Published online Sep 6, 2018. doi: 10.12998/wjcc.v6.i9.259
Peer-review started: March 30, 2018
First decision: April 26, 2018
Revised: July 18, 2018
Accepted: August 6, 2018
Article in press: August 7, 2018
Published online: September 6, 2018
Processing time: 161 Days and 18.1 Hours
To review the conversion therapy for initially unresectable hepatocellular carcinoma (HCC) patients and the suitable timing for subsequent salvage surgery.
A PubMed search was undertaken from 1987 to 2017 to identify articles using the keywords including “unresectable” “hepatocellular carcinoma”, ”hepatectomy”, ”conversion therapy”, “resection”, “salvage surgery” and “downstaging”. Additional studies were investigated through a manual search of the references from the articles. The exclusion criteria were duplicates, case reports, case series, videos, contents unrelated to the topic, comments, and editorial essays. The main and widely used conversion therapies and the suitable timing for subsequent salvage surgery were discussed in detail. Two members of our group independently performed the literature search and data extraction.
Liver volume measurements [future liver remnant (FLR)/total liver volume or residual liver volume/bodyweight ratio] and function tests (scoring systems and liver stiffness) were often performed in order to justify whether patients were suitable candidates for surgery. Successful conversion therapy was usually defined as downstaging the tumor, increasing FLR and providing subsequent salvage surgery, without increasing complications, morbidity or mortality. The requirements for performing salvage surgery after transcatheter arterial chemoembolization were the achievement of a partial remission in radiology, the disappearance of the portal vein thrombosis, and the lack of extrahepatic metastasis. Patients with a standardized FLR (sFLR) > 20% were good candidates for surgery after portal vein embolization, while other predictive parameters like growth rate, kinetic growth rate were treated as an effective supplementary. There was probably not enough evidence to provide a standard operation time after associating liver partition and portal vein ligation for staged hepatectomy or yttrium-90 microsphere radioembolization. The indications of any combinations of conversion therapies and the subsequent salvage surgery time still need to be carefully and comprehensively evaluated.
Conversion therapy is recommended for the treatment of initially unresectable HCC, and the suitable subsequent salvage surgery time should be reappraised and is closely related to its previous therapeutic effect.
Core tip: Since the treatment for initially unresectable hepatocellular carcinoma (HCC) patients is still controversial, we emphasize the importance and effectiveness of different conversion therapies and subsequent salvage surgery. We also introduce the common conversion therapies including their indications, advantages and shortcomings. Challengingly we try to elaborate on the suitable subsequent salvage surgery timing. We advocate the reasonable unified application of these to have the full effect of complementary advantages, to promote their therapeutic effect, and to increase the survival rate of the initially unresectable HCC patients.
- Citation: Zhang ZF, Luo YJ, Lu Q, Dai SX, Sha WH. Conversion therapy and suitable timing for subsequent salvage surgery for initially unresectable hepatocellular carcinoma: What is new? World J Clin Cases 2018; 6(9): 259-273
- URL: https://www.wjgnet.com/2307-8960/full/v6/i9/259.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i9.259
Hepatocellular carcinoma (HCC) is a primary cancer of the liver and is the fifth most prevalent cancer in men and the seventh in women worldwide[1]. The HCC incidence is the highest among malignancies in East Asia and sub-Saharan Africa and is closely related to hepatitis B virus (HBV) prevalence or consumption of aflatoxins[2].
The current standard classification system for HCC, the Barcelona clinic liver cancer (BCLC) classification, suggests that patients with very early or early stage HCC are candidates for curative surgery[3]. Curative therapy often refers to partial hepatectomy or liver transplantation (LT), bringing a positive prognosis to the selected HCC patients. Numerous staging systems provide patients with an estimated life expectancy, but only the BCLC staging system links staging with therapies. For patients meeting the Milan criteria, orthotopic liver transplantation (OLT) can provide an excellent 5-year survival of 70% or better[4]. Unfortunately, the unavailability of liver grafts minimizes the utilization of OLT, and most patients fail to meet the Milan criteria when waiting for an OLT[5]. Thus, hepatectomy is currently the first-line curative therapy, but only about 30% of lesions are resectable at the time of diagnosis[6].
In this context, conversion therapy is used to increase the resectability of initially unresectable HCC by increasing the size of the future liver remnant (FLR) or downstaging the tumor, followed by salvage surgery. It is usually defined as the therapy that renders some unresectable tumor surgical approachable in an attempt to improve the outcome of patients[7]. Recent studies have also demonstrated the 5-year survival rate after downstaging followed by hepatectomy varies from 24.9%-57%, which is comparable to primary liver resection (30%-60%)[8-11].
To challenge the limits of resectability, transcatheter arterial chemoembolization (TACE) is commonly used in initially unresectable HCC, featuring tumor size shrinking, satellite lesions disappearing and liver hypertrophy[12]. Portal vein embolization (PVE) is indicated for patients who are considered to have inadequate FLR, which induces hypertrophy of the FLR in an attempt to avoid liver failure. Currently, associating liver partition and portal vein ligation (ALPPS) has been regarded as an alternative, allowing for more rapid hypertrophy of the remnant liver, which induces a mean volume increase of 78.4%[13]. Radiotherapy such as hepatic intra-arterial yttrium-90 microsphere treatment has also demonstrated a promising effect on downstaging initially unresectable HCC and converting it into resectable HCC. Other conversion therapies can be any combination of the methods above. Tang et al[9] found that double and triple treatments produced a higher successful downstaging-resection rate and resulted in a better prognosis. Although various preoperative therapies provide initially unresectable HCC patients with the chance to undergo curative resection, the suitable timing of the subsequent salvage surgery remains uncertain and controversial. To review the selection of conversion therapy and the following suitable salvage surgery time, we conduct the review of the current literature.
A PubMed search was undertaken from 1987 to 2017 to identify articles using the keywords including “unresectable” “hepatocellular carcinoma”, ”hepatectomy”, ”conversion therapy”, “resection”, “salvage surgery” and “downstaging”. Additional studies were investigated through a manual search of the references from the articles. The exclusion criteria were duplicates, case reports, case series, videos, contents unrelated to the topic, comments, and editorial essays. The main and widely used conversion therapies and the suitable timing for subsequent salvage surgery were discussed in detail. Two members of our group independently performed the literature search and data extraction.
The typical procedures for a successful conversion therapy followed by salvage surgery are: (1) assessment of the patient’s condition, including tumor stage, liver function, FLR, and body tolerance; (2) selection of an effective conversion therapy to downstage the tumor, increase FLR and arrangement of long-term treatment by an experienced surgery; (3) assessment of timing for salvage surgery; and (4) an aggressive surgical approach to liver resection. The selection of conversion therapy depends on the tumor itself and the availability of expertise at the individual medical center, but we discuss the expertise or required skills here.
Similar to other tumors within an organ, it is essential to perform a preoperative assessment of the liver. The liver is a vital organ that possesses the functions of metabolism, detoxification, bile secretion, hematopoiesis and immune defense. Any therapies that may impair liver function can cause complications related to postoperative liver failure or increased mortality. As a result, liver insufficiency mostly occurs in patients with a decompensated liver, especially a cirrhotic liver. Based on this rationale, a liver assessment is performed in order to identify whether patients are suitable candidates for surgery, and the assessment typically consists of two aspects: Liver volume and function tests.
Liver volume test: FLR should be emphasized before any surgery as it is a significant predictor of post-hepatectomy liver failure (PHLF). With the advent of CT scans, a liver volumetric measurement can be achieved in a more accurate way. Although studies have verified that the difference between CT-guided liver volume assessment and real liver volume is minimal, the individual difference is not fully considered in CT-guided assessment[14,15]. In order to solve the problem, sFLR is suggested instead of FLR, which can be achieved by the ratio of FLR to total liver volume (TLV), calculated on the basis of Urata’s formula allowing for a comparison between patients[16]. TLV can be calculated by a formula that uses either body surface area (BSA) or weight, which is also designated as standard liver volume (SLT)[17]. Current studies on the safe limits of surgery outline the necessity of sFLR, and the details will be articulated below.
In addition to FLR, Truant et al[18] advocated a new calculating method, residual liver volume (RLV) to bodyweight ratio (RLV/BWR), to predict the postoperative complications and found that non-cirrhotic patients with RLV-BWR < 0.5% carried a higher risk of developing liver failure or postoperative mortality. Truant et al[19] further noted that RLV/BWR (0.5%) was as effective as the standardized RLV/sTLV (20%). From Lin et al[20], a retrospective study suggested that RLV/BWR (1.4%) had a certain predictive value for PHLF in patients with cirrhotic liver by a receiver operating characteristic curve (ROC). By dividing patients into an RLV/BWR > 1.4% group and an RLV/BWR < 1.4% group, a significant difference was found in the incidence of PHLF in the latter group (P = 0.006)[20].
Liver volume test is a viable and stable evaluation indirectly reflecting the quality and quantity of the hepatocyte and provide clinical guidance in a short time. But it still has its limitation under certain circumstance. For example, computed tomography-deriver liver volume (CTLV) is larger than SLT when the liver is under the situation of acute hypertrophy such as liver failure, liver resection, resulting in the misjudgment of real liver assessment.
In general, liver function tests can be classified into 3 types (as shown in Table 1: Biochemical parameters, dynamic quantitative tests to make liver function quantifiable, and scoring systems that incorporate laboratory tests with quantitative tests).
| Types | Contents |
| Biochemical parameters | Alanine transaminase-aspartate transaminase, gamma glutamyl transpeptidase, alkaline phosphatase, albumin, bilirubin (total and conjugated), coagulation test (INR), Serum glucose, lactate dehydrogenase, platelet count |
| Dynamic qualitative tests | 99-m TC-GSA scintigraphy (uptake), ICG test (clearance), aminopyrine breath test, MEGX, galactose elimination, LiMAX (metabolism) |
| Scoring systems | Child-Turcotte-Pugh systems, Model for end-stage liver disease, Model for end-stage liver disease-Na |
Liver biochemical parameters often indicate its function of metabolism or synthesis. The aminotransaminase enzymes, aspartate transferase (AST) and alanine transferase (ALT), are indicators of the extent of liver damage as well as necrosis. Usually the rise of these enzymes indicates the deterioration of the liver function. Albumin and clotting factors are synthesized by the liver whose concentration is closely related to the function of synthesis. Other parameters like plasma bilirubin, lactate dehydrogenase, and alkaline phosphatase can also reflect part of the liver function.
Relying too much on biochemical parameters is unreasonable, for that they only reflect the liver function indirectly and are easy to be influenced by other factors such as bile duct obstruction[21]. To assess the liver function more directly and quantitively, the dynamic quantitative liver function test is usually performed. Indocyanine Green (ICG) clearance test has been prevalent in Eastern country, featuring its non-toxic, water-soluble dye. Through applying ICG intravenously, clinicians are able to evaluate the liver function according to the clearance of ICG whose elimination is associated with the quantity of healthy hepatocyte. 99-m TC-GSA scintigraphy and 99-m TC-GSA PET/CT both are quantitative liver function tests which evaluate the liver morphologically and physiologically.
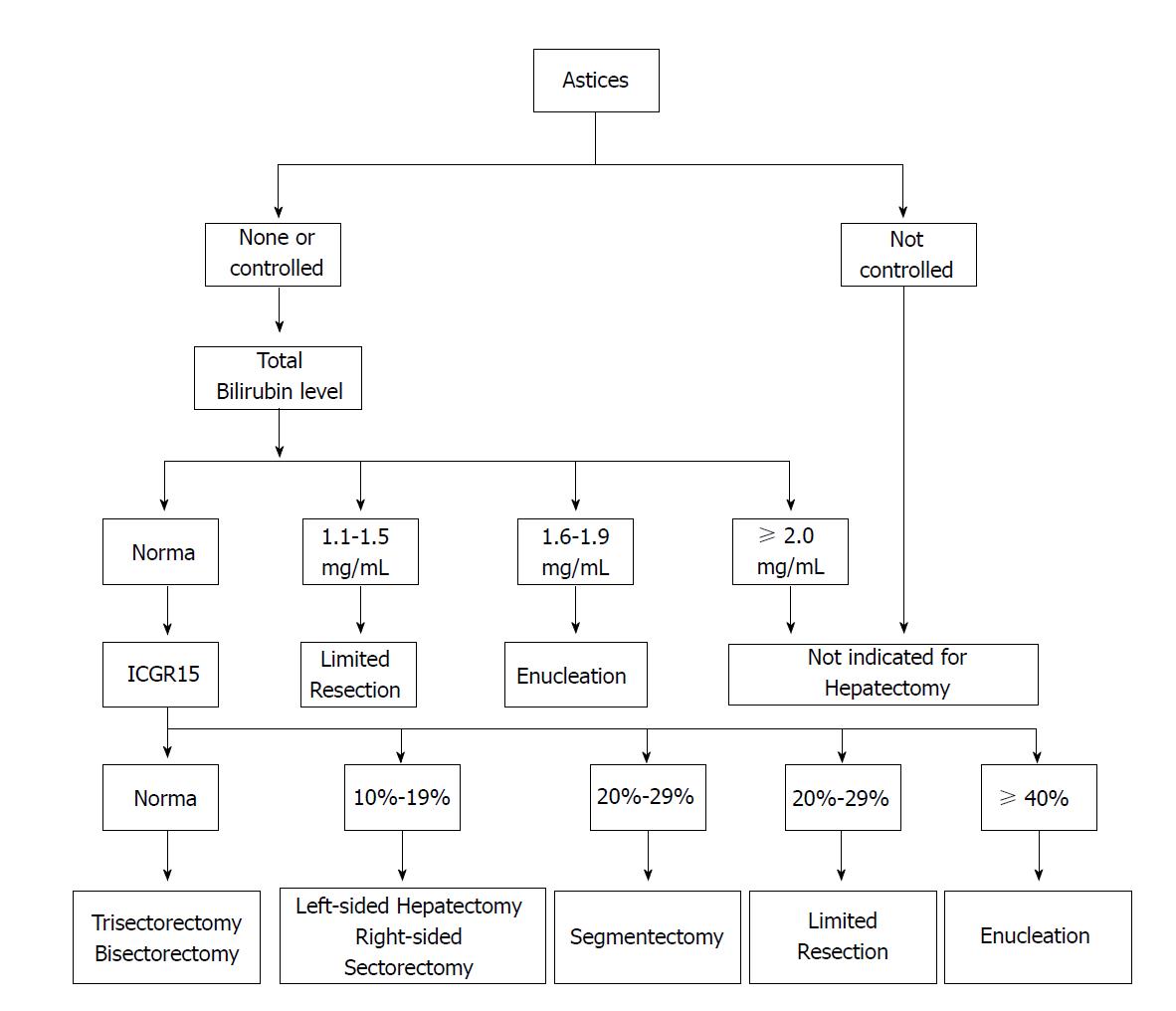
Clinicians are now focusing on a combination of heterogeneous assessment modalities because none of the single laboratory values can predict postoperative complications precisely. Therefore, scoring systems of liver function may offer an optimal choice for the patients scheduled for surgery. Among them, Child-Turcotte-Pugh (CTP) is frequently utilized in Asia, which is based on serum albumin, total bilirubin, prothrombin time and the presence and grade of ascites and hepatic encephalopathy. Although patients with CTP C can benefit from resection through careful selection, patients with CTP A are commonly considered to be good candidates for surgery[22]. However, this evaluation cannot identify “high risk” and “low risk” members of the CTP A group[23,24]. To address the issue, a decision tree (Figure 1) for hepatectomy has been proposed by Makuuchi et al[25]; the decision tree incorporates the presence or absence of ascites, the total bilirubin level, and the Indocyanine Green Clearance Test (ICGR15) into the criteria. A retrospective cohort study analyzing 1056 resections had also demonstrated that hepatic resection could be safely performed in patients who met the Makuuchi criteria[24].
Liver stiffness (LS) measured by transient elastography (TE) is also used to predict PHLF. We demonstrated that patients with LS ≥ 16.2 kPa carried a higher risk of PHLF (sensitivity = 71.43%, specificity = 85.11%) and recommended LS ≤ 16.2 kPa as the safe cutoff for surgery[26]. Analogously, a safe cutoff of LS of 15.7 kPa and 11.25 kPa were recommended by Cescon et al[27] and Chong et al[28], respectively.
All in all, liver function test still has its own limitation. Unlike liver volumetric assessment, biochemical parameters are too unstable to predict the PHLF. What’s more, clinicians should be cautious to use ICG clearance when patients have obstructive jaundice or cholestasis. ICG elimination combined with scoring systems or other dynamic quantitative test is recommended to fully assess the liver function because it alone doesn’t work well in a situation where the functional distribution is heterogeneity, like a damaged liver, cirrhotic liver, or liver after PVE.
TACE and the subsequent salvage surgery time: Transarterial chemoembolization (TACE), which was firstly reported by Yamada et al[29] in 1987, is a regime involving the injection of an embolic agent and a chemotherapeutic agent into the hepatic artery, resulting in ischemic necrosis of the tumor[30]. The underlying mechanism produces a selective ischemic and pharmacologic effect on the tumor. TACE is indicated for massive HCC (< 70% liver volume), multifocal tumors, major vascular invasion (MVI), and incomplete portal vein thrombosis (PVT), while it is contraindicated in patients with CTP C or extra-hepatic metastasis. According to the BCLC stage system, TACE is the standard therapy for BCLC stage B (intermediate HCC) patients and plays an important role in replacing other therapies that are not applicable regarding early or advanced HCC[31]. Many meta-analyses have demonstrated that pre-operative TACE has no significant effect on improving the survival of patients with resectable HCC[32-34], but none of them mentioned the effect on unresectable HCC.
TACE alone for initially unresectable HCC can achieve limited overall survival (OS). The 1-, 3-, 5- and 7-years survival rates were 82%, 47%, 26% and 16%, respectively, reported by Takayasu et al[35] in a prospective cohort study. The high recurrence rate may be because there are residual viable tumor cells after TACE that could not be detected radiologically. Thus, the subsequent salvage resection is needed to remove them to provide pathological evidence even when AFP normalizes after conversion therapy (≤ 20 mg/L). According to Zhang et al[36], the median OS in the S group (patients receiving resection after TACE) was 49 mo, which differed significantly from the median OS in the T group (not receiving resection after TACE), which was 31 mo. The 2-, 4-, and 5-year survival rates were 93%, 47%, and 26% in the S group and 74%, 18%, and 10% in the T group, respectively[36].
TACE followed by salvage surgery can prolong the OS of initially unresectable HCC patients. According to Majno et al[12], the conversion rate of initially unresectable HCC was 42%, but the OS was not mentioned. Fan et al[8] reported that 65 patients who received TACE followed by salvage surgery had a 5-year survival rate of 56%. Majno et al[12] also indicated that improved disease-free survival (DFS) after liver resection was closely related to a good response to TACE. A good response can be downstaging of the tumor or total necrosis. Downstaging was further defined as a 50% reduction of the product of the perpendicular diameters of the largest lesion detected by CT. Patients who met this criterion seemed to have a promising DFS[12]. The absence of PVT was also regarded as a criterion for downstaging[12]. We hypothesized that the response to TACE is an independent prognostic factor for survival and possible timing for salvage surgery. Two studies have reported on the possibility of this hypothesis. A prospective nonrandomized analysis from Luo et al[37] has revealed that subsequent resection prolonged survival time in patients who showed good response to TACE. However, this study was a nonrandomized study and it didn’t provide any details about which types of patients benefit a lot, CR or PR. In another study by Zhang et al[36], 82 patients with unresectable HCC were divided into 2 groups: S group (TACE followed by salvage surgery) and T group (TACE alone). The retrospect analysis showed that patients with TACE followed by surgery had better OS than TACE alone (49 mo vs 31 mo, P = 0.027). Furthermore, the author also made a subgroup analysis in S group, showing that the median OS for patients in the complete respond (CR) which was defined as achieving CR according to mRECIST with AFP normalized and partial response (PR) defined as PR in mRECIST was not significantly different (50 and 49 mo, respectively, P = 0.699)[36]. It is remarkable that the OS of patients who achieved CR to TACE in S group (salvage surgery) was comparable to that in and T group (TACE alone) (50 mo vs 54 mo, respectively). And those who achieved PR benefited a lot from salvage surgery in comparison to TACE alone (49 mo vs 24 mo)[36]. These findings suggest the suitable timing of surgery is achieving PR after TACE rather than CR. Here, we consider that not all patients undergo salvage surgery after TACE, especially those who achieve CR with respect to necrosis. The rationality might be that patients achieving CR in terms of radiologic necrosis actually have no or few viable tumor cells which potentially induce tumor recurrence, resulting in better survival. We also believe that the subsequent salvage time after TACE to be the time when patients achieve PR in radiology, because the considerable quantity of tumor cell still active in liver and resection is expected to remove the viable tumor cell in order to prolong the DFS. In the future, more efforts should be put on the following to evaluate the timing for salvage surgery: (1) diminishment of large HCC; (2) FLR; (3) disappearance of PVT or MVI; and (4) margin with tumor clearance > 2 cm.
PVE and the subsequent salvage surgery time: Preoperative PVE, which increases FLR through inducing hypertrophy, has been introduced to expand the indications for major resection or insufficient liver function. PVE in an attempt to increase FLR was firstly reported by Makuuchi et al[38] for hilar bile duct carcinoma, and its function in preventing postoperative liver failure has also been proven[38,39]. A meta-analysis from Abulkhir et al[40] reported that about 85% patients after PVE could be undertaken surgery, while 0.8% patients died after acute liver failure. PVE may induce atrophy in the embolized lobe and compensatory hypertrophy of the future remaining lobe after hepatectomy. Thus, PVE offers alternatives to patients with insufficient FLR and makes resection possible.
Generally, the salvage surgery time associated with liver assessment after PVE, in other words the FLR, is evaluated by three-dimensional CT. It is currently recommended that the minimum sFLR after hepatic resection are 20%-25% in normal livers, but 40% in compromised livers (such as cirrhosis, steatosis or chronic hepatitis)[41-44]. In Japan, PVE is performed when the non-tumor resection rate is > 60% for patients with normal ICGR15 and > 40% for patients with 10% < ICGR15 ≤ 20%[45]. Furthermore, PVE is rarely performed before an extended left hepatectomy or left trisectionectomy because the right posterior section often occupies approximately 30% of the TLV[46].
The sFLR is usually assessed 4-8 wk after PVE[47]. It is expected that the rapid growth of FLR can be achieved in the next 30-40 d. For patients with a normal liver, a sFLR ranging from 20%-25% is the minimal safe volume for surgery[42,48-50]. Two large studies have confirmed the 20% sFLR as the safe cutoff for surgery[48,51]. Abdalla[51] showed that 50% of patients with sFLR < 20% of TLV had postoperative complications while only 13% of patients with an FLR > 20%TLV had complications. In the study by Kishi et al[48], the incidences of hepatic insufficiency and death due to liver failure were not different between patients with 20% ≤ sFLR < 30% and patients with a sFLR ≥ 30% and only patients with sFLR < 20% had increased rate of complications. In addition, a sFLR ≥ 40% in patients with cirrhosis is often proposed as a safe minimal volume[52-54].
Unfortunately, the majority of assays mixed the safe cutoff with PVE with safe cutoff without PVE and only a few assays took a close look at what the safe cutoff of sFLR after PVE is. Vauthey et al[16] launched a research on a safe cutoff of FLR, which showed that subjects after PVE with sFLR ≤ 25% was a risk of experiencing major complications (60%) (P = 0.002), while those whose sFLR > 25% were free of major complications. It cannot formula a safe cutoff because the number of subjects in the study is only 5. Ribero et al[55] found that major and liver-related complications, hepatic dysfunction or insufficiency were greater in a patient with sFLR < 20% or with a degree of hypertrophy (DH) of not more than 5%. Both studies paid more attention to the difference between patients with PVE and patients without PVE, suggesting that the underlying risk of mixing them up.
It is very important to figure out what is the mechanism of the regenerative ability of the liver after PVE, especially the relation between liver function improvement and liver volume increment. Meier et al[56] retrospectively compared post-right hepatectomy outcomes in 28 patients with and 53 without PVE in a non-randomized study, suggesting that the immediate post-operative liver function per unit of volume in patients with PVE was better than those without PVE. This finding was also similar to Farges et al[47] who found the improved post-operative liver function in patients with PVE compared with those without PVE in terms of the chronic liver. The study of Hoekstra et al[21] also revealed that the increase in FLR function after PVE was more pronounced than the increase in FRL volume. Based on these three studies, we hypothesize that PVE is able to increase not only the liver volume but also the post-operative function per unit of volume, which has not been fully elucidated and we presume that the safe cutoff for surgery after PVE ought to be revaluated.
Apart from FLR, other factors are also used to predict post-operative complications. Leung et al[57] retrospectively analyzed 153 patients who underwent a major hepatectomy after PVE and calculated growth rate (GR = DH/weeks since PVE), finding that no patient with GR > 2.66%/wk developed liver failure. Shindoh et al[58] used degree of hypertrophy at initial volume assessment divided by number of weeks elapsed after PVE defined as the kinetic growth rate (KGR) to predict overall and liver-specific postoperative morbidity and mortality, whose study indicated that KGR of less than 2% per week vs ≥ 2% per week correlate with rates of hepatic insufficiency (21.6% vs 0%, P = 0.0001) and liver-related 90-d mortality (8.1% vs 0%, P = 0.04).
Methods to evaluate the suitable timing for surgery seem various, but the best remains uncertain. Technetium-99m-galactosyl human serum albumin (Tc-99m-GSA) scintigraphy might be a good candidate to assess the timing. In previous studies, Tc-99m-GSA scintigraphy is able to detect Tc-99m-GSA agent and determine the liver functional reserve in various physiological and pathological states which is usually performed 2 wk after PVE[59-61]. Hirai et al[59] reported that the functional increase in 99mTc-GSA uptake after PVE is superior to the degree of morphologic, which is similar to previous studies. It was shown in his study that patients with the ratio of the left lobe volume to the standard liver volume < 35% and a low 99mTc-GSAuptake (< 25%) in the non embolized lobe after PVE were a risk of developing postoperative liver failure. Beppu et al[61] prospectively performed an analysis on patients undergoing PVE and found that increment in the percentage of functional remnant liver volume was 7.5% greater for that of the non-tumorous RLV (P < 0.001). Kubo et al[62] performed 99mTc-GSA scintigraphy on 16 patients undergoing percutaneous transhepatic portal vein embolization (PTPE) and found that 12 patients with the left receptor index ≥ 0.35 were free of any major postoperative complication, which was calculated by dividing the radioactivity of the left lobe of the liver regions of interest (ROI) by that of the entire liver plus heart ROIs 15 min after the injection of the 99mTc-GSA[62]. Nishiyama et al[63] devised an original predictive residual index (PRI) by combining the k-value with functional liver volume which was measured by liver dynamic SPET for pre-operative assessment relevant to PTPE and reported that patients with PRI above 0.4 had a low incidence of hepatic failure after hepatectomy. As far as we are concerned, 99mTc-GSA scintigraphy is a good diagnostic tool for evaluation of functional liver volume and some safe cutoff could be used in assessing the suitable timing for surgery after PVE.
Wakabayashi et al[64] had previously noted a negative prognosis (complications or liver failure) for patients after PVE but prior to surgery was closely related to 5 factors: (1) a hypertrophic ratio of the left lobe < 1.21; (2) anICGR15 > 16% after PVE; (3) a portal pressure > 25 cmH2O immediately after PVE; (4) a post-PVE serum cholinesterase < 160 U/L; and (5) a serum hyaluronate > 160 ng/mL after PVE; however, we cannot obtain further information.
In our opinion, sFLR is usually used for evaluation of the appropriate surgery time; surgery may be safe when sFLR is > 20% for normal livers after PVE. Other predictive parameters like GR, KGR are treated as an effective supplementary to the assessment. Tc-99m-GSA scintigraphy might be a good candidate to accurately evaluate the suitable salvage surgery time.
ALPPS and the subsequent salvage surgery time: Associating liver partition and portal vein ligation (PVL) for staged hepatectomy (ALPPS) is a revolutionary strategy that combines liver partition with PVL followed by a second resection of the tumor part of the liver[65]. ALPPS consists of 2 stages. Stage 1 comprises surgical exploration, in situ splitting (ISS) of the liver parenchyma and exposure of the inferior vena cava. Stage 2 includes performing extended resection and ligating the disease-side hepatic artery, right bile duct, and hepatic vein. Unlike conventional PVE or PVL procedures, ALPPS occludes the blood supply (usually the portal vein) to the tumor part of the liver, blocks the collateral flow, and induces rapid growth of sFLR (40%-160%) in only 1 or 2 wk, while it takes more than 4 wk in PVE/PVL[66,67]. Therefore, ALPPS is often regarded as not only a remedy for PVE or PVL to accelerate FLR regeneration but also a strategy to prevent tumor progression. According to Erik Schadde et al[68], patients in the ALPPS group showed a 77% increase in FLR on average between stages compared to an increase of 34% in the PVE/PLE group. Moreover, patients in the ALPPS group (48/48) all achieved a 30% increase in FLR, which was the cut off proposed for safe liver resection, while the PVE/PVL group did not[68]. ALPPS could achieve a 100% feasibility of R0 resection by pathology, which was the aim of ALPPS and expanded the indication for extended resection[13]. It was also indicated that 83.3% of patients (10/12) achieved R0 in monosegment ALPPS hepatectomy[69].
ALPPS is often indicated for colorectal liver metastases (CRLM) or initially unresectable HCC with an insufficient FLR. Patients with a sFLR ≤ 30% in normal liver or a sFLR ≤ 40% in injured livers (such as livers with cirrhosis or macrosteatosis) are usually candidates for ALPPS. During the ALPPS, preoperative assessments before stage 2 will be performed 6-9 d after stage 1 to evaluate the FLR. CT is usually used for volumetric measurement. Studies have suggested an sFLR over 20% = 30% in patients with normal livers and over 40% = 50% in patients with diseased livers are safe for surgery[44,54,68,70-73]. We show in Table 2 (ALPPS over nearly five years, including FLR% changes between stage 1 and stage 2, morbidity and mortality) below in an attempt to determine the best cutoff. It seems that high morbidity occurs frequently when the sFLR is over 30%, indicating the safe cutoff in PVE might not be suitable for ALPPS. In view of this finding, Nadalin et al[70] used the growth rate of RLV/TLV > 30% or the RLVBWR > 0.5 as the safe cutoff for stage 2, for which morbidity and mortality were 66.7% and 28.7%, respectively. A multicenter study suggested that the 90-d mortality rate was 9%, and most deaths were related to liver failure[74]. Patients in the interval between stage 1 and stage 2 in ALPPS were reported to have a higher liver failure rate than those who underwent PVE[75]. The discrepancy between rapid volumetric hypertrophy and a high incidence of liver failure indicates a need to assess the intrinsic function of the liver.
| Ref. | Year | Cases | FLR%1 | FLR%2 | FLR%3 | Morbidity | Mortality | Feasibility |
| [106] | 2017 | 20 | 15 | 41 (24-67) | 88 | NM | 0 | 100 |
| [107] | 2016 | 295 | 26 | 39 | 74 | NM | 7.5 | NM |
| [108] | 2016 | 17 | 24.2 | 38.5 (27.9-56.9) | - | 11.8 | 5.9 | 100 |
| [69] | 2015 | 12 | 15 | 35 (26-53) | 160 | NM | 0 | 100 |
| [109] | 2015 | 9 | 21.1 | 32.2 (26.5-37.9) | 96 | 66.7 | 1 | 100 |
| [110] | 2015 | 62 | 24.2 | 39.1 (22.3-72.2) | 48.6 | 80 | 12.9 | 95.2 |
| [111] | 2015 | 11 | 33.9 | 46.3 (36.2-55.8) | 140 | 45 | 9.1 | 100 |
| [70] | 2014 | 15 | 22.6 | 36.3 (30-59.2) | 87.2 | 66.7 | 28.7 | 100 |
| [68] | 2014 | 48 | 23 | 41 (34-47) | 77.4 | NM | 17 | 100 |
| [13] | 2013 | 15 | 27 | 46.9 (31.7-67) | 78.4 | 53 | 0 | 100 |
| [112] | 2013 | 9 | 22.9 | NM | 87.2 | 68 | 12 | 100 |
| [66] | 2012 | 10 | 27.8 | NM | 82 | 40 | 0 | 100 |
Hepatobiliary scintigraphy (HBS) with 99-m TC-GSA is a quantitative regime that assesses the uptake function of the liver mass by calculating the density of specific receptors. Truant et al[76] identified a notable delay in a functional increase (12.5%) by HBS in ALPPS inter-stages phase, while the liver volume had achieved a remarkable rate of hypertrophy (41.7%). The hypothesis was presumably that the early stage of hypertrophy was carried by immature hepatocytes that lacked functional capacity. These findings suggest that liver failure could even occur after stage 1, which is supported by the International Study Group of Liver Surgery (ISGLS) criteria[77]. Although HBS is promising to assess the intrinsic liver function objectively, the lack of extensive studies makes it difficult to establish a safe cutoff for surgery. In previous studies, CTP Cor stages B, C and D of BCLC were regarded as predictors of death[23]. Schadde et al[68] proposed that liver failure meeting the ISGLS criteria after stage 1, or over 10 points of the model of end-stage liver disease (MELD) before stage 2, was an independent factor of a poor prognosis.
We believe that ALPPS may increase resectability and reduce unsatisfactory morbidity and mortality. There is insufficient evidence to sustain a safe cutoff not only in sFLR but also in intrinsic liver function. It is risky to apply the safe cutoff standard of FLR from PVE to ALPPS[78]. HBS combined with traditional assessments might be effective in distinguishing suitable candidates for stage 2.
Yttrium-90 microsphere radioembolization and the subsequent salvage surgery time: Yttrium-90 microsphere RE, a novel conversion therapy for initially unresectable HCC, is always indicated for insufficient FLR and lesions with close proximity to important structures such as portal veins that make R0 resection impossible. Nevertheless, yttrium-90 microsphere RE is inferior to PVE regarding the hypertrophy rate. PVE showed a higher hypertrophy compared with RE (PVE: 61.5% vs SIRT: 29.0%) within a shorter period [PVE: 33 (24-56) d vs SIRT: 46 (27-79) d][79].
We briefly classified the preoperative evaluation after RE into FLR and tumor response. Two previous studies had demonstrated that the increased rate of hypertrophy was unfavorable in RE[80,81]. To elucidate the dynamic change of FLR, one study observed that the FLR hypertrophy rate was 24% at 1.5-3 mo, 35% at 3-6 mo, and 45% after 9 mo. Despite the slow hypertrophy kinetic outcome, 9 of the 18 individuals achieved a sFLR > 25%. Additionally, the study indicated the volumetric hypertrophy after RE was likely to result in enough FLR for salvage surgery, although at a slow rate[79]. Regarding the tumor response, yttrium-90 microsphere RE is able to induce tumor necrosis. It is reported by many studies that the rate of CR and PR are 0%-10% and 35%-47% respectively according to WHO criterion in a patient with HCC after yttrium-90 microsphere RE[82-91]. However, the majority of the litertures took RE as a neoadjuvant therapy rather than conversion therapy. Only a few mentioned about the rate of downstaging to LT or resection, which is ranging from 29%-50%[92-94].
In our opinion, there are insufficient studies on the efficiency of the yttrium-90 microsphere RE as a conversion therapy for surgery. Also, the indications for yttrium-90 microsphere RE in an attempt to conversion therapy are uncertain. Due to the risk of tumor progression in patients undergoing PVE, we suggested that yttrium-90 microsphere RE might be considered when patients are contraindicated for PVE or vital structure is likely to get invaded because of tumor progression. In terms of those who only need adequate FLR, PVE is prior to RE.
As for safe cutoff, no prospective or retrospective study on timing for surgery is reported. A sFLR > 25% with a normal liver might be the safe cutoff[95]. For patients with cirrhosis, a sFLR > 40%is recommended. The tumor response evaluation is based on size (WHO criterion) or necrosis (EASL criterion) and ranges from 20% to 99%[88,90,96]. In spite of its promising effect on tumor necrosis, none of the studies evaluated the timing for surgery.
Sequential TACE and PVE and the subsequent salvage time: The feasibility and effectiveness of PVE to induce compensatory hypertrophy of the contra-lateral parenchyma for patients with insufficient FLR have been documented in numerous studies[42,97,98]. However, since the capacity for regeneration in cirrhotic patients is impaired, the hypertrophy rate often fails to meet the safe criterion for surgery. On the other hand, tumor progression could possibly occur based on the fact that the liver is a double blood-supply organ. In other words, when the portal vein is embolized, a compensatory increase in artery flow might occur[99].
To improve the insufficient FLR and reduce the risk of tumor progression, sequential TACE followed by PVE has been proposed. The rationale is that TACE not only augments the effect of PVE but also prevents the progression of the tumor through the double occlusion. An animal study of rabbit VX2 has documented that the TACE + PVE group has higher levels of IL-6, TNF-α and HGF than the TACE or PVE groups alone, indicating that combined treatment might induce stronger liver regeneration[100]. The reported rate of conversion to surgery is appreciable, ranging from 72% to 100% (Table 3). The 5-year OS rate was over 40%, which is comparable to the resection for resectable HCC[101,102]. It should be noted that TACE + PVE features not only a higher rate of increases in the percentage of FLR than PVE alone (12% vs 8%, respectively, P = 0.022) but also a better 5-year recurrence-free survival rate (37% vs 19%, respectively)[102]. A possible rationale for the appreciable hypertrophy is that TACE might attenuate the compensatory arterial flow in area embolized by PVE and induce severe damage in the embolized area, resulting in atrophy of FLR, which we also call double occulation effect[99]. However, Peng et al[103] reported that combined treatment did not induce significant increase in percent FLR compared with PVE alone [percent increase in FLR (PVE alone, 7.9% vs sequential intra-arterial therapy (IAT) + PVE, 7.4%; P = 0.203)] and the author assumed the different conclusion to the embolism agent and techniques[103]. Given the anti-tumor effect, TACE + PVE might induce more complete necrosis of tumor burden. It was reported by Ogata et al[102] that sequential TACE and PVE induced complete tumor necrosis in more than 80% of patients, compared with only 5% after PVE alone. The study also detected a higher 1-, 3- and 5-year recurrence-free survival rates in TACE + PVE group than PVE group (93%, 37% and 37% vs 63%, 19% and 19%; P = 0.041)[102].
| Ref. | Year | Cases | Types of tumor | Convert to surgery (%) | 5-yr disease-free survival rates (%) | Median survival time (mo) |
| [101] | 2004 | 17 | Hepatocellular carcinoma | 94 | 46.7 | NM |
| [102] | 2006 | 18 | Hepatocellular carcinoma | 100 | 37 | NM |
| [113] | 2011 | 71 | Hepatocellular carcinoma | 95.7 | 61 | NM |
| [103] | 2012 | 29 | Hepatocellular carcinoma and metastatic disease | 93.1 | NM | 58 |
| [104] | 2016 | 54 | Hepatocellular carcinoma | 72 | NM | 41 |
This combined approach was used for patients with unilobar HCC or impaired livers (such as livers with cirrhosis, fibrosis, steatohepatitis or steatosis) to undergo major hepatectomy. Patients with CTP A with a good performance status were simultaneously evaluated (ECOG 0-2)[104].
The TACE + PVE procedure usually consists of 2 steps: (1) TACE performed on selected patients; and (2) a follow-up PVE performed with measurements (liver volumetric assessment, ICGR15 and liver function tests) after an interval ranging from 1 wk to 4 wk[101,102,105]. Aoki et al[101] recommended two standards for resection: (1) the volumetric ratio of future remnant segments was nearly 40% (in cases with an ICGR15 of less than 10%) or 60% (in cases with an ICGR15 of 10%-20%) of the total liver parenchyma; and (2) the liver function test results had returned to the baseline. The results proved that patients who followed this cut-off had promising 5-year disease-free and OS rates of 46.7% and 55.6%, respectively[101]. Tumor progression, insufficient FLR hypertrophy (< 5%) and liver failure were used as exclusion criteria, and patients who met any of those criteria could achieve a median OS of 41 mo[104]. Ogata et al[102] identified F4 fibrosis and an increase in the percentage of FLR volume less than 10% as two important complication-related factors. In this study, he stratified the patients with cirrhosis and noted that a 5% and 10% increase in FLR should be achieved in F3 fibrosis and F4 fibrosis, respectively[102].
In the interval between TACE and PVE, the ALT and AST of almost all the patients were elevated but soon returned to a normal level over a short period; this finding could be explained by the necrosis of the liver parenchyma[105]. According to this rationale, careful selection of the timing for PVE is crucial. In other words, a short interval between TACE and PVE is likely to cause PHLF, while a long interval might result in disease progression. Here, we strongly advocate that a 5% increase in FLR and a normalized liver function tests can be used to determine a safe cutoff for salvage surgery time. If the patients can undergo liver cirrhotic assessment, then the safe cutoff of F4 fibrosis should be reappraised. Any patient who develops liver failure or tumor progression after conversion therapy should be excluded from the surgery list.
Are there any remaining problems that need to be solved? Firstly, the definition of unresectable is still subjective once T1 and T4 stages are excluded. However, the distribution of the nodules to both hepatic lobes, the presence of high alpha-feto levels, and the vascular involvement are substantial tumoral parameters that help in the evaluation of resectability beside residual liver function and patients general conditions. Moreover, the limit of unresectability depends on the level of the hospital and the experience of the operator or their expertise in surgery.
For the initially unresectable HCC patients, conversion therapies such as TACE, PVE, ALPPS, yttrium-90 RE, and sequential TACE and PVE have been demonstrated to be effective and should be performed. Both morphological and functional examinations need to be undertaken to estimate the therapeutic effect before salvage surgery. Controlling a good operative time and selecting a reasonable procedure are important for improving the operative efficacy. The reasonable unified application of conversion therapy and salvage surgery can improve the curative effect and increase the survival rate of patients.
Hepatocellular carcinoma (HCC) is a primary cancer of the liver and is the fifth most prevalent cancer in men and the seventh in women worldwide. Hepatectomy is currently the first-line curative therapy, but about 30% of lesions are resectable at the time of diagnosis. Conversion therapy is used to increase the resectability of initially unresectable HCC by increasing the size of the future liver remnant (FLR) or downstaging the tumor, followed by salvage surgery. Although various preoperative therapies provide initially unresectable HCC patients with the chance to undergo curative resection, the suitable timing of the subsequent salvage surgery remains uncertain and controversial.
Only 10%-30% HCC patients can obtain the chance to undergo surgery at the time of diagnosis. Those who are not suitable for curative surgery may benefit from conversion therapy and seize the opportunity to undergo salvage surgery when they reach the “timing”. Therefore, we review the types of conversion therapy and the suitable timing for salvage surgery.
To review the conversion therapy for initially unresectable HCC patients and the suitable timing for subsequent salvage surgery, and we finally hope to increase the 5-year survival rate of HCC patients.
A PubMed search was undertaken from 1987 to 2017 to identify articles using the key words including “unresectable” “hepatocellular carcinoma”, ”hepatectomy”, ”conversion therapy”, “resection”, “salvage surgery” and “downstaging”. Additional studies were investigated through a manual search of the references from the articles. The exclusion criteria were duplicates, case reports, case series, videos, contents unrelated to the topic, comments, and editorial essays. The main and widely used conversion therapies and the suitable timing for subsequent salvage surgery were discussed in detail. Two members of our group independently performed the literature search and data extraction.
Liver volume measurements (FLR/total liver volume or residual liver volume/bodyweight ratio) and function tests (scoring systems and liver stiffness) were often performed in order to justify whether patients were suitable candidates for surgery. Successful conversion therapy was usually defined as downstaging the tumor, increasing FLR and providing subsequent salvage surgery, without increasing complications, morbidity or mortality. The requirements for performing salvage surgery after transcatheter arterial chemoembolization (TACE) were the achievement of a partial remission in radiology, the disappearance of the portal vein thrombosis (PVT), and the lack of extrahepatic metastasis. Patients with a standardized FLR (sFLR) > 20% were good candidates for surgery after portal vein embolization (PVE), while other predictive parameters like growth rate (GR), kinetic growth rate (KGR) were treated as an effective supplementary. There was probably not enough evidence to provide a standard operation time after associating liver partition and portal vein ligation for staged hepatectomy (ALLPS) or yttrium-90 microsphere radioembolization (RE). The indications of any combinations of conversion therapies and the subsequent salvage surgery time still need to be carefully and comprehensively evaluated.
Conversion therapy is recommended for the treatment of initially unresectable HCC, and the suitable subsequent salvage surgery time should be reappraised and is closely related to its previous therapeutic effect.
For the initially unresectable HCC patients, conversion therapies such as TACE, PVE, ALPPS, yttrium-90 RE, and sequential TACE and PVE have been demonstrated to be effective and should be performed. Both morphological and functional examinations need to be undertaken to estimate the therapeutic effect before salvage surgery. Controlling a good operative time and selecting a reasonable procedure are important for improving the operative efficacy. The reasonable unified application of conversion therapy and salvage surgery can improve the curative effect and increase the survival rate of patients.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Agrawal S, Barone M, Ho MC, Memeo R S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11837] [Article Influence: 845.5] [Reference Citation Analysis (4)] |
| 2. | Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18694] [Cited by in RCA: 21371] [Article Influence: 2137.1] [Reference Citation Analysis (3)] |
| 3. | European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4059] [Cited by in RCA: 4521] [Article Influence: 347.8] [Reference Citation Analysis (2)] |
| 4. | Befeler AS, Hayashi PH, Di Bisceglie AM. Liver transplantation for hepatocellular carcinoma. Gastroenterology. 2005;128:1752-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Yao FY, Bass NM, Nikolai B, Davern TJ, Kerlan R, Wu V, Ascher NL, Roberts JP. Liver transplantation for hepatocellular carcinoma: analysis of survival according to the intention-to-treat principle and dropout from the waiting list. Liver Transpl. 2002;8:873-883. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 312] [Article Influence: 13.6] [Reference Citation Analysis (1)] |
| 6. | Llovet JM, Burroughs A, Bruix J. Hepatocellular carcinoma. Lancet. 2003;362:1907-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3241] [Cited by in RCA: 3282] [Article Influence: 149.2] [Reference Citation Analysis (0)] |
| 7. | Harding JJ, Connell LC, El Dika I, Abou-Alfa GK. Chapter 101 - Advances in systemic therapy for hepatocellular carcinoma. Blumgart’s Surgery of the Liver, Biliary Tract and Pancreas, 2-Volume Set. Sixth Edition. Philadelphia: Content Repository Only 2017; 1502-1513.e1504. |
| 8. | Fan J, Tang ZY, Yu YQ, Wu ZQ, Ma ZC, Zhou XD, Zhou J, Qiu SJ, Lu JZ. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg. 1998;15:674-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Tang ZY, Zhou XD, Ma ZC, Wu ZQ, Fan J, Qin LX, Yu Y. Downstaging followed by resection plays a role in improving the prognosis of unresectable hepatocellular carcinoma. Hepatobiliary Pancreat Dis Int. 2004;3:495-498. [PubMed] |
| 10. | Lau WY, Ho SK, Yu SC, Lai EC, Liew CT, Leung TW. Salvage surgery following downstaging of unresectable hepatocellular carcinoma. Ann Surg. 2004;240:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 112] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Sitzmann JV, Abrams R. Improved survival for hepatocellular cancer with combination surgery and multimodality treatment. Ann Surg. 1993;217:149-154. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Majno PE, Adam R, Bismuth H, Castaing D, Ariche A, Krissat J, Perrin H, Azoulay D. Influence of preoperative transarterial lipiodol chemoembolization on resection and transplantation for hepatocellular carcinoma in patients with cirrhosis. Ann Surg. 1997;226:688-701; discussion 701-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 392] [Cited by in RCA: 389] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 13. | Alvarez FA, Ardiles V, Sanchez Claria R, Pekolj J, de Santibañes E. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg. 2013;17:814-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 132] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 14. | Kalkmann J, Forsting M, Stattaus J. Liver volume variations as a parameter to assess therapy response in advanced metastatic liver disease. Onkologie. 2011;34:30-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 15. | Dubus L, Gayet M, Zappa M, Abaleo L, De Cooman A, Orieux G, Vilgrain V. Comparison of semi-automated and manual methods to measure the volume of liver tumours on MDCT images. Eur Radiol. 2011;21:996-1003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Vauthey JN, Chaoui A, Do KA, Bilimoria MM, Fenstermacher MJ, Charnsangavej C, Hicks M, Alsfasser G, Lauwers G, Hawkins IF. Standardized measurement of the future liver remnant prior to extended liver resection: methodology and clinical associations. Surgery. 2000;127:512-519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 494] [Cited by in RCA: 489] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 17. | Vauthey JN, Abdalla EK, Doherty DA, Gertsch P, Fenstermacher MJ, Loyer EM, Lerut J, Materne R, Wang X, Encarnacion A. Body surface area and body weight predict total liver volume in Western adults. Liver Transpl. 2002;8:233-240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 443] [Cited by in RCA: 465] [Article Influence: 20.2] [Reference Citation Analysis (0)] |
| 18. | Truant S, Oberlin O, Sergent G, Lebuffe G, Gambiez L, Ernst O, Pruvot FR. Remnant liver volume to body weight ratio > or =0.5%: A new cut-off to estimate postoperative risks after extended resection in the noncirrhotic liver. J Am Coll Surg. 2007;204:22-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 213] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 19. | Truant S, Boleslawski E, Sergent G, Leteurtre E, Duhamel A, Hebbar M, Pruvot FR. Liver function following extended hepatectomy can be accurately predicted using remnant liver volume to body weight ratio. World J Surg. 2015;39:1193-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 20. | Lin XJ, Yang J, Chen XB, Zhang M, Xu MQ. The critical value of remnant liver volume-to-body weight ratio to estimate posthepatectomy liver failure in cirrhotic patients. J Surg Res. 2014;188:489-495. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 21. | Hoekstra LT, de Graaf W, Nibourg GA, Heger M, Bennink RJ, Stieger B, van Gulik TM. Physiological and biochemical basis of clinical liver function tests: a review. Ann Surg. 2013;257:27-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 204] [Cited by in RCA: 250] [Article Influence: 20.8] [Reference Citation Analysis (0)] |
| 22. | Wu CC, Ho WL, Lin MC, Tang JS, Yeh DC, Liu TJ, P’eng FK. Is hepatic resection absolutely contraindicated for hepatocellular carcinoma in Child-Pugh class C cirrhotic patients? Hepatogastroenterology. 1999;46:635-639. [PubMed] |
| 23. | Franco D, Capussotti L, Smadja C, Bouzari H, Meakins J, Kemeny F, Grange D, Dellepiane M. Resection of hepatocellular carcinomas. Results in 72 European patients with cirrhosis. Gastroenterology. 1990;98:733-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 209] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 24. | Imamura H, Seyama Y, Kokudo N, Maema A, Sugawara Y, Sano K, Takayama T, Makuuchi M. One thousand fifty-six hepatectomies without mortality in 8 years. Arch Surg. 2003;138:1198-1206; discussion 1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 597] [Cited by in RCA: 615] [Article Influence: 29.3] [Reference Citation Analysis (0)] |
| 25. | Makuuchi M, Kosuge T, Takayama T, Yamazaki S, Kakazu T, Miyagawa S, Kawasaki S. Surgery for small liver cancers. Semin Surg Oncol. 1993;9:298-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 538] [Cited by in RCA: 597] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 26. | Wu D, Chen E, Liang T, Wang M, Chen B, Lang B, Tang H. Predicting the risk of postoperative liver failure and overall survival using liver and spleen stiffness measurements in patients with hepatocellular carcinoma. Medicine (Baltimore). 2017;96:e7864. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 27. | Cescon M, Colecchia A, Cucchetti A, Peri E, Montrone L, Ercolani G, Festi D, Pinna AD. Value of transient elastography measured with FibroScan in predicting the outcome of hepatic resection for hepatocellular carcinoma. Ann Surg. 2012;256:706-712; discussion 712-713. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 125] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 28. | Chong CC, Wong GL, Chan AW, Wong VW, Fong AK, Cheung YS, Wong J, Lee KF, Chan SL, Lai PB. Liver stiffness measurement predicts high-grade post-hepatectomy liver failure: A prospective cohort study. J Gastroenterol Hepatol. 2017;32:506-514. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Takayasu K, Shima Y, Muramatsu Y, Moriyama N, Yamada T, Makuuchi M, Hasegawa H, Hirohashi S. Hepatocellular carcinoma: treatment with intraarterial iodized oil with and without chemotherapeutic agents. Radiology. 1987;163:345-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 241] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 30. | Facciorusso A, Licinio R, Muscatiello N, Di Leo A, Barone M. Transarterial chemoembolization: Evidences from the literature and applications in hepatocellular carcinoma patients. World J Hepatol. 2015;7:2009-2019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 31. | Han K, Kim JH. Transarterial chemoembolization in hepatocellular carcinoma treatment: Barcelona clinic liver cancer staging system. World J Gastroenterol. 2015;21:10327-10335. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 131] [Cited by in RCA: 129] [Article Influence: 12.9] [Reference Citation Analysis (2)] |
| 32. | Tang YL, Qi XS, Guo XZ. Hepatic Resection after Initial Transarterial Chemoembolization Versus Transarterial Chemoembolization Alone for the Treatment of Hepatocellular Carcinoma: A Meta-analysis of Observational Studies. Asian Pac J Cancer Prev. 2015;16:7871-7874. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 33. | Wang X, Li J, Peng Y, Dai Y, Xu W. Influence of preoperative transarterial chemoembolization on the prognosis for patients with resectable hepatocellular carcinoma: a meta-analysis of randomized trials. Hepatogastroenterology. 2011;58:869-874. [PubMed] |
| 34. | Zhou Y, Zhang X, Wu L, Ye F, Su X, Shi L, Li B. Meta-analysis: preoperative transcatheter arterial chemoembolization does not improve prognosis of patients with resectable hepatocellular carcinoma. BMC Gastroenterol. 2013;13:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 35. | Takayasu K, Arii S, Ikai I, Omata M, Okita K, Ichida T, Matsuyama Y, Nakanuma Y, Kojiro M, Makuuchi M. Prospective cohort study of transarterial chemoembolization for unresectable hepatocellular carcinoma in 8510 patients. Gastroenterology. 2006;131:461-469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 580] [Cited by in RCA: 629] [Article Influence: 33.1] [Reference Citation Analysis (0)] |
| 36. | Zhang Y, Huang G, Wang Y, Liang L, Peng B, Fan W, Yang J, Huang Y, Yao W, Li J. Is Salvage Liver Resection Necessary for Initially Unresectable Hepatocellular Carcinoma Patients Downstaged by Transarterial Chemoembolization? Ten Years of Experience. Oncologist. 2016;21:1442-1449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 37. | Luo J, Peng ZW, Guo RP, Zhang YQ, Li JQ, Chen MS, Shi M. Hepatic resection versus transarterial lipiodol chemoembolization as the initial treatment for large, multiple, and resectable hepatocellular carcinomas: a prospective nonrandomized analysis. Radiology. 2011;259:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 57] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 38. | Makuuchi M, Thai BL, Takayasu K, Takayama T, Kosuge T, Gunvén P, Yamazaki S, Hasegawa H, Ozaki H. Preoperative portal embolization to increase safety of major hepatectomy for hilar bile duct carcinoma: a preliminary report. Surgery. 1990;107:521-527. [PubMed] |
| 39. | Lee KC, Kinoshita H, Hirohashi K, Kubo S, Iwasa R. Extension of surgical indications for hepatocellular carcinoma by portal vein embolization. World J Surg. 1993;17:109-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 112] [Cited by in RCA: 106] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 40. | Abulkhir A, Limongelli P, Healey AJ, Damrah O, Tait P, Jackson J, Habib N, Jiao LR. Preoperative portal vein embolization for major liver resection: a meta-analysis. Ann Surg. 2008;247:49-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 474] [Article Influence: 27.9] [Reference Citation Analysis (0)] |
| 41. | Fazakas J, Mándli T, Ther G, Arkossy M, Pap S, Füle B, Németh E, Tóth S, Járay J. Evaluation of liver function for hepatic resection. Transplant Proc. 2006;38:798-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 42. | Abdalla EK, Hicks ME, Vauthey JN. Portal vein embolization: rationale, technique and future prospects. Br J Surg. 2001;88:165-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 307] [Cited by in RCA: 304] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 43. | Abdalla EK, Barnett CC, Doherty D, Curley SA, Vauthey JN. Extended hepatectomy in patients with hepatobiliary malignancies with and without preoperative portal vein embolization. Arch Surg. 2002;137:675-80; discussion 680-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 356] [Cited by in RCA: 337] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 44. | Tucker ON, Heaton N. The ‘small for size’ liver syndrome. Curr Opin Crit Care. 2005;11:150-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 134] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 45. | Beppu T, Okabe H, Okuda K, Eguchi S, Kitahara K, Taniai N, Ueno S, Shirabe K, Ohta M, Kondo K. Portal Vein Embolization Followed by Right-Side Hemihepatectomy for Hepatocellular Carcinoma Patients: A Japanese Multi-Institutional Study. J Am Coll Surg. 2016;222:1138-1148.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 46. | She WH, Chok KSh. Strategies to increase the resectability of hepatocellular carcinoma. World J Hepatol. 2015;7:2147-2154. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 47. | Farges O, Belghiti J, Kianmanesh R, Regimbeau JM, Santoro R, Vilgrain V, Denys A, Sauvanet A. Portal vein embolization before right hepatectomy: prospective clinical trial. Ann Surg. 2003;237:208-217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 326] [Cited by in RCA: 429] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 48. | Kishi Y, Abdalla EK, Chun YS, Zorzi D, Madoff DC, Wallace MJ, Curley SA, Vauthey JN. Three hundred and one consecutive extended right hepatectomies: evaluation of outcome based on systematic liver volumetry. Ann Surg. 2009;250:540-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 339] [Cited by in RCA: 364] [Article Influence: 22.8] [Reference Citation Analysis (0)] |
| 49. | Shindoh J, Tzeng CW, Aloia TA, Curley SA, Huang SY, Mahvash A, Gupta S, Wallace MJ, Vauthey JN. Safety and efficacy of portal vein embolization before planned major or extended hepatectomy: an institutional experience of 358 patients. J Gastrointest Surg. 2014;18:45-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 94] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 50. | Wicherts DA, de Haas RJ, Adam R. Bringing unresectable liver disease to resection with curative intent. Eur J Surg Oncol. 2007;33 Suppl 2:S42-S51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 50] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 51. | Abdalla EK. Portal vein embolization (prior to major hepatectomy) effects on regeneration, resectability, and outcome. J Surg Oncol. 2010;102:960-967. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 52. | Kubota K, Makuuchi M, Kusaka K, Kobayashi T, Miki K, Hasegawa K, Harihara Y, Takayama T. Measurement of liver volume and hepatic functional reserve as a guide to decision-making in resectional surgery for hepatic tumors. Hepatology. 1997;26:1176-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 197] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 53. | Shirabe K, Shimada M, Gion T, Hasegawa H, Takenaka K, Utsunomiya T, Sugimachi K. Postoperative liver failure after major hepatic resection for hepatocellular carcinoma in the modern era with special reference to remnant liver volume. J Am Coll Surg. 1999;188:304-309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 317] [Cited by in RCA: 326] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 54. | Schnitzbauer AA, Lang SA, Goessmann H, Nadalin S, Baumgart J, Farkas SA, Fichtner-Feigl S, Lorf T, Goralcyk A, Hörbelt R. Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg. 2012;255:405-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 837] [Cited by in RCA: 934] [Article Influence: 71.8] [Reference Citation Analysis (0)] |
| 55. | Ribero D, Abdalla EK, Madoff DC, Donadon M, Loyer EM, Vauthey JN. Portal vein embolization before major hepatectomy and its effects on regeneration, resectability and outcome. Br J Surg. 2007;94:1386-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 330] [Cited by in RCA: 343] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 56. | Meier RP, Toso C, Terraz S, Breguet R, Berney T, Andres A, Jannot AS, Rubbia-Brandt L, Morel P, Majno PE. Improved liver function after portal vein embolization and an elective right hepatectomy. HPB (Oxford). 2015;17:1009-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 57. | Leung U, Simpson AL, Araujo RL, Gönen M, McAuliffe C, Miga MI, Parada EP, Allen PJ, D’Angelica MI, Kingham TP. Remnant growth rate after portal vein embolization is a good early predictor of post-hepatectomy liver failure. J Am Coll Surg. 2014;219:620-630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 75] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Shindoh J, Truty MJ, Aloia TA, Curley SA, Zimmitti G, Huang SY, Mahvash A, Gupta S, Wallace MJ, Vauthey JN. Kinetic growth rate after portal vein embolization predicts posthepatectomy outcomes: toward zero liver-related mortality in patients with colorectal liver metastases and small future liver remnant. J Am Coll Surg. 2013;216:201-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 252] [Cited by in RCA: 236] [Article Influence: 19.7] [Reference Citation Analysis (0)] |
| 59. | Hirai I, Kimura W, Fuse A, Suto K, Urayama M. Evaluation of preoperative portal embolization for safe hepatectomy, with special reference to assessment of nonembolized lobe function with 99mTc-GSA SPECT scintigraphy. Surgery. 2003;133:495-506. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 68] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 60. | Iimuro Y. ICG Clearance Test and 99mTc-GSA SPECT/CT Fusion Images. Visc Med. 2017;33:449-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 61. | Beppu T, Hayashi H, Okabe H, Masuda T, Mima K, Otao R, Chikamoto A, Doi K, Ishiko T, Takamori H. Liver functional volumetry for portal vein embolization using a newly developed 99mTc-galactosyl human serum albumin scintigraphy SPECT-computed tomography fusion system. J Gastroenterol. 2011;46:938-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 73] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 62. | Kubo S, Shiomi S, Tanaka H, Shuto T, Takemura S, Mikami S, Uenishi T, Nishino Y, Hirohashi K, Kawamura E. Evaluation of the effect of portal vein embolization on liver function by (99m)tc-galactosyl human serum albumin scintigraphy. J Surg Res. 2002;107:113-118. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 19] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 63. | Nishiyama Y, Yamamoto Y, Hino I, Satoh K, Wakabayashi H, Ohkawa M. 99mTc galactosyl human serum albumin liver dynamic SPET for pre-operative assessment of hepatectomy in relation to percutaneous transhepatic portal embolization. Nucl Med Commun. 2003;24:809-817. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 29] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 64. | Wakabayashi H, Yachida S, Maeba T, Maeta H. Evaluation of liver function for the application of preoperative portal vein embolization on major hepatic resection. Hepatogastroenterology. 2002;49:1048-1052. [PubMed] |
| 65. | de Santibañes E, Clavien PA. Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg. 2012;255:415-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 268] [Cited by in RCA: 282] [Article Influence: 21.7] [Reference Citation Analysis (0)] |
| 66. | Sala S, Ardiles V, Ulla M, Alvarez F, Pekolj J, de Santibañes E. Our initial experience with ALPPS technique: encouraging results. Updates Surg. 2012;64:167-172. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 67. | Alvarez FA, Iniesta J, Lastiri J, Ulla M, Bonadeo Lassalle F, de Santibañes E. [New method of hepatic regeneration]. Cir Esp. 2011;89:645-649. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 68. | Schadde E, Ardiles V, Slankamenac K, Tschuor C, Sergeant G, Amacker N, Baumgart J, Croome K, Hernandez-Alejandro R, Lang H. ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg. 2014;38:1510-1519. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 185] [Cited by in RCA: 209] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 69. | Schadde E, Malagó M, Hernandez-Alejandro R, Li J, Abdalla E, Ardiles V, Lurje G, Vyas S, Machado MA, de Santibañes E. Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery. 2015;157:676-689. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 70. | Nadalin S, Capobianco I, Li J, Girotti P, Königsrainer I, Königsrainer A. Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons Learned from 15 cases at a single centre. Z Gastroenterol. 2014;52:35-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 85] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 71. | Sanei B, Sheikhbahaei S, Sanei MH, Bahreini A, Jafari HR. Associating liver partition and portal vein ligation for staged hepatectomy: A surgical technique for liver resections. J Res Med Sci. 2017;22:52. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 72. | Tschuor Ch, Croome KP, Sergeant G, Cano V, Schadde E, Ardiles V, Slankamenac K, Clariá RS, de Santibaňes E, Hernandez-Alejandro R, Clavien PA. Salvage parenchymal liver transection for patients with insufficient volume increase after portal vein occlusion -- an extension of the ALPPS approach. Eur J Surg Oncol. 2013;39:1230-1235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 83] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 73. | Guglielmi A, Ruzzenente A, Conci S, Valdegamberi A, Iacono C. How much remnant is enough in liver resection? Dig Surg. 2012;29:6-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 275] [Cited by in RCA: 248] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
| 74. | Schadde E, Raptis DA, Schnitzbauer AA, Ardiles V, Tschuor C, Lesurtel M, Abdalla EK, Hernandez-Alejandro R, Jovine E, Machado M. Prediction of Mortality After ALPPS Stage-1: An Analysis of 320 Patients From the International ALPPS Registry. Ann Surg. 2015;262:780-785; discussion 785-786. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 179] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 75. | Schnitzbauer AA. A Comparison of Pitfalls after ALPPS Stage 1 or Portal Vein Embolization in Small-for-Size Setting Hepatectomies. Visc Med. 2017;33:435-441. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 76. | Truant S, Baillet C, Deshorgue AC, El Amrani M, Huglo D, Pruvot FR. Contribution of hepatobiliary scintigraphy in assessing ALPPS most suited timing. Updates Surg. 2017;69:411-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 77. | Olthof PB, Tomassini F, Huespe PE, Truant S, Pruvot FR, Troisi RI, Castro C, Schadde E, Axelsson R, Sparrelid E. Hepatobiliary scintigraphy to evaluate liver function in associating liver partition and portal vein ligation for staged hepatectomy: Liver volume overestimates liver function. Surgery. 2017;162:775-783. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 97] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 78. | Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Yamazaki K, Ishida Y, Tanaka K. Histologic features after surgery associating liver partition and portal vein ligation for staged hepatectomy versus those after hepatectomy with portal vein embolization. Surgery. 2016;159:1289-1298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 76] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 79. | Garlipp B, de Baere T, Damm R, Irmscher R, van Buskirk M, Stübs P, Deschamps F, Meyer F, Seidensticker R, Mohnike K. Left-liver hypertrophy after therapeutic right-liver radioembolization is substantial but less than after portal vein embolization. Hepatology. 2014;59:1864-1873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 80. | Vouche M, Lewandowski RJ, Atassi R, Memon K, Gates VL, Ryu RK, Gaba RC, Mulcahy MF, Baker T, Sato K. Radiation lobectomy: time-dependent analysis of future liver remnant volume in unresectable liver cancer as a bridge to resection. J Hepatol. 2013;59:1029-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 219] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 81. | Fernández-Ros N, Silva N, Bilbao JI, Iñarrairaegui M, Benito A, D’Avola D, Rodriguez M, Rotellar F, Pardo F, Sangro B. Partial liver volume radioembolization induces hypertrophy in the spared hemiliver and no major signs of portal hypertension. HPB (Oxford). 2014;16:243-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 69] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 82. | Mazzaferro V, Sposito C, Bhoori S, Romito R, Chiesa C, Morosi C, Maccauro M, Marchianò A, Bongini M, Lanocita R. Yttrium-90 radioembolization for intermediate-advanced hepatocellular carcinoma: a phase 2 study. Hepatology. 2013;57:1826-1837. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 340] [Cited by in RCA: 398] [Article Influence: 33.2] [Reference Citation Analysis (0)] |
| 83. | Salem R, Lewandowski RJ, Kulik L, Wang E, Riaz A, Ryu RK, Sato KT, Gupta R, Nikolaidis P, Miller FH. Radioembolization results in longer time-to-progression and reduced toxicity compared with chemoembolization in patients with hepatocellular carcinoma. Gastroenterology. 2011;140:497-507.e2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 498] [Cited by in RCA: 504] [Article Influence: 36.0] [Reference Citation Analysis (0)] |
| 84. | Hilgard P, Hamami M, Fouly AE, Scherag A, Müller S, Ertle J, Heusner T, Cicinnati VR, Paul A, Bockisch A. Radioembolization with yttrium-90 glass microspheres in hepatocellular carcinoma: European experience on safety and long-term survival. Hepatology. 2010;52:1741-1749. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 353] [Article Influence: 23.5] [Reference Citation Analysis (0)] |
| 85. | Salem R, Lewandowski RJ, Mulcahy MF, Riaz A, Ryu RK, Ibrahim S, Atassi B, Baker T, Gates V, Miller FH. Radioembolization for hepatocellular carcinoma using Yttrium-90 microspheres: a comprehensive report of long-term outcomes. Gastroenterology. 2010;138:52-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 738] [Cited by in RCA: 781] [Article Influence: 52.1] [Reference Citation Analysis (0)] |
| 86. | Kooby DA, Egnatashvili V, Srinivasan S, Chamsuddin A, Delman KA, Kauh J, Staley CA 3rd, Kim HS. Comparison of yttrium-90 radioembolization and transcatheter arterial chemoembolization for the treatment of unresectable hepatocellular carcinoma. J Vasc Interv Radiol. 2010;21:224-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 149] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 87. | Carr BI, Kondragunta V, Buch SC, Branch RA. Therapeutic equivalence in survival for hepatic arterial chemoembolization and yttrium 90 microsphere treatments in unresectable hepatocellular carcinoma: a two-cohort study. Cancer. 2010;116:1305-1314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 148] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 88. | Kulik LM, Carr BI, Mulcahy MF, Lewandowski RJ, Atassi B, Ryu RK, Sato KT, Benson A 3rd, Nemcek AA Jr, Gates VL, Abecassis M, Omary RA, Salem R. Safety and efficacy of 90Y radiotherapy for hepatocellular carcinoma with and without portal vein thrombosis. Hepatology. 2008;47:71-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 447] [Cited by in RCA: 457] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 89. | Sangro B, Bilbao JI, Boan J, Martinez-Cuesta A, Benito A, Rodriguez J, Panizo A, Gil B, Inarrairaegui M, Herrero I. Radioembolization using 90Y-resin microspheres for patients with advanced hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2006;66:792-800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 157] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 90. | Salem R, Lewandowski RJ, Atassi B, Gordon SC, Gates VL, Barakat O, Sergie Z, Wong CY, Thurston KG. Treatment of unresectable hepatocellular carcinoma with use of 90Y microspheres (TheraSphere): safety, tumor response, and survival. J Vasc Interv Radiol. 2005;16:1627-1639. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 285] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 91. | Carr BI. Hepatic arterial 90Yttrium glass microspheres (Therasphere) for unresectable hepatocellular carcinoma: interim safety and survival data on 65 patients. Liver Transpl. 2004;10:S107-S110. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 188] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 92. | Iñarrairaegui M, Pardo F, Bilbao JI, Rotellar F, Benito A, D’Avola D, Herrero JI, Rodriguez M, Martí P, Zozaya G. Response to radioembolization with yttrium-90 resin microspheres may allow surgical treatment with curative intent and prolonged survival in previously unresectable hepatocellular carcinoma. Eur J Surg Oncol. 2012;38:594-601. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 106] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 93. | Ettorre GM, Laurenzi A, Vennarecci G. Downstaging Hepatocellular Carcinoma with Yttrium-90 radioembolization: resection or transplantation? Eur J Surg Oncol. 2014;40:789-790. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 94. | Tohme S, Sukato D, Chen HW, Amesur N, Zajko AB, Humar A, Geller DA, Marsh JW, Tsung A. Yttrium-90 radioembolization as a bridge to liver transplantation: a single-institution experience. J Vasc Interv Radiol. 2013;24:1632-1638. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 95. | Lewandowski RJ, Donahue L, Chokechanachaisakul A, Kulik L, Mouli S, Caicedo J, Abecassis M, Fryer J, Salem R, Baker T. (90) Y radiation lobectomy: Outcomes following surgical resection in patients with hepatic tumors and small future liver remnant volumes. J Surg Oncol. 2016;114:99-105. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 86] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 96. | Gaba RC, Lewandowski RJ, Kulik LM, Riaz A, Ibrahim SM, Mulcahy MF, Ryu RK, Sato KT, Gates V, Abecassis MM. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587-1596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 183] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 97. | Vyas S, Markar S, Partelli S, Fotheringham T, Low D, Imber C, Malago M, Kocher HM. Portal vein embolization and ligation for extended hepatectomy. Indian J Surg Oncol. 2014;5:30-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 29] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 98. | Aoki T, Kubota K. Preoperative portal vein embolization for hepatocellular carcinoma: Consensus and controversy. World J Hepatol. 2016;8:439-445. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 99. | Nagino M, Nimura Y, Kamiya J, Kanai M, Hayakawa N, Yamamoto H. Immediate increase in arterial blood flow in embolized hepatic segments after portal vein embolization: CT demonstration. AJR Am J Roentgenol. 1998;171:1037-1039. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 49] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Guo WC, He XF, Li YH, Li ZH, Mei QL, Chen Y. The effect of sequential transcatheter arterial chemoembolization (TACE) and portal venous embolizations (PVE) vs TACE or PVE alone on rabbit VX2 liver carcinoma and on liver regeneration. Eur Rev Med Pharmacol Sci. 2016;20:3186-3193. [PubMed] |
| 101. | Aoki T, Imamura H, Hasegawa K, Matsukura A, Sano K, Sugawara Y, Kokudo N, Makuuchi M. Sequential preoperative arterial and portal venous embolizations in patients with hepatocellular carcinoma. Arch Surg. 2004;139:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 137] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 102. | Ogata S, Belghiti J, Farges O, Varma D, Sibert A, Vilgrain V. Sequential arterial and portal vein embolizations before right hepatectomy in patients with cirrhosis and hepatocellular carcinoma. Br J Surg. 2006;93:1091-1098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 186] [Cited by in RCA: 184] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 103. | Peng PD, Hyder O, Bloomston M, Marques H, Corona-Villalobos C, Dixon E, Pulitano C, Hirose K, Schulick RD, Barroso E. Sequential intra-arterial therapy and portal vein embolization is feasible and safe in patients with advanced hepatic malignancies. HPB (Oxford). 2012;14:523-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 104. | Ronot M, Cauchy F, Gregoli B, Breguet R, Allaham W, Paradis V, Soubrane O, Vilgrain V. Sequential transarterial chemoembolization and portal vein embolization before resection is a valid oncological strategy for unilobar hepatocellular carcinoma regardless of the tumor burden. HPB (Oxford). 2016;18:684-690. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 105. | Xu C, Lv PH, Huang XE, Wang SX, Sun L, Wang FA, Wang LF. Safety and efficacy of sequential transcatheter arterial chemoembolization and portal vein embolization prior to major hepatectomy for patients with HCC. Asian Pac J Cancer Prev. 2014;15:703-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 106. | Enne M, Schadde E, Björnsson B, Hernandez Alejandro R, Steinbruck K, Viana E, Robles Campos R, Malago M, Clavien PA, De Santibanes E. ALPPS as a salvage procedure after insufficient future liver remnant hypertrophy following portal vein occlusion. HPB (Oxford). 2017;19:1126-1129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 107. | Olthof PB, Huiskens J, Wicherts DA, Huespe PE, Ardiles V, Robles-Campos R, Adam R, Linecker M, Clavien PA, Koopman M. Survival after associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) for advanced colorectal liver metastases: A case-matched comparison with palliative systemic therapy. Surgery. 2017;161:909-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 108. | Chan AC, Poon RT, Chan C, Lo CM. Safety of ALPPS Procedure by the Anterior Approach for Hepatocellular Carcinoma. Ann Surg. 2016;263:e14-e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 109. | Vivarelli M, Vincenzi P, Montalti R, Fava G, Tavio M, Coletta M, Vecchi A, Nicolini D, Agostini A, Ahmed EA. ALPPS Procedure for Extended Liver Resections: A Single Centre Experience and a Systematic Review. PLoS One. 2015;10:e0144019. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 36] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 110. | Truant S, Scatton O, Dokmak S, Regimbeau JM, Lucidi V, Laurent A, Gauzolino R, Castro Benitez C, Pequignot A, Donckier V, Lim C, Blanleuil ML, Brustia R, Le Treut YP, Soubrane O, Azoulay D, Farges O, Adam R, Pruvot FR; e-HPBchir Study Group from the Association de Chirurgie Hépato-Biliaire et de Transplantation (ACHBT). Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): impact of the inter-stages course on morbi-mortality and implications for management. Eur J Surg Oncol. 2015;41:674-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 105] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 111. | Tanaka K, Matsuo K, Murakami T, Kawaguchi D, Hiroshima Y, Koda K, Endo I, Ichikawa Y, Taguri M, Tanabe M. Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): short-term outcome, functional changes in the future liver remnant, and tumor growth activity. Eur J Surg Oncol. 2015;41:506-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 112. | Li J, Girotti P, Königsrainer I, Ladurner R, Königsrainer A, Nadalin S. ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg. 2013;17:956-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 102] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 113. | Yoo H, Kim JH, Ko GY, Kim KW, Gwon DI, Lee SG, Hwang S. Sequential transcatheter arterial chemoembolization and portal vein embolization versus portal vein embolization only before major hepatectomy for patients with hepatocellular carcinoma. Ann Surg Oncol. 2011;18:1251-1257. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |