatobiliary fistula. The patient was unable to be diagnosed by radiologic imaging including cholangiography. The diagnosis was made through pancreatography and SpyGlass cholangiopancreatoscopy.
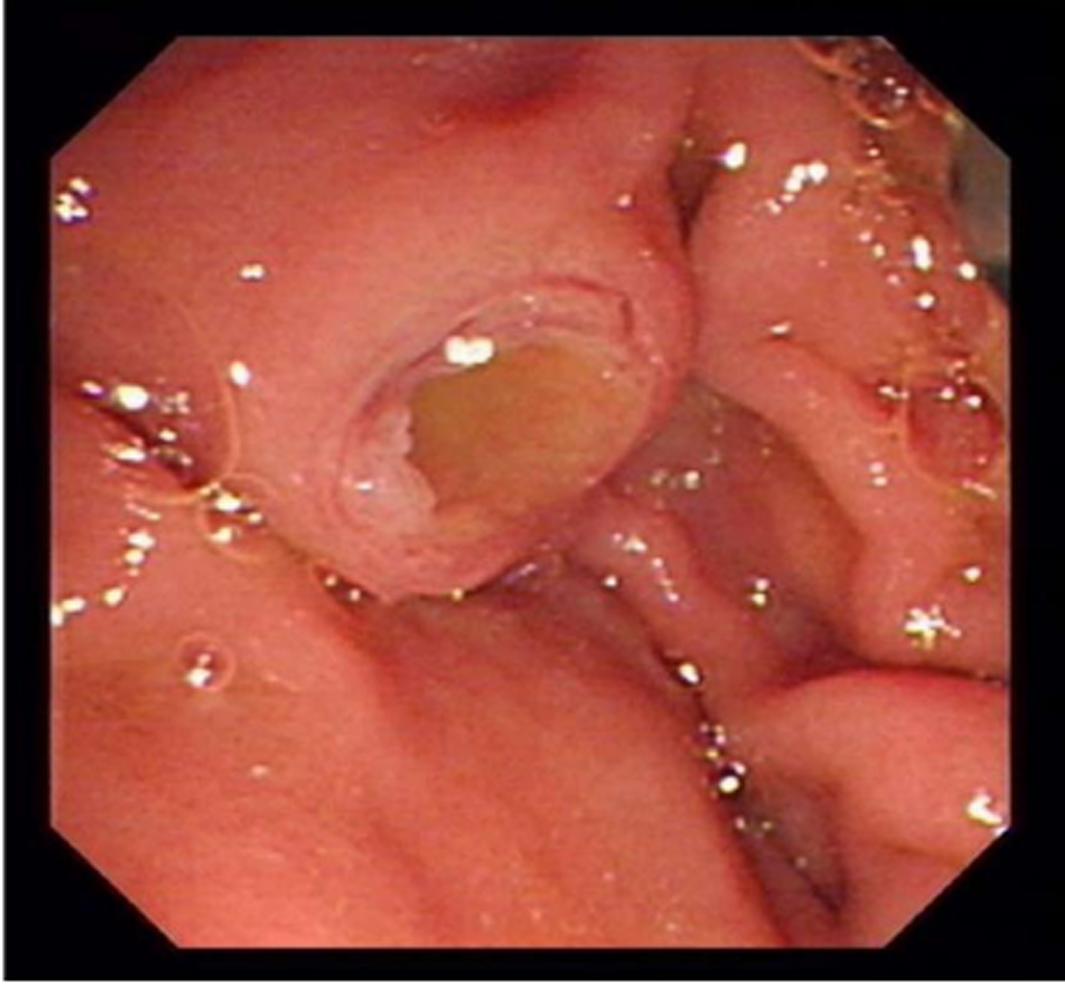
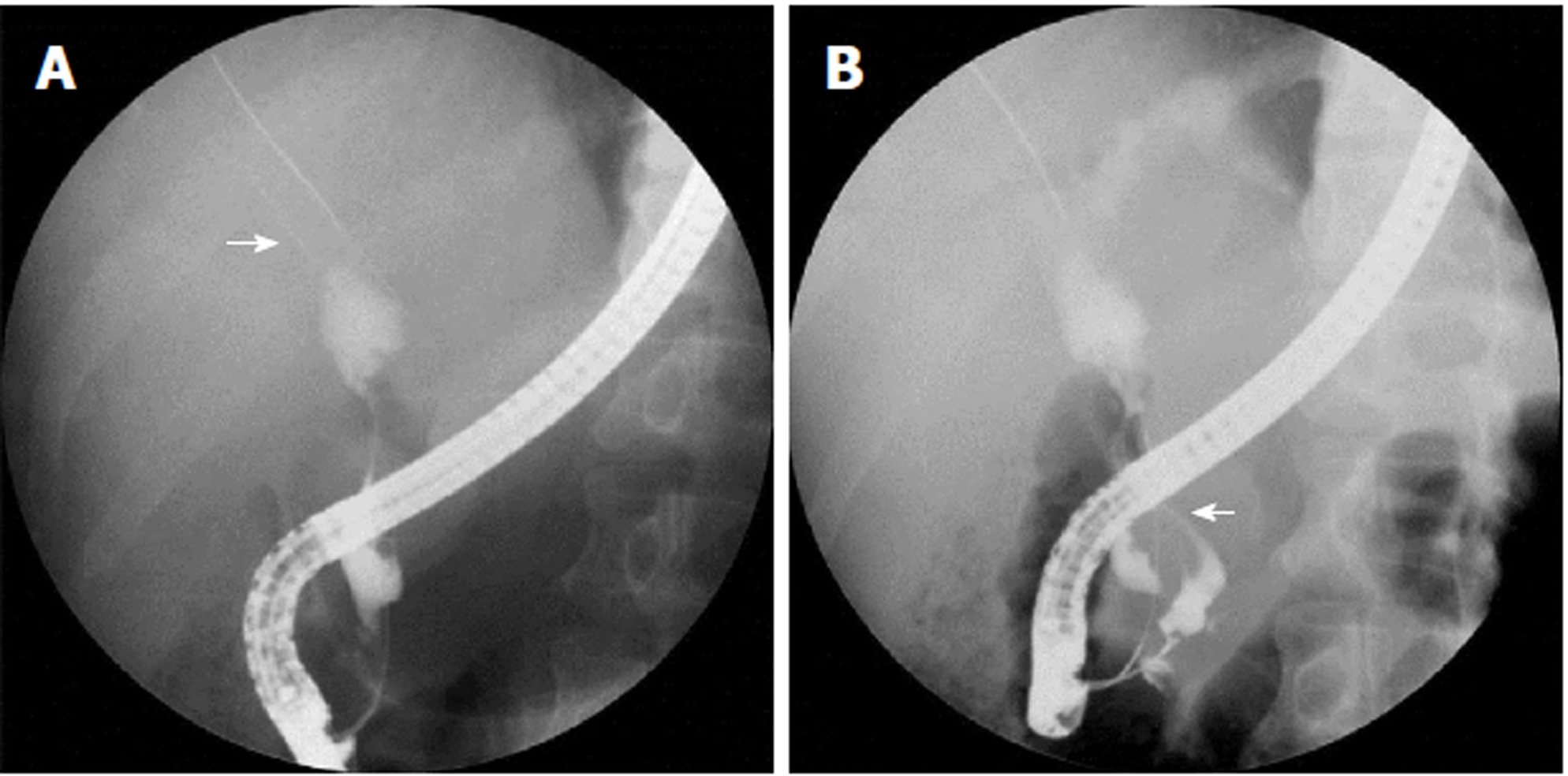
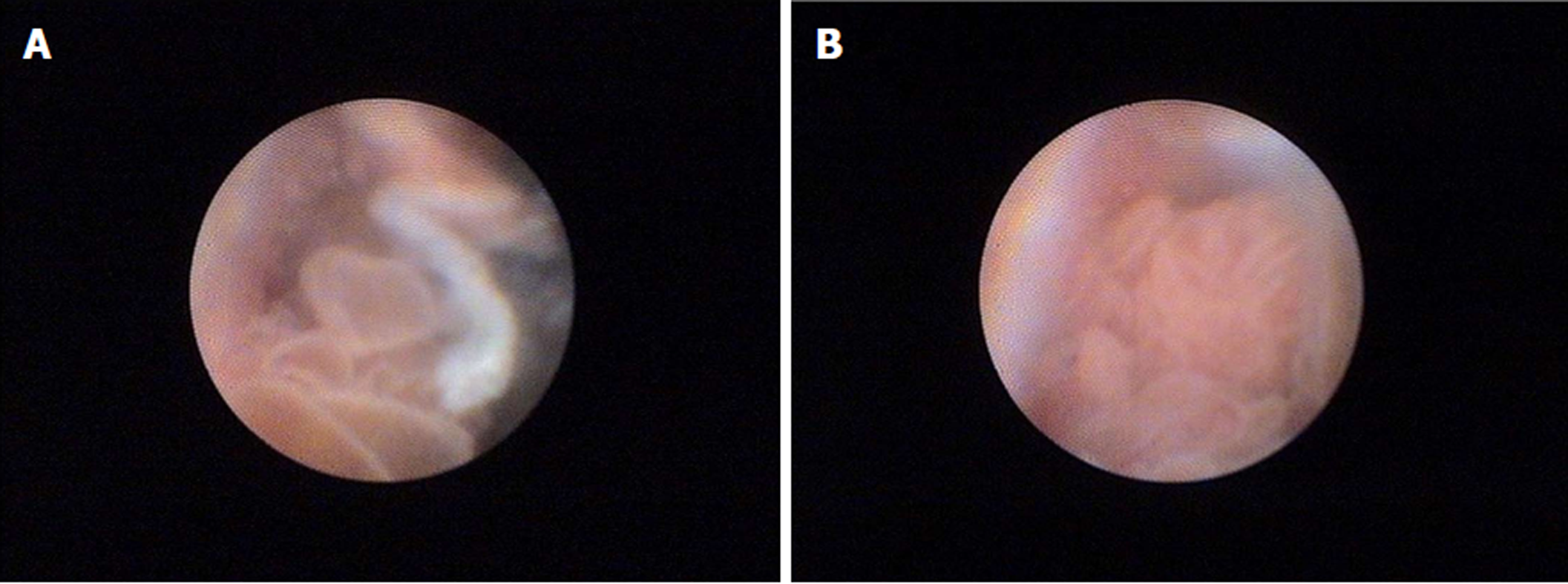
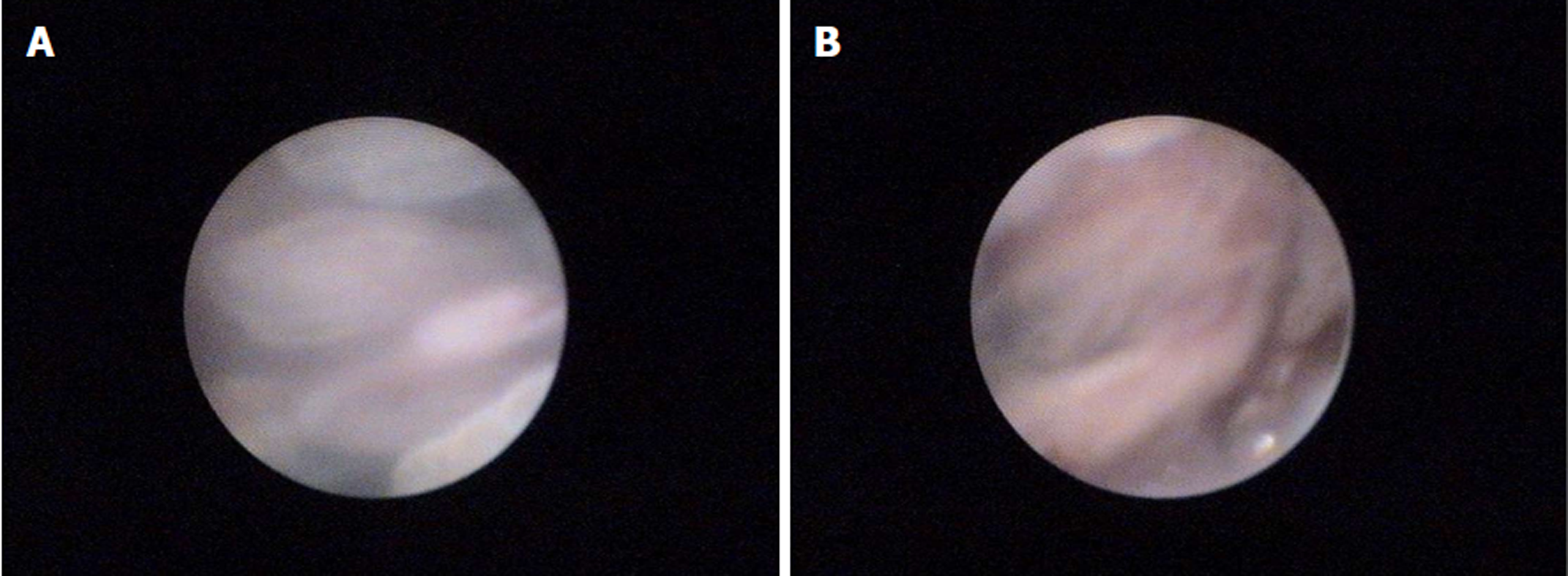
A 52-year-old man presented symptoms of recurrent upper abdominal pain, fever, jaundice, and weight loss for nine months. Four months prior, the patient was suspected of having malignant biliary obstruction and underwent percutaneous transhepatic biliary metal stenting at another hospital. However, due to recurrent episodes of cholangitis and jaundice, the patient was referred to our hospital for further examination. Physical examinations at admission were unremarkable except for jaundice and mild tenderness in the upper abdomen. Laboratory examinations revealed markedly elevated GGT (1593 IU/L) and ALP (1091 IU/L) levels, mildly elevated transaminase and TBiL levels, with normal serum amylase, blood glucose, CA19-9, and CEA. Ultrasonography (US), computed tomography (CT), and magnetic resonance cholangiopancreatography (MRCP) showed common bile duct stricture with significantly dilated intra-extra biliary tract and main pancreatic duct, without intraductal masses or mural nodules on both ducts. Pathogenesis could not be identified based on CT and MRCP. Hence, endoscopic retrograde cholangiopancreatography (ERCP) was performed. Mucus discharge from the enlarged orifice of the major papilla with fish eye-like appearance was observed (Figure 1). Duodenoscopy showed a normal stomach and duodenum. Cholangiography revealed bile duct stricture and proximal bile duct dilation, and a displaced metal stent in the intrahepatic bile duct (Figure 2A). The metal stent was retrieved using a snare. Pancreatography revealed a pancreatobiliary fistula between the main pancreatic duct and common bile duct, with a dilated pancreatic duct (8 mm in diameter) (Figure 2B). A 0.025 inch guide wire was inserted through the fistula orifice of the pancreatic duct into the bile duct. SpyGlass cholangiopancreatoscopy (Boston Scientific Corp, Natick, Massachusetts, United States) was performed via a duodenoscope during ERCP. This was due to a suspected mucin-producing biliary tumor and indeterminate main pancreatic duct dilatation. SpyGlass imaging showed excessive mucus in the bile duct. Multiple fish egg-like protrusions and a cauliflower-like protrusion with vascular images in the common bile duct (Figure 3A and B) were observed after mucus removal. Afterwards, a pancreatoscope with SpyGlass over the guide wire via the pancreatic orifice was inserted into the main pancreatic duct after pancreatic sphincterotomy. Excessive mucus and multiple longilineal papillary protrusions, as well as leaf-like projections with vascular images in the main duct of the pancreatic head were observed (Figure 4A and B). The extent of the lesions in the pancreaticobiliary duct was identified through SpyGlass. The protruding lesions in both the biliary and pancreatic duct were biopsied under direct vision. Afterwards, a nasobiliary drainage catheter and a pancreatic 7-Fr stent were temporarily placed to prevent SpyGlass-related complications. Both IPMN (intestinal type) with intermediate-grade dysplasia and IPNB (pancreaticobiliary type) with moderate to high-grade dysplasia were diagnosed via biopsy. Pylorus-preserving pancreaticoduodenectomy was performed three months after percutaneous transhepatic biliary drainage (PTBD) due to poor drainage. Postoperative pathological diagnosis of IPNB with moderate to high-grade dysplasia, with focal invasion of the bile duct wall without lymph node metastasis, and IPMN with moderate-grade dysplasia was determined. The patient had a good prognosis with weight gain without any clinical symptoms or pancreaticobiliary dilation during the 52-month postoperative follow-up period.
Synchronous malignant IPNB and benign IPMN associated with a pancreatobiliary fistula.
Pylorus-preserving pancreaticoduodenectomy was performed.
The patient was successfully treated by surgical management with an excellent prognosis. The patient had weight gain without any clinical symptoms or pancreaticobiliary dilation during the 52-month post-surgical follow-up period.
Patients with IPMN have a greater risk of developing benign and malignant extrapancreatic neoplasms compared to patients with pancreatic disorders such as pancreatic ductal adenocarcinoma and mucinous cystic neoplasm[9]. The etiology of increased extrapancreatic malignancies in patients with IPMN is unclear. It could be related to gene mutations or environmental factors. The majority of IPMN with extrapancreatic malignancies are cancers of the digestive system, with 2.4% of them being cholangiocarcinomas. Gastrointestinal cancer is common in Asia, while skin, breast, and prostate carcinomas are common in the United States[3]. We report a patient with co-occurrence of benign IPMN and extrapancreatic malignancy (malignant IPNB). Both IPMN and IPNB exhibited excessive mucin secretion, but had different histological subtypes and growth morphologies. Patients having simultaneous occurrence of IPMN and IPNB are exceedingly rare[4,5]. To the best of our knowledge, only nine cases have been reported in the literature[10-18]. In previously reported patients with synchronous IPMN and IPNB, all IPNB lesions were localized at the intrahepatic duct without IPMN developed fistulas (Table 1). However, in our patient, IPNB was localized in the common bile duct and IPMN was associated with a pancreatobiliary fistula. This is the first incidence to be reported with such a finding.
| Ref. | Year | Sex | Age | IPNB | IPMN | Pancreatobiliary fistula | ||
| Location | Pathology | Location | Pathology | |||||
| Joo et al[10] | 2000 | M | 60 | Left IHD | LGD | Branch duct | LGD | No |
| Ishida et al[11] | 2002 | M | 67 | B1 | Without dysplasia | Branch duct | Without dysplasia | No |
| Yamaguchi et al[12] | 2005 | M | 69 | Left IHD | IC | Branch duct | MIC | No |
| Zalinski et al[13] | 2007 | F | 65 | Bilateral | IHD | Main duct | HGD | No |
| Park et al[14] | 2010 | M | 67 | Left IHD | LGD | Mixed duct | LGD | No |
| Valente et al[15] | 2012 | M | 76 | Right IHD | IC | Branch duct | LGD | No |
| Xu et al[16] | 2012 | F | 68 | Left IHD | LGD | Branch duct | Without dysplasia | No |
| Moon et al[17] | 2014 | F | 66 | Left IHD | HGD | Main duct | HGD | No |
| Bansal et al[18] | 2016 | M | 70 | Right IHD | IC | Main duct | IC | No |
| Ren X et al | 2018 | M | 52 | CBD | MIC | Main duct | LGD | Yes |
IPMN rarely fistulate into adjacent organs, such as the duodenum, stomach, common bile duct, and more rarely, the colon and small intestine[3,6]. The frequency of fistulas with IPMN is reported to be around 1.9%–6.6%[6,8]. IPMN-related fistulas are often multiple, and the majority of pancreatobiliary fistulas with IPMN develop from malignant IPMN due to direct tumor invasion into the bile duct. Our patient developed pancreatobiliary fistulization from benign IPMN perhaps due to pancreatic perforation into the bile duct due to the mechanical pressure exerted by excessive mucin accumulation, or may have developed due to epithelial stripping caused by auto-digestion by enzyme-rich fluids or by inflammatory cytokines[3,6,7]. In patients with IPMN, the most common histopathological type is gastric type with excessive mucin secretion, followed by intestinal type. About 94% of fistulas with IPMN are of the intestinal type as reported by Kobayashi et al[5]. Our patient with benign IPMN was also diagnosed with an intestinal type pancreatobiliary fistula. Whether the fistula was caused by the intestinal type IPMN of the four histologic subtypes remains unclear. In addition, pancreatobiliary fistulas can also be associated with pancreatic pseudocyst, acute necrotizing pancreatitis, and pancreatic abscess[7].
IPNB or IPMN with ductal dilatation are easily revealed through radiologic imaging. Intraductal masses, mural nodules, or fistulas with IPMN can be generally detected by radiologic imaging[6]. However, it is not always helpful for detecting pancreatobiliary fistulas[8], hence further examinations may be required for diagnosis. Excessive mucin secretion is observed in the majority of patients with IPMN and in about one-third of IPNB patients. If mucus is observed in the biliopancreatic ducts, it is generally indicative of mucin-producing biliopancreatic tumors. However, mucin cannot be detected by US, CT, or MRCP, because the signal intensity of mucin is the same as that of water. When IPMN develops pancreatobiliary fistulas, excessive mucin flows into the bile duct from the pancreatic duct due to the pressure in the pancreatic duct being higher than that of the bile duct. This ultimately leads to biliary drainage obstruction and cholangitis and results in the clinical presentation of patients with IPMN being similar to that of IPNB. If mucus discharge from the dilated bile duct and pancreatic duct orifice of the major papilla has a pig-nosed appearance during ERCP, it may indicate a pancreatobiliary fistula with IPMN[8]. In our patient, IPNB was suspected due to repeated episodes of cholangitis and mucobilia extruding from the dilated biliary duct orifice with fish eye-like appearance, without dilated pancreatic orifice during duodenoscopy. Because of this, a pancreatobiliary fistula with IPMN was not diagnosed. When pancreatobiliary fistulas related to IPMN are unable to be detected by radiologic imaging including cholangiography, the best diagnostic option would be IDUS of the biliary tract[8]. Pancreatobiliary fistula was unexpectedly discovered in our patient using pancreatography rather than by cholangiography.
Both IPMN and IPNB have a tendency to grow longitudinally along the lumen. IPNB is generally associated with superficial spreading. Cholangiopancreatoscopy could be useful to preoperatively assess the extent of the neoplasm, and is superior to other diagnostic tools. The patient underwent SpyGlass examination due to suspected IPNB and indeterminate pancreatic duct dilatation. Our patient was diagnosed with concurrent IPNB and IPMN. The extent of neoplasms spreading along the biliopancreatic ductal lumen and the pancreatobiliary fistula was determined by SpyGlass direct visualization. The majority of IPMN projective lesions with vascular images are often malignant. However, in our patient, although IPMN showed multiple papillary protrusions with vascular images, it was confirmed as benign by biopsy and surgery. Results from SpyGlass biopsy specimens of the bile duct were consistent with postoperative pathological diagnosis of malignant IPNB, with the exception of focal tumor invasion on the duct wall. Although SpyGlass biopsy is useful to distinguish between a benign lesion and malignancy, it cannot represent the actual staging for the disease.
Pancreatobiliary fistulas with IPMN can be treated using biliary metal stents for the fistula, and surgical biliary bypass to drain bile duct obstruction. When only mucin from the fistula obstructs biliary drainage, the obstruction could be resolved by both biliary and pancreatic sphincterotomy[7]. However, in our patient, pancreatic sphincterotomy and PTBD including metal stenting did not have significant therapeutic efficacy. Displacement of the metal stent into the intrahepatic duct was observed, which may be related to both IPNB and IPMN with the fistula secreting excessive mucin leading to severe biliary tree enlargement.
Surgical resection for IPMN with non-invasive carcinoma has an excellent prognosis[2]. However, diffuse IPNB located at the perihilar bile duct has a poor prognosis with a 5-year survival rate of only 20.8% after surgical resection[19]. In our patient, radical resection was performed because of concurrent malignant IPNB localized at the common bile duct, along with pancreatobiliary fistula and IPMN.
We recommend pancreatography and SpyGlass cholangiopancreatoscopy to effectively diagnose pancreatobiliary fistula with IPMN, when a suitable diagnosis cannot be made based on radiologic imaging. For radical resection, it is important to identify the extent of IPNB and IPMN lesions in the pancreaticobiliary duct by SpyGlass.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bonavina L, Maurea S S- Editor: Ma YJ L- Editor: Wang TQ E- Editor: Bian YN
| 1. | Ohtsuka M, Shimizu H, Kato A, Yoshitomi H, Furukawa K, Tsuyuguchi T, Sakai Y, Yokosuka O, Miyazaki M. Intraductal papillary neoplasms of the bile duct. Int J Hepatol. 2014;2014:459091. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 99] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Larghi A, Panic N, Capurso G, Leoncini E, Arzani D, Salvia R, Del Chiaro M, Frulloni L, Arcidiacono PG, Zerbi A, Manta R, Fabbri C, Ventrucci M, Tarantino I, Piciucchi M, Carnuccio A, Boggi U, Costamagna G, Delle Fave G, Pezzilli R, Bassi C, Bulajic M, Ricciardi W, Boccia S. Prevalence and risk factors of extrapancreatic malignancies in a large cohort of patients with intraductal papillary mucinous neoplasm (IPMN) of the pancreas. Ann Oncol. 2013;24:1907-1911. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 41] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 3. | Machado NO, Al Qadhi H, Al Wahibi K. Intraductal Papillary Mucinous Neoplasm of Pancreas. N Am J Med Sci. 2015;7:160-175. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 70] [Cited by in RCA: 74] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 4. | Riall TS, Stager VM, Nealon WH, Townsend CM Jr, Kuo YF, Goodwin JS, Freeman JL. Incidence of additional primary cancers in patients with invasive intraductal papillary mucinous neoplasms and sporadic pancreatic adenocarcinomas. J Am Coll Surg. 2007;204:803-13; discussion 813-4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 57] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 5. | Tanaka M, Kobayashi K, Mizumoto K, Yamaguchi K. Clinical aspects of intraductal papillary mucinous neoplasm of the pancreas. J Gastroenterol. 2005;40:669-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 107] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 6. | Ravaud S, Laurent V, Jausset F, Cannard L, Mandry D, Oliver A, Claudon M. CT and MR imaging features of fistulas from intraductal papillary mucinous neoplasms of the pancreas to adjacent organs: A retrospective study of 423 patients. Eur J Radiol. 2015;84:2080-2088. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.7] [Reference Citation Analysis (1)] |
| 7. | Sung KF, Chu YY, Liu NJ, Hung CF, Chen TC, Chen JS, Lin CH. Direct peroral cholangioscopy and pancreatoscopy for diagnosis of a pancreatobiliary fistula caused by an intraductal papillary mucinous neoplasm of the pancreas: a case report. Dig Endosc. 2011;23:247-250. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Koizumi M, Kumagi T, Kuroda T, Azemoto N, Yamanishi H, Ohno Y, Yokota T, Ochi H, Tange K, Ikeda Y, Hiasa Y. Difficulty in management of intraductal papillary mucinous neoplasm-associated pancreatobiliary fistulas and the role of “pig-nose” appearance and intraductal ultrasonography in diagnosis. Endosc Int Open. 2016;4:E446-E450. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 9. | Reid-Lombardo KM, Mathis KL, Wood CM, Harmsen WS, Sarr MG. Frequency of extrapancreatic neoplasms in intraductal papillary mucinous neoplasm of the pancreas: implications for management. Ann Surg. 2010;251:64-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 10. | Joo YH, Kim MH, Lee SK, Seo DW, Yoo KS, Min YI, Chang JJ, Yu E. A case of mucin-hypersecreting intrahepatic bile duct tumor associated with pancreatic intraductal papillary mucinous tumor. Gastrointest Endosc. 2000;52:409-412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 11. | Ishida M, Seki K, Honda K, Kimura T, Katayama K, Hirose K, Dojo M, Azuma T, Imamura Y, Hutchins RR, Yamaguchi A. Intraductal mucinous tumors occurring simultaneously in the liver and pancreas. J Gastroenterol. 2002;37:1073-1078. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Yamaguchi Y, Abe N, Imase K, Mizuno H, Chinen K, Mori H, Sugiyama M, Atomi Y, Ishida H, Takahashi S. A case of mucin hypersecreting intraductal papillary carcinomas occurring simultaneously in liver and pancreas. Gastrointest Endosc. 2005;61:330-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 13. | Zalinski S, Paradis V, Valla D, Belghiti J. Intraductal papillary mucinous tumors of both biliary and pancreatic ducts. J Hepatol. 2007;46:978-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 14. | Park BH, Suh JH, Cha HJ, Kim YM, Choi HJ. Intraductal papillary mucinous tumor simultaneously involving the liver and pancreas-A case report. Korean J Pathol. 2010;44:86. |
| 15. | Valente R, Capurso G, Pierantognetti P, Iannicelli E, Piciucchi M, Romiti A, Mercantini P, Larghi A, Federici GF, Barucca V, Osti MF, Di Giulio E, Ziparo V, Delle Fave G. Simultaneous intraductal papillary neoplasms of the bile duct and pancreas treated with chemoradiotherapy. World J Gastrointest Oncol. 2012;4:22-25. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 13] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Xu XW, Li RH, Zhou W, Wang J, Zhang RC, Chen K, Mou YP. Laparoscopic resection of synchronous intraductal papillary mucinous neoplasms: a case report. World J Gastroenterol. 2012;18:6510-6514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 17. | Moon DB, Lee SG, Jung DH, Park GC, Park YH, Park HW, Kim MH, Lee SK, Yu ES, Kim JH. [Synchronous malignant intraductal papillary mucinous neoplasms of the bile duct and pancreas requiring left hepatectomy and total pancreatectomy]. Korean J Gastroenterol. 2014;63:129-133. [PubMed] |
| 18. | Bansal A, Thung SN, Zhu H, Schwartz M, Lewis S. Synchronous pancreatic adenocarcinoma and intrahepatic cholangiocarcinoma arising in the context of intraductal papillary neoplasms. Clin Imaging. 2016;40:897-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 19. | Kang MJ, Jang JY, Lee KB, Han IW, Kim SW. Impact of macroscopic morphology, multifocality, and mucin secretion on survival outcome of intraductal papillary neoplasm of the bile duct. J Gastrointest Surg. 2013;17:931-938. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |