Published online Dec 6, 2018. doi: 10.12998/wjcc.v6.i15.952
Peer-review started: August 24, 2018
First decision: October 11, 2018
Revised: October 28, 2018
Accepted: November 7, 2018
Article in press: November 7, 2018
Published online: December 6, 2018
Processing time: 105 Days and 0.4 Hours
To examine whether second generation of colon capsule endoscopy (CCE-2) is acceptable for assessing the severity of mucosal inflammation and evaluating mucosal healing using CCE-2 is able to predict outcome in ulcerative colitis (UC) patients, especially in clinical remission.
A total of 30 consecutive UC patients in clinical remission were enrolled to undergo CCE-2. Clinical remission was defined as clinical activity index (CAI) ≤ 4 according to Rachmilewitz index. The rate of total colon observation and colon cleansing level were evaluated. Severity of mucosal inflammation in UC was assessed according to the Mayo endoscopic subscore (MES) and Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Relapse-free survival was assessed. Acceptability of CCE-2 was assessed using a questionnaire survey.
The rate of total colon observation within its battery life was 93.3%. The proportion of “excellent” plus “good” cleansing level was 73.3%. The rate of mucosal healing (MES 0, 1) assessed by CCE-2 was 77.0%. The relapse-free survival rate was significantly higher in MES 0, 1 than in MES 2, 3 (P = 0.0435), and in UCEIS 0-3 than in UCEIS 4-8 (P = 0.0211), whereas there was no significant difference between CAI 0 and CAI 1-4 groups. A questionnaire survey revealed an overall acceptability of CCE.
CCE-2 is acceptable for assessing the severity of mucosal inflammation in UC patients, especially in clinical remission. Evaluating mucosal healing using CCE-2 was able to predict outcome.
Core tip: Although mucosal healing is a newly established therapeutic goal in ulcerative colitis (UC), it remains unclear whether evaluating endoscopic activity using colon capsule endoscopy (CCE-2) is able to predict outcome. The present study was a prospective study to evaluate the usefulness of CCE-2 in patients with UC, especially in clinical remission. We revealed that our reduced-volume preparation regimen for CCE-2 could attain a high rate of total colon observation and high acceptability, and that assessment of endoscopic activity by CCE-2 using Mayo endoscopic subscore and Ulcerative Colitis Endoscopic Index of Severity can predict outcome.
- Citation: Takano R, Osawa S, Uotani T, Tani S, Ishida N, Tamura S, Yamade M, Iwaizumi M, Hamaya Y, Furuta T, Miyajima H, Sugimoto K. Evaluating mucosal healing using colon capsule endoscopy predicts outcome in patients with ulcerative colitis in clinical remission. World J Clin Cases 2018; 6(15): 952-960
- URL: https://www.wjgnet.com/2307-8960/full/v6/i15/952.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i15.952
Ulcerative colitis (UC) is a chronic idiopathic inflammatory bowel disease with a relapsing and remitting course, and is associated with impaired quality of life[1]. Conventional colonoscopy (CS) plays a major role in the diagnosis and assessment of disease severity and extent as well as surveillance for dysplasia in patients with UC[2-4]. In recent years, besides symptom control, mucosal healing has been established as a new therapeutic goal. It predicts clinical remission and the requirement for hospitalization and surgery[5-8]. However, conventional CS has several limitations, including adverse events, low patient compliance and has manpower restrictions[9,10]. Therefore, an alternative approach that can overcome these limitations is required.
In 2009, the second generation of colon capsule endoscopy (CCE-2) was released, providing a larger number of images per second and a broader viewing angle[11]. CCE-2 has several benefits for patients with UC in assessing mucosal inflammation as the procedure is relatively non-invasive without direct trauma to the mucosa or air insufflation[12,13]. Therefore, it has a high level of patient acceptance without anaesthesia. To date, the accuracy of CCE-2 for assessment of mucosal inflammation in UC appears to be comparable with that of CS[14-16]. However, there have been a limited number of studies. It remains unclear which UC patients may benefit from the use of CCE-2.
The Mayo endoscopic subscore (MES) is widely used in clinical trials to describe the degree of endoscopic activity in patients with UC. In clinical trials as well as in practise, a MES of 0 or 1 is a commonly accepted criterion for mucosal healing and predicts a better outcome[17-19]. More recently, another score index, Ulcerative Colitis Endoscopic Index of Severity (UCEIS), was validated to measure endoscopic severity in UC[20,21]. UCEIS is more sensitive in detecting mucosal inflammation and is superior to other scoring systems in detecting treatment response and predicting disease outcomes[22,23]. However, it is not yet confirmed whether assessment of mucosal inflammation by CCE-2 using MES or UCEIS is able to predict outcome in clinical practise.
Conventional bowel preparations may be excessive for patients with severe or fulminant UC, leading to increased diarrhea and bleeding. Therefore, the preparation should be tailored to the patient in such cases. In the present study, we developed a novel reduced-volume regimen for CCE-2 examination in patients with UC, especially those in clinical remission, and assessed the feasibility of evaluating the severity of mucosal inflammation. Furthermore, we examined whether evaluation of endoscopic activity by CCE-2 using MES and UCEIS was able to predict outcome.
This was a single-center, prospective study conducted in UC patients with clinical remission, carried out in accordance with the Declaration of Helsinki. Approval for the study was obtained from the ethics committee of Hamamatsu University School of Medicine, Japan. Written informed consent for participation in the study was obtained from all patients. This study was registered with the University Hospital Medical Information Network (UMIN), UMIN000030539.
Enrolment of patients aged 16 to 80 years began in October 2015 and was completed in December 2017. Eligible patients had a histologically confirmed diagnosis of UC with clinical remission (Rachmilewitz index ≤ 4)[24]. Patients with the following criteria were excluded: dysphagia; pregnant or possibly pregnant women; a pacemaker or other implanted electromedical device; presence or history of small and large bowel obstruction; a contraindication to bowel preparation (congestive heart failure, renal insufficiency, life-threatening condition); allergic to polyethylene glycol (PEG), magnesium citrate, sennoside, metoclopramide or mosapride citrate; those undergoing magnetic resonance imaging 2 wk after CCE-2; and inappropriate for this study by other reasons judged by the investigators.
The present study used a CCE-2 known as PillCam COLON 2 (Medtronic Japan Co., Ltd., Tokyo, Japan). A modified regimen of bowel preparation was developed to improve patient’s acceptability by reducing the volume and shortening the time of examination using low-volume PEG (MoviPrep, EA Pharma, Tokyo, Japan). Details of the CCE-2 procedure are presented in Table 1. On the day before the capsule procedure, patients ate a low-fiber diet and drank 50 g of magnesium citrate mixed with 180 mL of water and received 48 mg oral sennosides after dinner. On the procedure day, patients swallowed a colon capsule with 20 mg mosapride citrate at 9:00 am. If the capsule had moved out from the stomach to the duodenum, 1 L of low-volume PEG plus 0.5 L of water was administered as a first booster. After 1 h, 1 L of low-volume PEG plus 0.5 L of water was administered again as a second booster. Three hours later, if the capsule was not excreted outside the body, 50 g of magnesium citrate mixed with 180 mL of water was administrated as a third booster. Optional use of bisacodyl suppository was allowed only if the capsule was not excreted outside the body after a third booster. Recording was continued until the battery ran down or the capsule was excreted.
| Day | Procedure | |
| Previous day | Diet | Low-fibre diet |
| After dinner | Magnesium citrate 50 g/180 mL + Sennoside 48 mg | |
| Examination | 09:00 | Mosapride citrate 20 mg |
| Swallowing of CCE-2 capsule | ||
| Booster (1) | 1 L of low-volume PEG (MoviPrep) + 0.5 L water | |
| Booster (2) | 1 L of low-volume PEG (MoviPrep) + 0.5 L water | |
| Booster (3) | Magnesium Citrate 50 g/180 mL |
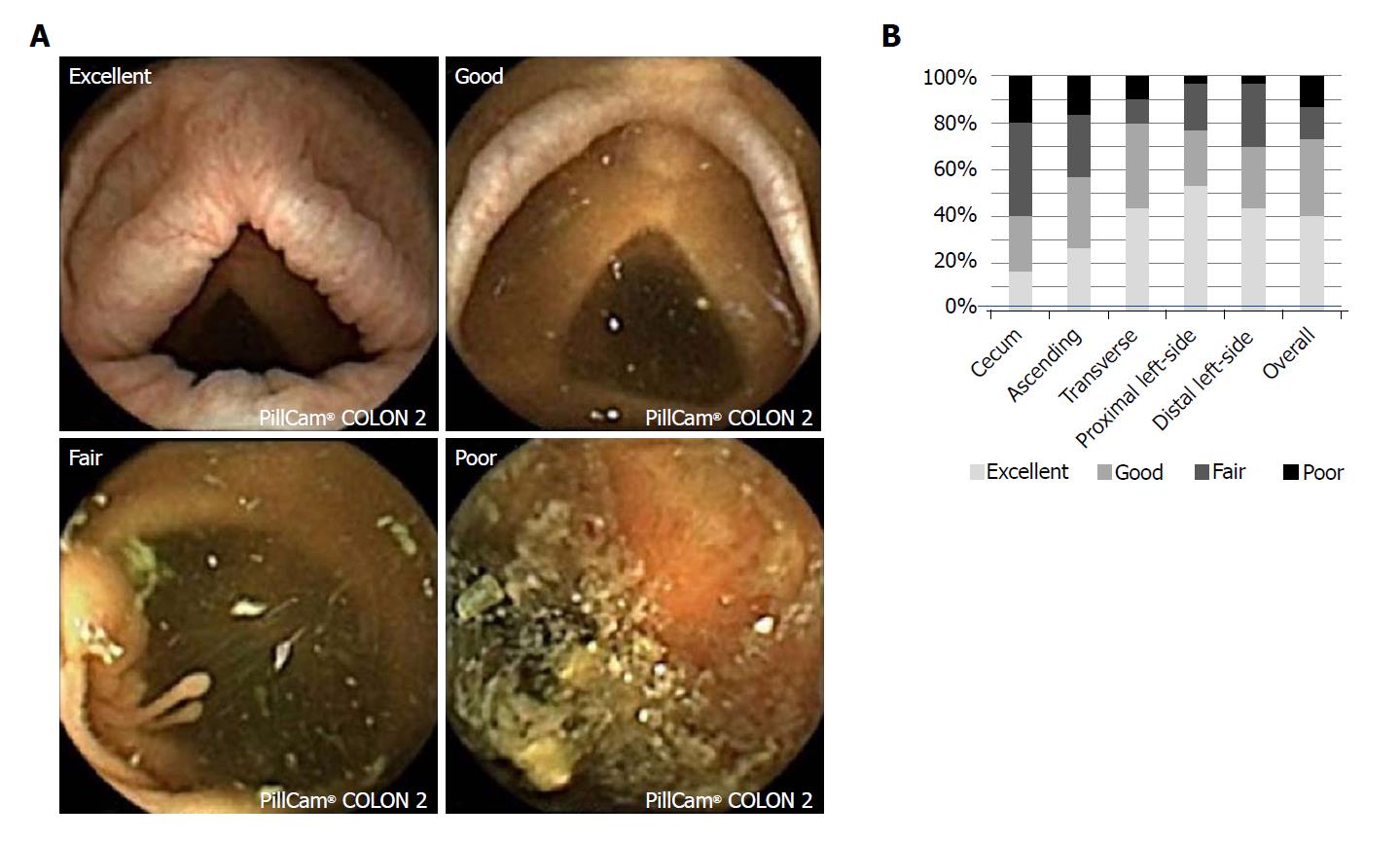
The rate of CCE-2 excretion was calculated, and the transit time for each part of the gastrointestinal tract was recorded. The level of colonic cleansing was scored according to a four-point grading scale, as previously reported[25]. The hepatic flexure and splenic flexure which had been automatically determined by the software were reconfirmed and used as markers to separate the segment in the colon. Each segment was scored as cecum, ascending colon, transverse colon, proximal left-sided colon and distal left-sided colon. Representative images are shown in Figure 1A. Adverse effects were also recorded. CCE-2 images were reviewed independently by two experts of capsule endoscopy (Osawa S and Takano R). One (Osawa S) had eight years of clinical experience in capsule endoscopy and the other (Takano R) had four years of clinical experience, and both had read more than 200 capsule endoscopy videos. The final reports involving endoscopic activity score and cleansing effectiveness were prospectively made based on a consensus between the two experts.
Patients were evaluated using the clinical activity index (CAI) according to Rachmilewitz[24]. Clinical remission was defined as CAI ≤ 4. Relapse was defined as an increase in the CAI score, CAI > 4, after achieving clinical remission. Exacerbation was defined as any additional treatment for clinical symptoms.
The endoscopic activity of UC was evaluated by MES and UCEIS. MES is a four-point scale (0-3). The UCEIS is a nine-point scale (0-8) of three descriptors, calculated as a simple sum: vascular pattern (0-2), bleeding (0-3) and erosions and ulcers (0-3)[20]. The highest score among segments was determined as the overall score.
A questionnaire survey was conducted to evaluate the acceptability of the CCE-2 procedure, asking patients about following five items: physical pain, mental distress, bowel preparation, next examination and overall acceptability. Each question comprised five-grade evaluations.
Statistical analysis was carried out using SPSS (SPSS 17.0; SPSS Inc., Chicago, Illinois, United States). Results were expressed as mean ± SD with minimum and maximum values, and categorical data were expressed as percentage. Pearson’s Chi-square test was used to compare distribution of the activity score assessed by CCE. Kaplan-Meier plots with log-rank test were used to compare between two groups in relapse-free and exacerbation-free survival.
A total of 30 patients were enrolled in the study. Patients’ demographics are shown in Table 2. The mean age was 48.6 ± 13.3 years; 18 subjects were male and 12 were female. The mean disease duration was 13.9 ± 9.5 years, and the clinical UC activity of the enrolled patients assessed by Rachmilewitz index was 0.73 ± 1.14 (63.3% in CAI = 0 and 36.7% in CAI = 1-4). Regarding types of disease, 19 patients (63.3%) had total colitis, 10 (33.3%) had left-sided colitis and one (3.3%) had proctitis. Most of the patients were treated with 5-aminosalicylate drugs. The median observational period was 20.5 mo (range 5-27 mo).
| Numbers of patients | 30 |
| Gender (male/female) | 18/12 |
| Age [mean ± SD (range), yr] | 48.6 ± 13.3 (24-67) |
| Disease duration [mean ± SD (range), yr] | 13.9 ± 9.5 (1-32) |
| Inpatient/outpatient | 0/30 |
| History of abdominal surgery | 2 (6.7) |
| Type of disease | |
| Total colitis | 19 (63.3) |
| Left-sided | 10 (33.3) |
| Proctitis | 1 (3.3) |
| Disease activity (Rachmilewitz index) | |
| CAI = 0 | 19 (63.3) |
| CAI = 1 | 4 (13.3) |
| CAI = 2 | 4 (13.3) |
| CAI = 3 | 2 (6.7) |
| CAI = 4 | 1 (3.3) |
| Serum albumin (mean ± SD, g/dL) | 4.2 ± 0.5 |
| Serum CRP (mean ± SD, mg/dL) | 0.11 ± 0.15 |
| Medications | |
| 5-ASA | 24 (80.0) |
| Steroid | 4 (13.3) |
| Thiopurines | 12 (40.0) |
| Anti-TNF-Ab | 1 (3.3) |
| No medication | 1 (3.3) |
| Observation period after CCE; median (range) | 20.5 (5-27) |
CCE-2 performance is shown in Table 3. The rate of total colon observation within its battery life in UC patients were 93.3% and 27 patients (90.0%) excreted the CCE-2 within 8 h. The mean total transit time was 263.8 ± 228.2 min (range 54-952 min). The mean colonic and small intestinal transit times were 163.9 ± 211.0 min (range 9-775 min) and 72.7 ± 34.3 min (range 23-155 min), respectively. The total liquid volume on the examination day was 2329 ± 854 mL (range 500-3180 mL). No severe adverse events were observed in this study.
| Total colon observation1, % | 93.3% (28/30) |
| Excretion within 8 h, % | 90.0% (27/30) |
| Capsule retention rate, % | 0% (0/30) |
| Mean transit time ± SD (range), min | |
| Stomach | 27.2 ± 15.4 (4-63) |
| Small intestine | 72.7 ± 34.3 (23-155) |
| Colon2 | 163.9 ± 211.0 (9-775) |
| Cecum and ascending colon | 50.4 ± 84.2 (1-386) |
| Transverse colon | 11.7 ± 17.6 (5-80) |
| Left-side colon2 | 101.9 ± 186.3 (5-751) |
| Total time2 | 263.8 ± 228.2 (54-952) |
| Total liquid volume of the examination day | 2329 ± 854 (500-3180) |
The effectiveness of cleansing using our bowel preparation regimen is shown in Figure 1B. The percentages of “excellent” plus “good” were 40% in the cecum, 57% in the ascending colon, 80% in the transverse colon, 77% in the proximal left-sided colon and 70% in the distal left-sided colon. As a whole, the proportion of “excellent” plus “good” cleansing level was 73.3%.
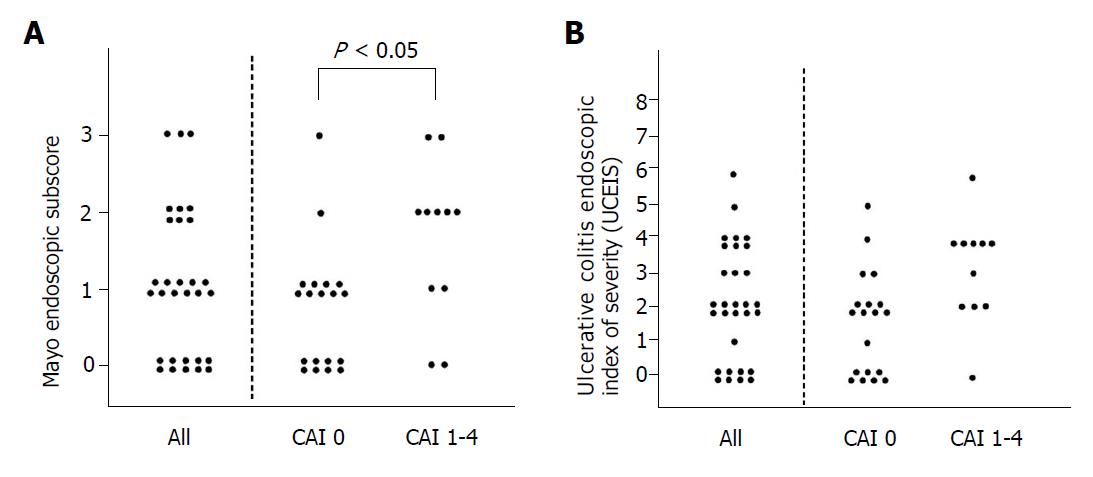
We examined the distribution of endoscopic activity score assessed by CCE-2 in clinical remission. As shown in Figure 2A, the rate of mucosal healing (MES 0, 1) assessed by CCE-2 was 77.0%. When we evaluated the distribution of endoscopic activity score in between CAI 0 and CAI 1-4 groups, statistical difference was observed in the distribution of MES, whereas distribution of UCEIS by CCE-2 was not statistically different in between CAI 0 and CAI 1-4 groups (Figure 2B).
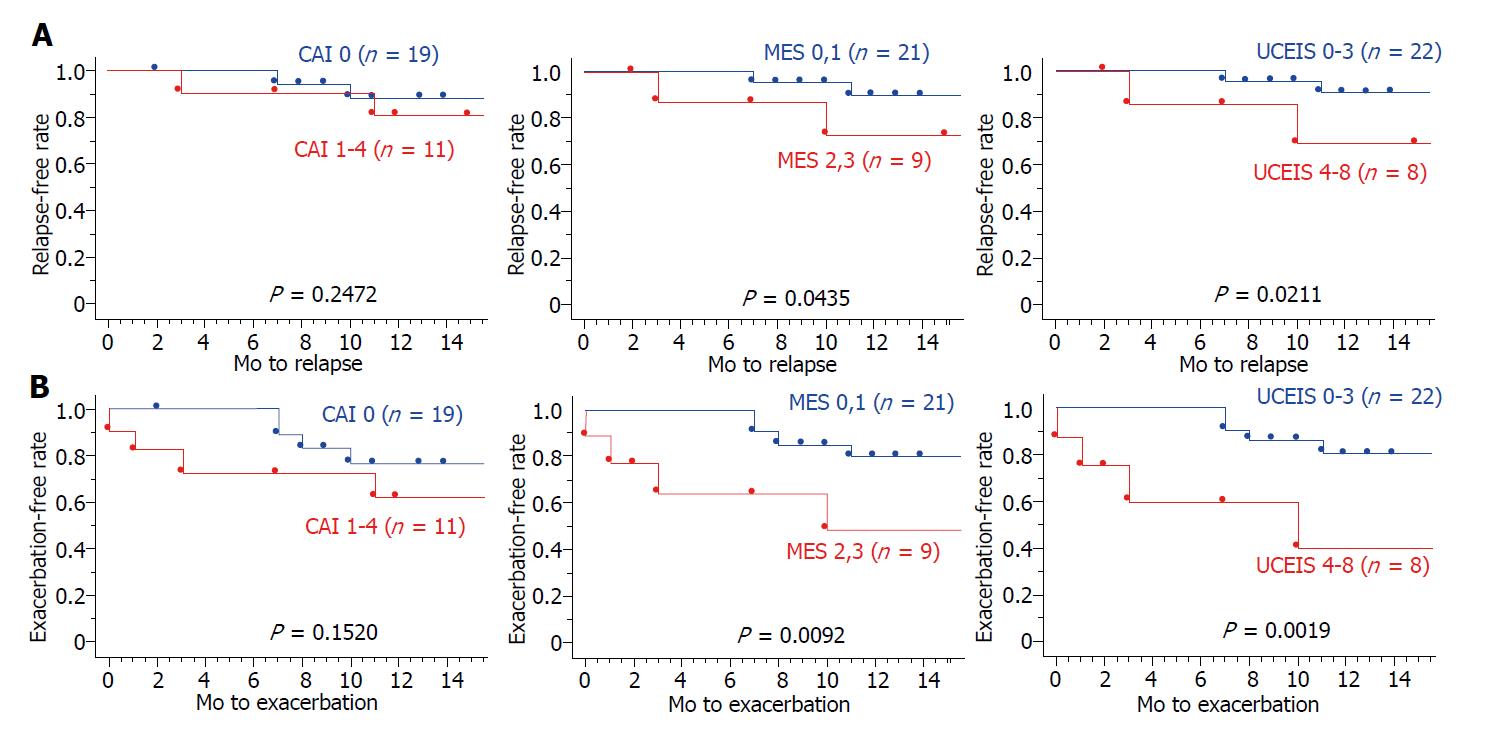
Based on the Kaplan–Meier survival estimator graphs (Figure 3), the overall cumulative relapse-free and exacerbation-free survival rates at 12 mo were 85.2% and 71.2%, respectively. The relapse-free survival rate was significantly higher in MES 0, 1 than in MES 2, 3 (P < 0.05; log-rank test), and in UCEIS 0-3 than in UCEIS 4-8 (P < 0.05; log-rank test). Furthermore, the exacerbation-free survival rate was significantly higher in MES 0, 1 than in MES 2, 3 (P < 0.01; log-rank test), and in UCEIS 0-3 than in UCEIS 4-8 (P < 0.01; log-rank test). However, in both survival rates, there was no significant difference between CAI 0 and CAI 1-4 groups. These results indicated that indices for predicting UC relapse risk are endoscopic scores rather than clinical scores.
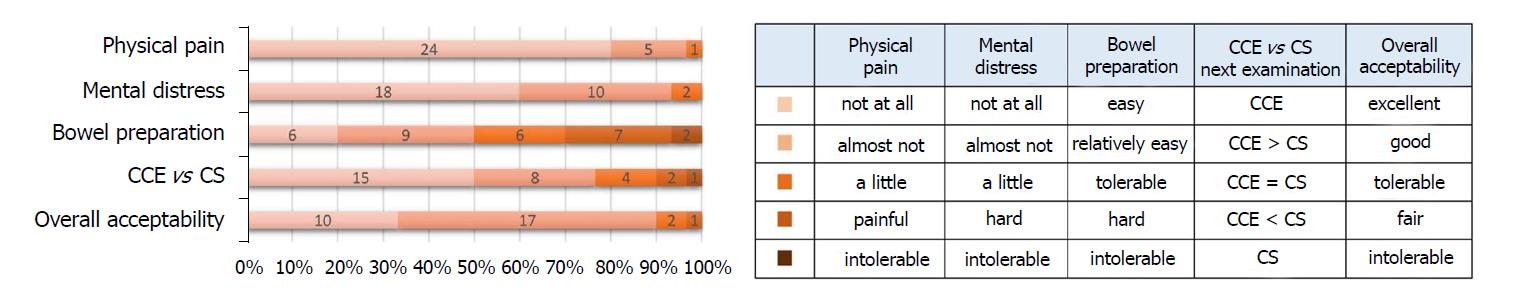
To evaluate the acceptability of the CCE-2 procedure, we conducted a questionnaire survey about the following five items: Physical pain, mental distress, bowel preparation, next examination and overall acceptability. The results are shown in Figure 4. For overall acceptability, the proportion of “excellent” plus “good” was 90%. For physical pain and mental distress, most patients felt almost nothing or nothing at all. In the pre-treatment, patients’ opinion varied regarding the tolerance of bowel preparation. The questionnaire survey showed that 77% of patients would choose CCE-2 rather than CS for future scheduled endoscopies.
The present study was a prospective study to evaluate the usefulness of CCE-2 in patients with UC, especially in clinical remission, and revealed the following novel findings: (1) our reduced-volume preparation regimen for CCE-2 could attain a high rate of total colon observation, and high acceptability; and (2) assessment of endoscopic activity by CCE-2 using MES and UCEIS can predict outcome. These results suggested that CCE-2 could be an alternative to endoscopic examination for follow-up of UC, especially in clinical remission.
The current European Society of Gastrointestinal Endoscopy recommendation for CCE preparation is use of 4 L of PEG solution administered as a split-dose (2 L the day before the examination and 2 L before capsule ingestion) combined with oral use of prokinetics, low-volume sodium phosphate (NaP) boosters[26]. However, most Japanese patients are not able to tolerate it in clinical practise because of the high volume. Although reduced-volume regimens have been reported for UC patients previously[14,27], there was still room for improvement in terms of cleansing level and rate of total colon observation, Usui et al[27] reported that the proportion of “excellent” plus “good” cleansing was approximately 60%. They discussed that a fair level of colonic cleansing was adequate for the evaluation of UC mucosal severity, whereas it is not sufficient for surveying colon polyps. In this study, we developed a novel reduced-volume regimen of bowel preparation for CCE-2 examination in patients with UC, especially in clinical remission expecting receptive improvement without bowel preparation before swallowing a capsule endoscopy on the examination day. As a result, a shortened transit time through the stomach and colon, and high rates of total colon observation with adequate cleansing could be obtained. More recently, Okabayashi et al[28] reported a simple 1-d CCE-2 procedure using castor oil added to the booster without dietary restrictions, which successfully achieved a high excretion rate of 93.9% (31/33) and high acceptance. It is attractive regimen enabled the volume of bowel preparation to be reduced to 1.45 ± 0.07 L whereas the cleansing level was lower than our procedure.
In this study, the rate of mucosal healing assessed by CCE-2 seemed to be equivalent to that of CS. First-generation CCE (CCE-1) displayed a sensitivity and specificity of 89% and 75%, respectively, for the diagnosis of active UC. Although the procedure was safe, the usefulness of CCE-1 for evaluation of UC activity was controversial among studies because of its low specificity[14,29-32]. CCE-2 equipped with an accelerated frame rate and larger angle of view has improved the accuracy for detecting intraluminal abnormality. Oliva et al[15] investigated the performance of CCE-2 in 29 paediatric UC patients, and reported that the sensitivity, specificity, positive predictive value and negative predictive value for inflammation detection were 95%, 100%, 100% and 85%, respectively. A recent prospective study in 150 patients revealed that CCE-2 had a sensitivity of 97% and 94% to detect mucosal inflammation (MES ≥ 1) and moderate to severe inflammation (MES ≥ 2), respectively. To detect moderate-to-severe mucosal inflammation, the negative predictive value was improved substantially from 65% with the first-generation capsule to 96% with CCE-2[16]. These studies using CCE-2 support our findings of high detectability using CCE-2.
Until now, there has not been an established scoring system of CCE used worldwide for evaluating endoscopic activity of UC[12]. Recently, the largest-scale study consisting of 150 patients using CCE-2 showed substantial agreement between CCE-2 and CS for either MES [intraclass correlation coefficient (ICC) 0.69; 95% confidence interval (CI), 0.46–0.81] or UCEIS (ICC 0.64; 95%CI: 0.38-0.78) with almost perfect (ICC > 0.80) intra- and inter-observer agreement[16]. However, there have been no studies evaluating whether score of capsule endoscopic activity contributes to the prediction of the clinical course in patients with UC. In our study, assessing mucosal healing by CCE-2 using MES, which is most frequently used in clinical trials and practice, was able to predict outcome in the same way as CS. That is, so-called mucosal healing of MES 0-1 was significantly associated with low relapse-free survival rate and exacerbation rate. Furthermore, we also revealed that UCEIS, which has been validated to be more sensitive in detecting mucosal inflammation, was able to predict outcome in the same way as CS. In this score the threshold for mucosal healing has yet to be determined. Remission is defined as UCEIS 0-1 in some studies[22,33,34]. According to our analysis, MES 0-1 by CCE-2 was equivalent to UCEIS 0-3.
There were several limitations to this study. First, since this study was designed as a preliminary study, a small number of patients were enrolled. Second, this study was conducted in a single center setting that might have involved some bias for selecting patients and the details of the CCE-2 procedure. Third, as all of the enrolled patients were Japanese, it is not confirmed whether bowel preparation regimen of this study is suitable for patients with UC worldwide. Fourth, there was no direct comparison between CCE-2 and CS findings in our study, by which the value of this study would be further increased. Finally, although endoscopic surveillance for colitis-associated cancer is another important issue in the management of UC, the end points of this study did not involve this as it requires tissue sampling for histology.
Nevertheless, despite the limitations and disadvantages of tissue sampling for histology, our study strongly suggests, even in small sample size, that CCE-2 with our regimen of bowel preparation showed high acceptability in UC patients and endoscopic activity by CCE-2 using MES and UCEIS was significantly associated with outcome in clinical remission. This painless, much less invasive tool may be routinely used instead of CS in the near future to monitor inflammation in UC patients, especially those in clinical remission.
Mucosal healing is a newly established therapeutic goal in ulcerative colitis (UC). The accuracy of the second generation of colon capsule endoscopy (CCE-2) for assessment of mucosal inflammation in UC appears to be comparable with that of colonoscopy (CS). It remains unclear which UC patients may benefit from the use of CCE-2, and whether evaluating endoscopic activity using CCE-2 is able to predict outcome. Further, a standard preparation regimen validated for UC patients in clinical remission has not been established.
Conventional CS has several limitations, such as adverse events and low patient compliance. To clarify the usefulness of less-invasive CCE-2 would provide a new option in clinical practice in UC patients.
To assess the feasibility of CCE-2 with a novel reduced-volume regimen in patients with UC in clinical remission, and to examine whether evaluation of endoscopic activity by CCE-2 is able to predict outcome.
The study was conducted as single-center, prospective setting. A total of 30 consecutive patients were enrolled. CCE-2 performance was evaluated, and acceptability was assessed using a questionnaire survey. Endoscopic activity was assessed according to both Mayo endoscopic subscore (MES) and Ulcerative Colitis Endoscopic Index of Severity (UCEIS).
The rate of total colon observation was 93.3% and the proportion of “excellent” plus “good” cleansing level was 73.3% with the reduced-volume regimen. The relapse-free survival rate was significantly correlated with MES and UCEIS, whereas it was not correlated with clinical activity index. A questionnaire survey revealed an overall acceptability of CCE-2.
CCE-2 was acceptable for UC patients in clinical remission. Evaluating mucosal healing using CCE-2 was able to predict outcome.
Despite the small sample size, this study certainly suggested the usefulness of CCE-2 in UC patients in clinical remission. CCE-2 could serve as an alternative modality to CS for follow up of UC. Further extensive study with a larger sample size is expected to be conducted to spread this novel modality widely.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Japan
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Christodoulou DK, Guglielmi FW, Triantafyllou K S- Editor: Dou Y L- Editor: A E- Editor: Wu YXJ
| 1. | Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Ulcerative colitis. Lancet. 2012;380:1606-1619. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1151] [Cited by in RCA: 1543] [Article Influence: 118.7] [Reference Citation Analysis (5)] |
| 2. | Dignass A, Lindsay JO, Sturm A, Windsor A, Colombel JF, Allez M, D’Haens G, D’Hoore A, Mantzaris G, Novacek G. Second European evidence-based consensus on the diagnosis and management of ulcerative colitis part 2: current management. J Crohns Colitis. 2012;6:991-1030. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 728] [Cited by in RCA: 702] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 3. | Annese V, Daperno M, Rutter MD, Amiot A, Bossuyt P, East J, Ferrante M, Götz M, Katsanos KH, Kießlich R. European evidence based consensus for endoscopy in inflammatory bowel disease. J Crohns Colitis. 2013;7:982-1018. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 640] [Cited by in RCA: 584] [Article Influence: 48.7] [Reference Citation Analysis (1)] |
| 4. | Hata K, Kishikawa J, Anzai H, Shinagawa T, Kazama S, Ishii H, Nozawa H, Kawai K, Kiyomatsu T, Tanaka J. Surveillance colonoscopy for colitis-associated dysplasia and cancer in ulcerative colitis patients. Dig Endosc. 2016;28:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Neurath MF, Travis SP. Mucosal healing in inflammatory bowel diseases: a systematic review. Gut. 2012;61:1619-1635. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 676] [Cited by in RCA: 662] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 6. | Shah SC, Colombel JF, Sands BE, Narula N. Mucosal Healing Is Associated With Improved Long-term Outcomes of Patients With Ulcerative Colitis: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2016;14:1245-1255.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 259] [Article Influence: 28.8] [Reference Citation Analysis (0)] |
| 7. | Theede K, Kiszka-Kanowitz M, Nordgaard-Lassen I, Mertz Nielsen A. The Impact of Endoscopic Inflammation and Mucosal Healing on Health-related Quality of Life in Ulcerative Colitis Patients. J Crohns Colitis. 2015;9:625-632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 31] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 8. | Mazzuoli S, Guglielmi FW, Antonelli E, Salemme M, Bassotti G, Villanacci V. Definition and evaluation of mucosal healing in clinical practice. Dig Liver Dis. 2013;45:969-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 109] [Article Influence: 9.1] [Reference Citation Analysis (1)] |
| 9. | Vienne A, Simon T, Cosnes J, Baudry C, Bouhnik Y, Soulé JC, Chaussade S, Marteau P, Jian R, Delchier JC. Low prevalence of colonoscopic surveillance of inflammatory bowel disease patients with longstanding extensive colitis: a clinical practice survey nested in the CESAME cohort. Aliment Pharmacol Ther. 2011;34:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 10. | Verschuren EC, Ong DE, Kamm MA, Desmond PV, Lust M. Inflammatory bowel disease cancer surveillance in a tertiary referral hospital: attitudes and practice. Intern Med J. 2014;44:40-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Spada C, Hassan C, Costamagna G. Colon capsule endoscopy. Gastrointest Endosc Clin N Am. 2015;25:387-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Shi HY, Ng SC, Tsoi KK, Wu JC, Sung JJ, Chan FK. The role of capsule endoscopy in assessing mucosal inflammation in ulcerative colitis. Expert Rev Gastroenterol Hepatol. 2015;9:47-54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 13. | Singeap AM, Stanciu C, Cojocariu C, Sfarti C, Trifan A. Capsule Endoscopy in Inflammatory Bowel Disease: Current Applications. Arch Iran Med. 2015;18:379-383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Hosoe N, Matsuoka K, Naganuma M, Ida Y, Ishibashi Y, Kimura K, Yoneno K, Usui S, Kashiwagi K, Hisamatsu T. Applicability of second-generation colon capsule endoscope to ulcerative colitis: a clinical feasibility study. J Gastroenterol Hepatol. 2013;28:1174-1179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 15. | Oliva S, Di Nardo G, Hassan C, Spada C, Aloi M, Ferrari F, Redler A, Costamagna G, Cucchiara S. Second-generation colon capsule endoscopy vs. colonoscopy in pediatric ulcerative colitis: a pilot study. Endoscopy. 2014;46:485-492. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 16. | Shi HY, Chan FKL, Higashimori A, Kyaw M, Ching JYL, Chan HCH, Chan JCH, Chan AWH, Lam KLY, Tang RSY. A prospective study on second-generation colon capsule endoscopy to detect mucosal lesions and disease activity in ulcerative colitis (with video). Gastrointest Endosc. 2017;86:1139-1146.e6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 33] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 17. | Samaan MA, Mosli MH, Sandborn WJ, Feagan BG, DʼHaens GR, Dubcenco E, Baker KA, Levesque BG. A systematic review of the measurement of endoscopic healing in ulcerative colitis clinical trials: recommendations and implications for future research. Inflamm Bowel Dis. 2014;20:1465-1471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 72] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Ikeya K, Sugimoto K, Kawasaki S, Iida T, Maruyama Y, Watanabe F, Hanai H. Tacrolimus for remission induction in ulcerative colitis: Mayo endoscopic subscore 0 and 1 predict long-term prognosis. Dig Liver Dis. 2015;47:365-371. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 19. | Papamichael K, Baert F, Tops S, Assche GV, Rutgeerts P, Vermeire S, Gils A, Ferrante M. Post-Induction Adalimumab Concentration is Associated with Short-Term Mucosal Healing in Patients with Ulcerative Colitis. J Crohns Colitis. 2017;11:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 20. | Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lémann M, Lichtenstein GR. Developing an instrument to assess the endoscopic severity of ulcerative colitis: the Ulcerative Colitis Endoscopic Index of Severity (UCEIS). Gut. 2012;61:535-542. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 353] [Cited by in RCA: 451] [Article Influence: 34.7] [Reference Citation Analysis (0)] |
| 21. | Travis SP, Schnell D, Krzeski P, Abreu MT, Altman DG, Colombel JF, Feagan BG, Hanauer SB, Lichtenstein GR, Marteau PR. Reliability and initial validation of the ulcerative colitis endoscopic index of severity. Gastroenterology. 2013;145:987-995. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 277] [Cited by in RCA: 341] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 22. | Ikeya K, Hanai H, Sugimoto K, Osawa S, Kawasaki S, Iida T, Maruyama Y, Watanabe F. The Ulcerative Colitis Endoscopic Index of Severity More Accurately Reflects Clinical Outcomes and Long-term Prognosis than the Mayo Endoscopic Score. J Crohns Colitis. 2016;10:286-295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 133] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 23. | Travis SP, Schnell D, Feagan BG, Abreu MT, Altman DG, Hanauer SB, Krzeski P, Lichtenstein GR, Marteau PR, Mary JY. The Impact of Clinical Information on the Assessment of Endoscopic Activity: Characteristics of the Ulcerative Colitis Endoscopic Index Of Severity [UCEIS]. J Crohns Colitis. 2015;9:607-616. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 47] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 24. | Rachmilewitz D. Coated mesalazine (5-aminosalicylic acid) versus sulphasalazine in the treatment of active ulcerative colitis: a randomised trial. BMJ. 1989;298:82-86. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 771] [Cited by in RCA: 806] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 25. | Leighton JA, Rex DK. A grading scale to evaluate colon cleansing for the PillCam COLON capsule: a reliability study. Endoscopy. 2011;43:123-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 26. | Spada C, Hassan C, Galmiche JP, Neuhaus H, Dumonceau JM, Adler S, Epstein O, Gay G, Pennazio M, Rex DK. Colon capsule endoscopy: European Society of Gastrointestinal Endoscopy (ESGE) Guideline. Endoscopy. 2012;44:527-536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 174] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 27. | Usui S, Hosoe N, Matsuoka K, Kobayashi T, Nakano M, Naganuma M, Ishibashi Y, Kimura K, Yoneno K, Kashiwagi K. Modified bowel preparation regimen for use in second-generation colon capsule endoscopy in patients with ulcerative colitis. Dig Endosc. 2014;26:665-672. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 20] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 28. | Okabayashi S, Kobayashi T, Nakano M, Toyonaga T, Ozaki R, Tablante MC, Kuronuma S, Takeuchi O, Hibi T. A Simple 1-Day Colon Capsule Endoscopy Procedure Demonstrated to be a Highly Acceptable Monitoring Tool for Ulcerative Colitis. Inflamm Bowel Dis. 2018;24:2404-2412. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 29. | Sung J, Ho KY, Chiu HM, Ching J, Travis S, Peled R. The use of Pillcam Colon in assessing mucosal inflammation in ulcerative colitis: a multicenter study. Endoscopy. 2012;44:754-758. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 73] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 30. | Manes G, Ardizzone S, Cassinotti A. PillCam Colon and ulcerative colitis: what do physicians need to know? Endoscopy. 2013;45:325. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 31. | Ye CA, Gao YJ, Ge ZZ, Dai J, Li XB, Xue HB, Ran ZH, Zhao YJ. PillCam colon capsule endoscopy versus conventional colonoscopy for the detection of severity and extent of ulcerative colitis. J Dig Dis. 2013;14:117-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 32. | San Juan-Acosta M, Caunedo-Álvarez A, Argüelles-Arias F, Castro-Laria L, Gómez-Rodríguez B, Romero-Vázquez J, Belda-Cuesta A, Pellicer-Bautista F, Herrerías-Gutiérrez JM. Colon capsule endoscopy is a safe and useful tool to assess disease parameters in patients with ulcerative colitis. Eur J Gastroenterol Hepatol. 2014;26:894-901. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 19] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 33. | Arai M, Naganuma M, Sugimoto S, Kiyohara H, Ono K, Mori K, Saigusa K, Nanki K, Mutaguchi M, Mizuno S. The Ulcerative Colitis Endoscopic Index of Severity is Useful to Predict Medium- to Long-Term Prognosis in Ulcerative Colitis Patients with Clinical Remission. J Crohns Colitis. 2016;10:1303-1309. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 34. | Saigusa K, Matsuoka K, Sugimoto S, Arai M, Kiyohara H, Takeshita K, Mizuno S, Mori K, Nanki K, Takeshita T. Ulcerative colitis endoscopic index of severity is associated with long-term prognosis in ulcerative colitis patients treated with infliximab. Dig Endosc. 2016;28:665-670. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 3.1] [Reference Citation Analysis (0)] |