Published online Dec 26, 2018. doi: 10.12998/wjcc.v6.i16.1155
Peer-review started: October 7, 2018
First decision: November 8, 2018
Revised: November 10, 2018
Accepted: November 23, 2018
Article in press: November 24, 2018
Published online: December 26, 2018
Processing time: 78 Days and 7.9 Hours
Aspergillosis is a frequent invasive fungal infection in liver recipients (affecting 1%-9.2% of all patients), second only to candidiasis. Significant risk factors for invasive aspergillosis in liver recipients include corticosteroid therapy, neutropenia, T-cell dysfunction, renal failure and requirement for renal replacement therapy. Aspergillus infection usually affects the lungs of liver recipients, with hematogenous dissemination occurring in 50%-60% of cases. Renal involvement is rare and is considered to occur in 0.4% of all cases of invasive aspergillosis.
This paper describes a case of a liver recipient presenting with a newly formed renal mass a year after liver transplantation. The patient underwent liver transplantation due to alcoholic liver cirrhosis, with preoperative corticosteroid therapy and postoperative immunosuppressants (tacrolimus and mycophenolate mofetil). His 1-year follow-up was uneventful, with a satisfying graft function and lack of any symptoms. During a routine follow-up abdominal ultrasound, he was diagnosed with a renal tumor. The renal imaging findings were inconclusive (with a differential diagnosis to renal cell carcinoma), while the computed tomography (CT) of the chest showed scar tissue in the lungs suggestive of previous inflammation. The patient underwent radical nephrectomy, with histopathological analysis showing renal aspergilloma, yielding postoperative treatment with voriconazole. His follow up was uneventful, and the chest CT did not show any change in pulmonary lesions. This case illustrates the possibility of aspergillosis affecting the lungs of liver recipients, subsequently affecting the kidney and forming an aspergilloma.
Clinicians should be aware of aspergilloma mimicking solid organ tumors in organ recipients.
Core tip: Renal aspergilloma should be suspected in cases of newly formed renal mass in immunosuppressed patients (e.g., after organ transplantation). Imaging findings in renal aspergilloma are frequently inconclusive, with a possible differential diagnosis to renal cell carcinoma or other tumors. Reduction of immunosuppression and antifungal therapy is required in the treatment of invasive aspergillosis. Surgical treatment should be considered in cases of renal aspergillosis.
- Citation: Smolovic B, Vukcevic B, Muhovic D, Ratkovic M. Renal aspergillosis in a liver transplant patient: A case report and review of literature. World J Clin Cases 2018; 6(16): 1155-1159
- URL: https://www.wjgnet.com/2307-8960/full/v6/i16/1155.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i16.1155
The suppressed immune system of transplant recipients represents fertile ground for invasive aspergillosis (IA), most commonly caused by Aspergillus fumigatus, a saprophytic species seldom pathogenic in healthy hosts[1]. IA contributes to 9.3%-16.9% of all deaths in the first year after transplantation[2]. Aspergillosis affects 1%-9.2% of liver recipients[3], being the second most common invasive fungal infection (after candidiasis). The usual entry site of Aspergillus is the respiratory tract[4], and hematogenous dissemination from the lungs occurs in 50%-60% of cases. Significant risk factors for IA include corticosteroid therapy (suppressing polymorphonuclear and macrophage function), neutropenia, T-cell dysfunction[2], renal failure, and the use of renal replacement therapy (RRT)[3]. Other risk factors described in the literature include: prolonged surgery, excessive blood loss, retransplantation, steroid-resistant rejection, and the use of broad-spectrum antibiotics[5]. Despite the fact that the disease can affect almost all tissues and organs, renal involvement is rare[4,6], and it is estimated to occur in 0.4% of all IA cases[7]. Imaging findings in renal aspergillosis are often inconclusive[1], and the definitive diagnosis requires histopathological and microbiological analysis of biopsy material or surgical specimens[6]. A case of IA forming renal aspergilloma after liver transplantation (LT) is presented here, with the intent to inspire the clinicians to suspect this infectious complication in liver recipients.
A 58-year-old asymptomatic male LT recipient was diagnosed with a newly formed renal mass during a routine follow-up ultrasound examination of the abdomen.
The patient suffered from alcoholic liver cirrhosis and entered terminal liver insufficiency with multiorgan failure. Prior to LT, he presented with esophageal varices (grade II), portal hypertensive gastroduodenopathy and ascites, as well as positive IgG for cytomegalovirus (CMV). Other viral antibodies tests were negative. We performed an extensive pretransplant preparation (owing to his Model For End-Stage Liver Disease score of 32), including corticosteroid treatment (as indicated in the treatment of alcoholic liver disease[8]), after which orthotopic LT was performed in December of 2013. Two weeks after LT, CMV infection reactivated, and he was treated with valganciclovir for two weeks, after which CMV polymerase chain reaction (PCR) test was negative. Immunosuppressants (tacrolimus and mycophenolate mofetil) were started after the remission of CMV infection. His 1-year follow-up was uneventful, with a satisfying graft function and lack of any symptoms. However, he exhibited leukopenia due to hypersplenism.
The patient’s past illnesses (besides liver cirrhosis treated with LT) only included the presence of an atrial septal defect.
Besides alcohol abuse prior to the onset of liver cirrhosis (followed by permanent abstinence from alcohol to this day), other personal and family history was unremarkable.
The patient was re-admitted in January 2015 for a routine follow-up. On admission, the patient’s physical examination was normal, without any significant pathological findings.
Bronchoalveolar lavage was sterile. Urinalysis showed microhematuria. Other laboratory and microbiological examinations were unremarkable, with blood and urine cultivations negative for both bacteria and fungi.
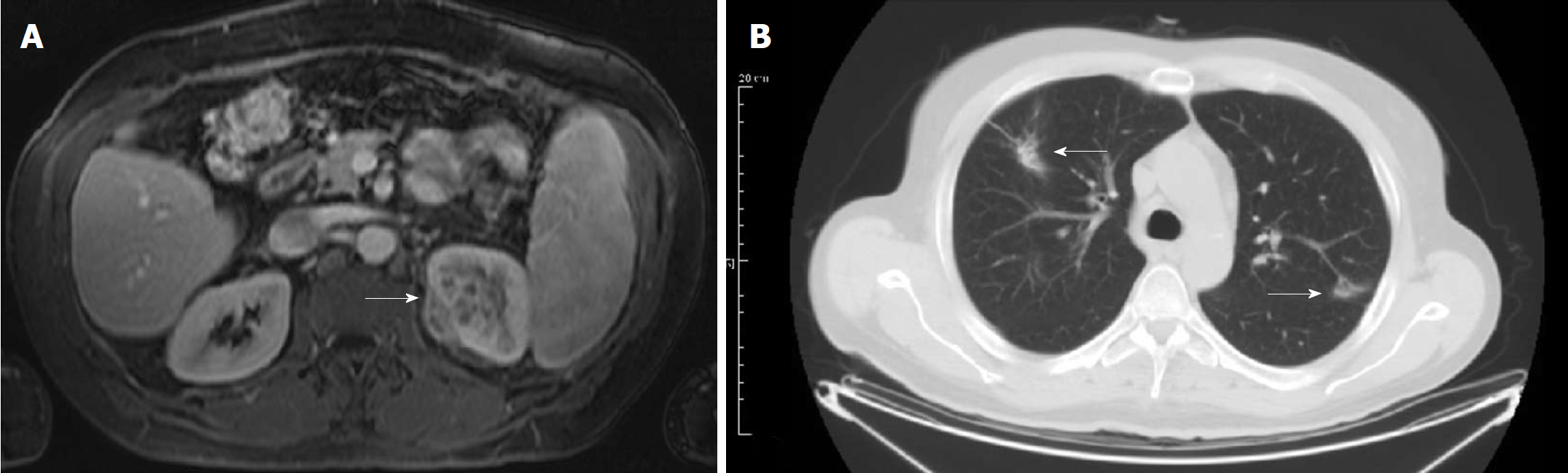
Ultrasound revealed mass was described in the superior pole of the left kidney, confirmed by computed tomography (CT). Magnetic resonance imaging (MRI) of the lesion was inconclusive, with a description of an expansive lesion infiltrating the perirenal fat–suggestive of a renal cell carcinoma. After the diagnosis of a renal tumor, a chest CT was performed, showing bilateral lesions in lung apices suggestive of scar tissue from previous inflammation (Figure 1). The hospital in which the patient was treated was not equipped with a positron-emission tomography (PET) scanner, and the patient refused to be transferred to another institution–so, unfortunately, a PET scan was not performed.
The patient refused a percutaneous renal biopsy, so a radical nephrectomy was performed a month after. Preoperative tests included blood urea nitrogen, serum creatinine, cystatin C and electrolyte levels, as well as creatinine clearance and urinalysis (testing for albuminuria/proteinuria). Histopathological and microbiological analysis of the removed kidney showed a mycotic pseudotumor caused by A. fumigatus.
After radical nephrectomy, postoperative treatment included voriconazole for 12 wk. 4 mo after surgery and finished antifungal therapy, the patient’s urine and bronchoalveolar lavage were both sterile.
To this day, the patient continues to exhibit normal graft function, without any radiographic findings suggestive of aspergillosis relapse or a change in the chest CT findings.
Renal aspergillosis may develop as single/multiple parenchymal abscesses due to hematogenous dissemination, or as a fungal ball in the renal pelvis. Clinical presentation may include hematuria, abdominal pain and fever[9]. The most common consequences of renal aspergillosis are pelvic obstruction and perinephric abscess[10]. Renal aspergillosis occurs due to hematogenous spreading from the lung, ascending from urinary tract infections, or secondary to obstructive uropathy[11].
In high-risk patients, IA usually occurs in the first month posttransplant[3,12]. However, a recent trend of late IA is noticed - probably due to cytomegalovirus (CMV) infection or recurrent hepatitis C virus. LT candidates are at high risk of acquiring Aspergillus infection even during pre-transplant period, whereas kidney recipients are at an increasingly high risk for fungal infections (such as aspergillosis or cryptococcosis) after kidney transplantation, due to a longer and more intense immunosuppression (particularly the use of corticosteroids)[13].
In a systematic review of 116 liver recipients suffering from IA, Barchiesi et al[4] described kidney involvement in 13 cases. It is not known whether renal aspergillosis was the only active lesion of IA in these cases. Meng et al[6] reported two cases of isolated renal aspergillosis after LT (with tacrolimus and methylprednisolone as immunosuppressants), diagnosed 9 and 15 mo after surgery. None of the patients had active pulmonary lesions on chest radiography. The first patient had unilateral renal lesions which were suspected to be malignant (according to CT), treated with radical nephrectomy. The other patient presented with severe urethral stenosis (after fulguration for urethral meatus condyloma) causing mild bilateral hydronephrosis, as well as bilateral renal lesions, diagnosed as abscesses (on MRI). The diagnosis of IA was made by cultures of abscess aspiration. The patient underwent nephrectomy of the left kidney (which was more affected by IA). Both patients were treated with azoles (fluconazole and voriconazole) postoperatively, and they showed complete remission of IA. The authors concluded that the first patient showed hematogenous dissemination of Aspergillus, while the second patient’s obstructive uropathy contributed to renal aspergillosis. To the best of our knowledge, the patient presented herein is the third described case of renal aspergilloma after liver transplantation without any other confirmed active lesions. It is interesting to consider the pulmonary scar tissue as the remnants of previous lung lesions that led to the hematogenous spreading of Aspergillus.
The initial delay in establishing the diagnosis of aspergillosis often affects the success rate of the treatment[4]. Radiographic studies must be performed within 5 d from the onset of infection because the specific but poorly sensitive “halo” sign usually disappears after a week. On the other hand, the “air crescent“ sign appears after three weeks[3]. Ultrasound, CT and MRI findings of renal aspergilloma are often non-specific, showing a heterogenous mass with secondary changes (abscess formation or necrosis)-difficult to distinguish from renal cell carcinoma or pyelonephritic abscess[1]. Laboratory studies include cultures from the respiratory tract secretions, assays for detecting galactomannan, as well as the detection of fungal DNA by PCR assay[10]. Isolation of Aspergillus from the respiratory tract is rare, but it has a high positive predictive value of IA development (41%-72%)[3].
Mortality from IA in liver recipients ranges from 83%-88%, with RRT and CMV infection as independent predictors of mortality[3]. However, more recent studies have reported better outcomes[2,4]. Reduction of immunosuppression is crucial in the treatment of IA[6]. There are reports on success from adjunctive immunotherapy. Systemic treatment includes amphotericin, azoles, and echinocandins; as well as combination regimens[2,10]. Voriconazole is regarded as the primary treatment of IA in solid organ recipients, as endorsed by the Clinical Practice Guidelines of the Infectious Disease Society of America; with amphotericin B deoxycholate and its lipid derivatives as second-line treatment[10,14]. There are opinions that amphotericin should be the drug of choice even when the microbiological confirmation of Aspergillus is lacking, owing to the drug’s coverage of other fungal infections which might mimic aspergillosis (such as mucormycosis)[2]. The effect of combined antifungal treatment is not yet investigated, and it should be used as salvage therapy[3].
Due to the fact that renal aspergillosis after solid organ transplantation is a rare event, not many recommendations regarding therapeutic strategies (both medicamentous and surgical) are available. Meng et al[6] suggested that the high mortality rate of IA in solid organ recipients dictates the surgical resection (a radical or partial nephrectomy in the case of renal aspergillosis) to be the main therapeutic strategy, followed by adjuvant antifungal therapy. Treatment of renal aspergillosis depends on the form of the disease. If the abscesses are small, medicamentous treatment should be considered, while larger abscesses or complications should be treated surgically, with nephrectomy as the last option[10]. Irrigation with amphotericin via a nephrostomy tube is useful in pelvic disease but has no role in the treatment of parenchymal abscesses[15].
Regarding the treatment of renal allograft aspergilloma, Linden et al[16] advocate surgical drainage and antifungal therapy, with the intent to avoid transplant nephrectomy, similar to the opinion of Johnston et al[17], who described a kidney recipient treated with nephrostomy for a fungus ball in the allograft pelvis. Unlike these reported outcomes, a paper by Kamal et al[18] contains a description of a HIV-positive kidney recipient who suffered allograft aspergillosis (with diffuse necrosis of the renal parenchyma) treated with nephrectomy. In their review of 10 patients with an additional original case reported, Rey et al[19] stated that early nephrectomy is the treatment of choice in immunocompromised AIDS patients suffering from renal Aspergillus abscess, especially if medical treatment fails to eliminate the infection. Halpern et al[9] also suggested early surgical removal of the aspergilloma in immunocompromised patients. In their opinion, the benefit of this approach lies in the histopathological evaluation of the tissue, which is useful in the choice of antimicrobial therapy. Carlesse et al[20] described a 6-year-old immunocompromised patient with Burkitt’s lymphoma, presenting with an asymptomatic renal aspergilloma unsuccessfully treated with amphotericin B and voriconazole followed by a nodulectomy (there was a relapse of aspergilloma after the surgery). The infection was afterward successfully controlled with a nephrectomy. The authors concluded that the suppressed immune system and low tissue penetration of the drugs in the renal lesion caused the failure of the medical treatment. Therefore, they suggested that a radical procedure such as nephrectomy is required to control the infection in immunocompromised patients. However, Oosten et al[11] reported a case of an AIDS patient suffering from bilateral renal aspergillosis, successfully treated with percutaneous drainage and voriconazole. The authors stated that this therapeutic strategy was employed in order to avoid life-long dialysis (due to the bilateral distribution of the infection). There is clearly a need for future research on therapeutic strategies and the effect of immunosuppression on the outcome of renal aspergillosis treatment.
Regarding the patient presented herein, several factors (the negative microbiological tests, the imaging findings suggestive of renal cell carcinoma, and the failure to perform a kidney biopsy) resulted in the patient undergoing a nephrectomy with a clinical suspicion of a malignant renal tumor. The fact that a radical procedure was performed to treat a non-malignant disease should spark some interest in researching the potential usefulness of conservative therapy (e.g., percutaneous drainage) in the diagnosis and treatment of encapsulated renal aspergillomas.
In conclusion, this case illustrates how IA in organ recipients may present with difficulties in differential diagnosis. The possibility of IA forming aspergillomas in different organs and tissues should be kept in mind when approaching an immunocompromised patient with an imaging finding of a tumor-like lesion.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Montenegro
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Giorgakis E, Hanna R, Hibberd AD, Kohla MAS S- Editor: Cui LJ L- Editor: A E- Editor: Wu YXJ
| 1. | Bulakçı M, Kartal MG, Çelenk E, Tunçer S, Kılıçaslan I. Multimodality Imaging Findings of a Renal Aspergilloma. Balkan Med J. 2016;33:701-705. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Singh N, Paterson DL. Aspergillus infections in transplant recipients. Clin Microbiol Rev. 2005;18:44-69. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 458] [Cited by in RCA: 429] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 3. | Singh N, Husain S; AST Infectious Diseases Community of Practice. Aspergillosis in solid organ transplantation. Am J Transplant. 2013;13 Suppl 4:228-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 4. | Barchiesi F, Mazzocato S, Mazzanti S, Gesuita R, Skrami E, Fiorentini A, Singh N. Invasive aspergillosis in liver transplant recipients: epidemiology, clinical characteristics, treatment, and outcomes in 116 cases. Liver Transpl. 2015;21:204-212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 60] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Kusne S, Torre-Cisneros J, Mañez R, Irish W, Martin M, Fung J, Simmons RL, Starzl TE. Factors associated with invasive lung aspergillosis and the significance of positive Aspergillus culture after liver transplantation. J Infect Dis. 1992;166:1379-1383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 131] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Meng XC, Jiang T, Yi SH, Xie PY, Guo YF, Quan L, Zhou J, Zhu KS, Shan H. Renal aspergillosis after liver transplantation: clinical and imaging manifestations in two cases. World J Gastroenterol. 2014;20:18495-18502. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 13] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 7. | Paterson DL, Singh N. Invasive aspergillosis in transplant recipients. Medicine (Baltimore). 1999;78:123-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 298] [Cited by in RCA: 268] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 8. | Stickel F, Datz C, Hampe J, Bataller R. Pathophysiology and Management of Alcoholic Liver Disease: Update 2016. Gut Liver. 2017;11:173-188. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 170] [Article Influence: 21.3] [Reference Citation Analysis (0)] |
| 9. | Halpern M, Szabo S, Hochberg E, Hammer GS, Lin J, Gurtman AC, Sacks HS, Shapiro RS, Hirschman SZ. Renal aspergilloma: an unusual cause of infection in a patient with the acquired immunodeficiency syndrome. Am J Med. 1992;92:437-440. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 10. | Patterson TF, Thompson GR 3rd, Denning DW, Fishman JA, Hadley S, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Nguyen MH, Segal BH, Steinbach WJ, Stevens DA, Walsh TJ, Wingard JR, Young JA, Bennett JE. Practice Guidelines for the Diagnosis and Management of Aspergillosis: 2016 Update by the Infectious Diseases Society of America. Clin Infect Dis. 2016;63:e1-e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1848] [Article Influence: 205.3] [Reference Citation Analysis (0)] |
| 11. | Oosten AW, Sprenger HG, van Leeuwen JT, Meessen NE, van Assen S. Bilateral renal aspergillosis in a patient with AIDS: a case report and review of reported cases. AIDS Patient Care STDS. 2008;22:1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 16] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Shi SH, Lu AW, Shen Y, Jia CK, Wang WL, Xie HY, Zhang M, Liang TB, Zheng SS. Spectrum and risk factors for invasive candidiasis and non-Candida fungal infections after liver transplantation. Chin Med J (Engl). 2008;121:625-630. [PubMed] |
| 13. | Zicker M, Colombo AL, Ferraz-Neto BH, Camargo LF. Epidemiology of fungal infections in liver transplant recipients: a six-year study of a large Brazilian liver transplantation centre. Mem Inst Oswaldo Cruz. 2011;106:339-345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 14. | Walsh TJ, Anaissie EJ, Denning DW, Herbrecht R, Kontoyiannis DP, Marr KA, Morrison VA, Segal BH, Steinbach WJ, Stevens DA. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1903] [Cited by in RCA: 1877] [Article Influence: 110.4] [Reference Citation Analysis (0)] |
| 15. | Poll LW, Koch J, Medve M, May P, Sarbia M, Engelbrecht V, Mödder U. CT appearance of a renal aspergilloma in a patient with the acquired immunodeficiency syndrome. Urol Int. 1999;62:110-113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Linden E, Restrepo D, Dikman S, Murphy B, Huprikar S. Aspergillus infection limited to renal allograft: case report and review of literature. Transpl Infect Dis. 2006;8:177-181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Johnston O, Little DM, Hickey D, Conlon PJ. Aspergillus ‘fungus ball’ within a cadaveric renal transplant graft. Nephrol Dial Transplant. 2004;19:1317-1318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 18. | Kamal M, Apewokin S, Anand M, Abu Jawdeh BG, Govil A, Sheikh MM, Shah S. Late-Onset Allograft Aspergillosis in an HIV-Positive Renal Transplant Recipient: A Case Report. Transplant Proc. 2017;49:1570-1573. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 19. | Rey D, de Mautort E, Saussine C, Hansmann Y, Waller J, Herbrecht R, Christmann D, Lindner V, Letscher-Bru V, Koenig H. Isolated renal Aspergillus abscess in an AIDS patient with a normal CD4+ cell count on highly active antiretroviral therapy. Eur J Clin Microbiol Infect Dis. 1999;18:137-141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 15] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Carlesse F, Marcos AC, Seber A, Petrilli AS, Luisi FA, Ricci G, Lederman HM, Alves MT, Gonçalves SS, Abib SC. Renal aspergillosis in a 6-year-old male with Burkitt’s lymphoma. Pediatr Infect Dis J. 2015;34:679-680. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |