Published online Nov 26, 2018. doi: 10.12998/wjcc.v6.i14.753
Peer-review started: July 13, 2018
First decision: July 24, 2018
Revised: September 4, 2018
Accepted: October 17, 2018
Article in press: October 16, 2018
Published online: November 26, 2018
Processing time: 136 Days and 21.3 Hours
To investigate the benefits of the Seattle protocol in the diagnosis of Chinese individuals with Barrett’s esophagus.
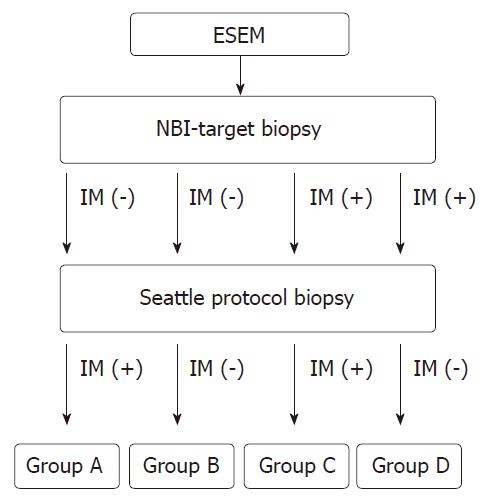
Subjects enrolled were patients from one center with endoscopically-suspected esophageal metaplasia. These patients first received narrow-band imaging-targeted biopsy, and later, the Seattle protocol-guided biopsy, within a period from October 2012 to December 2014. Those cases without initial pathologic patterns of intestinal metaplasia (IM) and then appearance or loss of IM tissue were designated as Group A or B, respectively. Those with initial pathologic patterns of IM, which then persisted or were lost were designated as Group C or D, respectively.
The number of cases for each group was as follows: A: 20, B: 78, C: 31 and D: 14. The distribution of the Prague criteria M levels of Group A was significantly higher than Group B (P = 0.174). Among these groups, Group C had the highest proportions of hiatus hernia (54.8%), long segment Barrett’s esophagus (29%), and also the highest Prague criteria M levels. The sensitivity of IM detection was 69.2% for the narrow-band imaging-targeted biopsy and 78.5% for the Seattle protocol-guided biopsy. The difference was not significant (P = 0.231). The number of detectable dysplasias increased from one case via the NBI-target biopsy to five cases via the Seattle protocol-guided biopsy, including one case of adenocarcinoma.
The Seattle protocol improved the IM detection in our subjects with higher Prague criteria M levels and disclosed more cases with dysplastic tissues.
Core tip: While comparing the diagnosis of Barrett’s esophagus in a Chinese population via narrow-band imaging-targeted biopsy or the Seattle protocol-guided biopsy, the sensitivity of intestinal metaplasia detection was 69.2% and 78.5%, respectively. The number of detectable dysplasias increased from one case via the narrow-band imaging-targeted biopsy to five cases via the Seattle protocol-guided biopsy. These results concluded that the Seattle protocol identified more cases with dysplastic tissues.
- Citation: Lee SW, Lien HC, Chang CS, Lin MX, Chang CH, Ko CW. Benefits of the Seattle biopsy protocol in the diagnosis of Barrett’s esophagus in a Chinese population. World J Clin Cases 2018; 6(14): 753-758
- URL: https://www.wjgnet.com/2307-8960/full/v6/i14/753.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i14.753
Endoscopically, the current definition of Barrett’s esophagus (BE) includes the presence of an area of salmon-colored mucosa in the distal esophagus, plus the histological finding of intestinal metaplasia (IM) in the esophagus[1]. BE is clinically important because it is a major risk factor for the development of esophageal adenocarcinoma (EAC), and the number of EAC cases has been growing in Western countries[2]. EAC is typically diagnosed at an advanced stage when it also has a poor prognosis, as its 5-year survival rate is low (17%)[3]. Therefore, early detection of BE, especially those with dysplastic tissue, attracted recent research interests. Regarding the choice of biopsy methods in detecting BE, the use of narrow band imaging (NBI) has been compared with traditional white light endoscopy (WLE), and results showed its superiority over WLE[1,3]. According to the American Gastroenterological Association guidelines, the standard for diagnosing BE is the endoscopic evaluation performed with 4-quadrant biopsy specimens taken every 1-2 cm, which is described as the Seattle biopsy protocol[1,4]. However, some in this field considered disadvantages of the Seattle protocol as being relatively inefficient, time-consuming and providing a low diagnostic rate[5-7]. In Europe, reportedly only half of endoscopists follow the Seattle protocol for biopsy of BE patients[8,9].
Our present study was aimed to evaluate the benefits of the Seattle protocol on BE cases in a Chinese population of Taiwan.
We collected data from subjects who had endoscopically-suspected esophageal metaplasia followed by NBI-targeted biopsy conducted at the Medical Screening Center at Taichung Veteran General Hospital. Over a period from October 2012 to December 2014, these patients were asked to repeat another open-access transoral upper gastrointestinal endoscopy together with the Seattle protocol-guided biopsy. NBI-target biopsy was defined as surveillance of the gastroesophageal junction (GEJ) with NBI, and a biopsy was taken according to the individual endoscopist’s expertise. Seattle protocol-guided biopsy was performed with 4-quadrant biopsy specimens taken every 1-2 cm at the GEJ. We recorded the general patient data, which included their age, gender, and body mass index (BMI). In addition, we collected their endoscopic findings, including hiatus hernia, erosive esophagitis (EE), short segment BE (SSBE, extending < 3 cm into the esophagus) and long segment BE (LSBE, extending ≥ 3 cm into the esophagus)[10], the Prague C and M criteria[11], and pathologic dysplasia appearances, including low-grade dysplasia (LGD), high grade dysplasia and EAC.
Exclusion criteria included total esophagectomy, severe cardiopulmonary deficiency, malignancy, other unsuitable conditions for upper gastrointestinal scope, and segments of metaplastic columnar epithelium < 1 cm, which were classified as “specialized IM of the esophagogastric junction”.
The flow-chart of the study is shown in Figure 1. Subjects without initial pathologic patterns of IM, but with IM tissue detected later by the Seattle protocol were classified into “Group A”. Subjects without initial pathologic patterns of IM and without IM tissue detected later by the Seattle protocol were classified into “Group B”. Subjects with initial pathologic patterns of IM and again confirmed by the Seattle protocol later were classified into “Group C”. Subjects with initial pathologic patterns of IM that were not detected later by the Seattle protocol were classified into “Group D”. After grouping, inter-group differences were analyzed statistically as described below.
Data of each measured parameter were first expressed as mean and standard deviation. Gender, hiatus hernia, endoscopic and pathologic findings of BE tissue of the stratified groups were expressed as percentages of patients in their respective groups. Comparisons were made using Pearson’s chi-square test to evaluate the contributions of gender and positive ratio of each stratified group. Independent t-tests were used to analyze age, BMI, and numbers of biopsy pieces. A P-value < 0.05 was considered statistically significant.
From the total of 143 enrolled subjects, the number of patients in each group was as follows: A: 20 (14%), B: 78 (54.5%), C: 31 (21.7%) and D: 14 (9.8%).
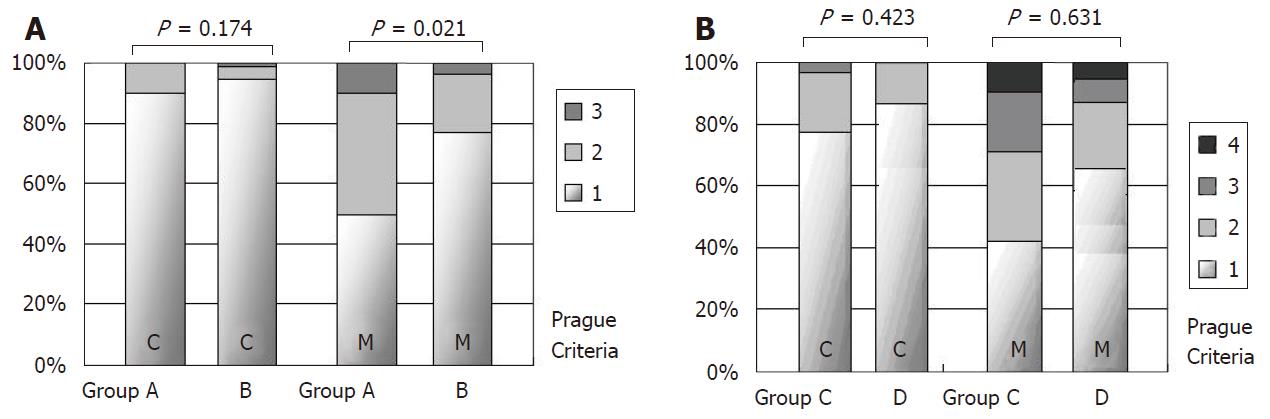
First, for Groups A and B, their general data and endoscopic appearances are shown in Table 1. The levels of age, gender and BMI were similar between the two groups. Compared to Group B, Group A had slightly higher proportions of EE (35% vs 19.2%, P = 0.132), hiatus hernia (40% vs 19.2%, P = 0.051), and LSBE (10% vs 3.8%, P = 0.269), but none of the differences were statistically significant. However, we found significantly more endoscopic biopsy pieces at the GEJ in Group A than in Group B (mean 5.69 vs 4.00, P = 0.039). The Prague C and M criteria of these subjects in Groups A and B are shown in Figure 2A. The distributions of “C” levels were similar between groups (P = 0.174), but the “M” levels were significantly higher in Group A (P = 0.021).
| Group A (n = 20) | Group B (n = 78) | P-value | |||
| mean ± SD | n (%) | mean ± SD | n (%) | ||
| Age (yr) | 60.95 ± 17.29 | 55.19 ± 13.28 | 0.1891 | ||
| Gender (male) | 9 (45.0) | 35 (44.9) | 0.9922 | ||
| BMI (kg/m2) | 25.76 ± 4.52 | 23.69 ± 3.59 | 0.0791 | ||
| EE | 7 (35.0) | 15 (19.2) | 0.1322 | ||
| Hiatus hernia | 8 (40.0) | 15 (19.2) | 0.0512 | ||
| LSBE | 2 (10.0) | 3 (3.8) | 0.2692 | ||
| Biopsy pieces | 4.58 ± 1.57 | 3.39 ± 1.74 | 0.0091 | ||
The general data, endoscopic appearances and the Prague C and M criteria for Groups C and D are shown in Table 2 and Figure 2B. Group C had, among all groups, the highest proportion of hiatus hernia (54.8%), LSBE (29%) and the highest Prague criteria “M” levels. Group D, compared to Group C, had a significantly higher proportion of EE (57.1% vs 19.4%, P = 0.011) and fewer biopsy pieces (mean 4 vs 5.69, P = 0.039).
| Group C (n = 31) | Group D (n = 14) | P-value | |||
| mean ± SD | n (%) | mean ± SD | n (%) | ||
| Age (yr) | 66.53 ± 12.47 | 65.79 ± 14.18 | 0.8601 | ||
| Gender (male) | 25 (80.6) | 12 (85.7) | 0.5182 | ||
| BMI (kg/m2) | 25.65 ± 3.70 | 23.14 ± 3.64 | 0.0511 | ||
| EE | 6 (19.4) | 8 (57.1) | 0.0112 | ||
| Hiatus hernia | 17 (54.8) | 6 (42.9) | 0.4572 | ||
| LSBE | 9 (29.0) | 2 (14.3) | 0.2872 | ||
| Biopsy pieces | 5.69 ± 2.82 | 4.00 ± 2.18 | 0.0391 | ||
The results of the two different biopsy protocols in terms of sensitivity of IM detection are listed in Table 3. Among all subjects with pathologic appearance of IM at the GEJ (n = 65 in Groups A, C and D), 45 of them (in Groups C and D) yielded positive IM results via NBI-target biopsy at a sensitivity of 69.2%. In comparison, 51 subjects (in Groups A and C) yielded positive IM results via the Seattle protocol-guided biopsy, with a higher sensitivity of 78.5%. However, the difference of IM detection rates between these two biopsy protocols was not significant (P = 0.231).
As shown in Table 3, the number of detectable dysplasias in the GEJ of all the enrolled subjects was only one case at the beginning with NBI-target biopsy, which later increased to five cases using the Seattle protocol. Among the five subjects, four were LGD and one was EAC arising from the BE tissue.
BE, a condition in which the squamous epithelium of the distal esophagus is replaced by columnar epithelium with IM, is a well-established precursor of EAC. Regarding the risk of EAC, BE has a > 40-fold increase over the general population. Although BE has been a disease primarily found in the Western world, it has become more frequently reported in Asian countries[12]. The underlying etiology of BE is still unclear. In the majority of cases, BE is associated with a combined reflux of acid and bile, even in the absence of symptoms. The prevalence of BE has been estimated at 1%–2% in patients undergoing endoscopy for any indication, and the prevalence rises to 5%–15% in patients with gastroesophageal reflux disease (commonly known as GERD) symptoms[13]. Risk factors for BE include older ages, male gender, Caucasian race, GERD symptoms, central abdominal obesity, and possibly tobacco smoking[14].
Due to the extremely poor outcome of EAC, the early and accurate diagnosis of BE, especially with the presentation of dysplastic tissue, is particularly important. Currently, the use of electronic chromoendoscopy, like NBI, is a standard protocol for detecting BE. According to previous reports, NBI, compared to WLE, could better detect dysplasia in BE, and higher grades of dysplasia with fewer biopsy samples[3,15]. It is the recommendation of the American Gastroenterological Association guidelines that 4-quadrant biopsy specimens be obtained at intervals of 1-cm in BE patients with known or suspected dysplasia, and this is consistent with the Seattle protocol[1]. However, the Seattle protocol is considered by some as relatively inefficient, requiring additional biopsy procedures, longer procedure times, and higher operating costs. This technique is also vulnerable to sampling errors, consequently lower diagnostic rates, suboptimal disease management, and poorer adherence to the practice guidelines[5-9].
In a multicenter, randomized, crossover trial of 123 BE patients, the NBI-targeted biopsy was compared with the 4-quadrant biopsy with WLE. No difference was found between the two techniques in terms of the frequency of detecting IM tissues (both 85%) and dysplasia (71% for NBI vs 55% for WLE; P = 0.15)[3]. However, the procedure of “4-quadrant biopsy” in that study is not fully compatible with the Seattle protocol. As a result, the IM or dysplasia detection rate might have been underestimated. Another study enrolling 2245 BE patients from the U.S. pathology database reported that only 51.2% of cases had adhered to the Seattle protocol, and more cases of LSBE were associated with poorer adherence. Furthermore, non-adherence was associated with fewer detections of dysplasia (OR = 0.53, 95%CI: 0.35-0.82)[5].
For our patients, the Seattle-protocol guided biopsy, when compared with NBI-target biopsy, slightly improved the sensitivity rate of IM at the GEJ (78.5% vs 69.2%), although the difference was not significant (P = 0.231). Further analysis showed that the Seattle-protocol-guided biopsy had improved IM detection in those cases with higher “M” levels, according to the Prague criteria. On the contrary, the Seattle biopsy protocol had limitations regarding the correct diagnoses of BE in those individuals with EE. Unsurprisingly, the more biopsy samples were obtained during endoscopy, the better the diagnosis of IM became. This could account for the lack of difference between these two biopsy protocols in detecting IM in most of our cases with LSBE, and support that the more biopsy pieces that were taken, the less likely sampling errors occurred.
The mean biopsy pieces obtained per patient in our study ranged between 3.39 and 5.69. The majority of cases with fewer biopsy pieces were SSBE (89.5%). This finding is compatible with previous studies on Asian patients[12]. According to one study, biopsies of at least eight pieces at the GEJ was required for an adequate detection of IM[16]. However, it is technically difficult to obtain as many as eight samples per SSBE patient.
One study in the UK enrolling 220 BE subjects reported a significant increase in the detection rate of all types of dysplasia when the Seattle Protocol was adapted (LGD: 12% vs 3.6%, advanced dysplasia: 5.2% vs 0.8%, P < 0.00001)[17]. In our study, the number of cases with dysplastic tissue over the GEJ increased from one via the NBI-target biopsy to five via the Seattle protocol-guided biopsy, although the increment was not significant. For the one patient with EAC detected early by the Seattle protocol-guided biopsy, treatments were started promptly with curative therapy, like the endoscopic submucosal dissection.
Recently, other advanced endoscopic techniques have been introduced to improve the detection of IM and dysplastic tissue in BE. These techniques include cytosponge, esophageal capsule endoscopy, dye-based chromoendoscopy, and confocal laser endomicroscopy[18,19]. However, they have not yet reached routine clinical application due to their higher costs and uncertain efficacies.
There are some limitations in our study. Firstly, the endoscopic and pathologic appearance were diagnosed or confirmed by individual endoscopists and pathologists, with inevitable inter-observer and intra-observer variations. Secondly, this study was hospital-based and data were derived from a single tertiary care center. Selection bias of cases could not be ruled out. Thirdly, some BE-associated variables, like reflux symptom, smoking habit and Helicobacter pylori, were not analyzed. Finally, most of our cases belonged to SSBE, and this may not reflect populations in the Western countries. Further work is still needed to confirm or reinforce our present results.
In conclusion, we found that the Seattle protocol showed improvements in IM detection in subjects with high Prague criteria “M” levels, and disclosed more cases, including EAC, with dysplastic tissue.
According to the American Gastroenterological Association guidelines, the definition of Barrett’s esophagus (BE) includes the histological finding of intestinal metaplasia (IM) in the esophagus. BE is clinically important because it is a major risk factor for the development of esophageal malignancy. Therefore, early detection of BE, especially those with dysplastic tissue, attracted recent research interests.
The standard for diagnosing BE is endoscopic evaluation performed with the Seattle biopsy protocol. However, some in this field consider disadvantages of the Seattle protocol as being relatively inefficient, time-consuming and providing low diagnostic rates. In Western countries, reportedly only half of endoscopists follow the Seattle protocol for biopsy of BE patients.
The aim of this study is to investigate the benefits of the Seattle protocol in the diagnosis of Chinese individuals with BE.
Subjects enrolled were cases of Taichung Veterans General Hospital with endoscopically-suspected esophageal metaplasia. These patients first received the narrow-band imaging (NBI)-targeted biopsy and later the Seattle protocol-guided biopsy, within a period from October 2012 to December 2014. Cases without initial pathologic patterns of IM and then appearance or loss of IM tissue were designated as Group A or B, respectively. Those with initial pathologic patterns of IM, which then persisted or were lost were designated as Group C or D, respectively.
The number of cases for each group was as follows: A: 20, B: 78, C: 31 and D: 14. The distribution of the Prague criteria M levels of Group A was significantly higher than Group B (P = 0.174). The sensitivity of IM detection was 69.2% for the NBI-targeted biopsy and 78.5% for the Seattle protocol-guided biopsy. The difference was not significant (P = 0.231). The number of detectable dysplasia increased from one case via the NBI-targeted biopsy to five cases via the Seattle protocol-guided biopsy, including one case of adenocarcinoma.
The Seattle protocol improved the IM detection in the subjects with higher Prague criteria M levels, and it disclosed more cases with dysplastic tissues.
In the future, the prospective studies in the selected BE patients should be conducted to evaluate the usefulness of the Seattle protocol in clinical practice.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Taiwan
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Fujita T, Zhao J, Zhu SB S- Editor: Wang JL L- Editor: Filipodia E- Editor: Song H
| 1. | Shaheen NJ, Falk GW, Iyer PG, Gerson LB; American College of Gastroenterology. ACG Clinical Guideline: Diagnosis and Management of Barrett’s Esophagus. Am J Gastroenterol. 2016;111:30-50; quiz 51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 990] [Cited by in RCA: 1058] [Article Influence: 117.6] [Reference Citation Analysis (0)] |
| 2. | Rustgi AK, El-Serag HB. Esophageal carcinoma. N Engl J Med. 2014;371:2499-2509. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 817] [Cited by in RCA: 997] [Article Influence: 90.6] [Reference Citation Analysis (0)] |
| 3. | Sharma P, Hawes RH, Bansal A, Gupta N, Curvers W, Rastogi A, Singh M, Hall M, Mathur SC, Wani SB. Standard endoscopy with random biopsies versus narrow band imaging targeted biopsies in Barrett’s oesophagus: a prospective, international, randomised controlled trial. Gut. 2013;62:15-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 245] [Article Influence: 20.4] [Reference Citation Analysis (0)] |
| 4. | Levine DS. Management of dysplasia in the columnar-lined esophagus. Gastroenterol Clin North Am. 1997;26:613-634. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 59] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 5. | Abrams JA, Kapel RC, Lindberg GM, Saboorian MH, Genta RM, Neugut AI, Lightdale CJ. Adherence to biopsy guidelines for Barrett’s esophagus surveillance in the community setting in the United States. Clin Gastroenterol Hepatol. 2009;7:736-742; quiz 710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 283] [Cited by in RCA: 260] [Article Influence: 16.3] [Reference Citation Analysis (0)] |
| 6. | Eloubeidi MA, Provenzale D. Does this patient have Barrett’s esophagus? The utility of predicting Barrett’s esophagus at the index endoscopy. Am J Gastroenterol. 1999;94:937-943. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 98] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 7. | Dulai GS. Surveying the case for surveillance. Gastroenterology. 2002;122:820-823. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 8. | Amamra N, Touzet S, Colin C, Ponchon T. Current practice compared with the international guidelines: endoscopic surveillance of Barrett’s esophagus. J Eval Clin Pract. 2007;13:789-794. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | Mandal A, Playford RJ, Wicks AC. Current practice in surveillance strategy for patients with Barrett’s oesophagus in the UK. Aliment Pharmacol Ther. 2003;17:1319-1324. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 10. | Sharma P, Morales TG, Sampliner RE. Short segment Barrett’s esophagus--the need for standardization of the definition and of endoscopic criteria. Am J Gastroenterol. 1998;93:1033-1036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 176] [Cited by in RCA: 159] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Sharma P, Dent J, Armstrong D, Bergman JJ, Gossner L, Hoshihara Y, Jankowski JA, Junghard O, Lundell L, Tytgat GN. The development and validation of an endoscopic grading system for Barrett’s esophagus: the Prague C & M criteria. Gastroenterology. 2006;131:1392-1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 731] [Cited by in RCA: 716] [Article Influence: 37.7] [Reference Citation Analysis (0)] |
| 12. | Shiota S, Singh S, Anshasi A, El-Serag HB. Prevalence of Barrett’s Esophagus in Asian Countries: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol. 2015;13:1907-1918. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 66] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 13. | Shaheen NJ, Richter JE. Barrett’s oesophagus. Lancet. 2009;373:850-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 234] [Cited by in RCA: 240] [Article Influence: 15.0] [Reference Citation Analysis (0)] |
| 14. | Runge TM, Abrams JA, Shaheen NJ. Epidemiology of Barrett’s Esophagus and Esophageal Adenocarcinoma. Gastroenterol Clin North Am. 2015;44:203-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 164] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 15. | Wolfsen HC, Crook JE, Krishna M, Achem SR, Devault KR, Bouras EP, Loeb DS, Stark ME, Woodward TA, Hemminger LL. Prospective, controlled tandem endoscopy study of narrow band imaging for dysplasia detection in Barrett’s Esophagus. Gastroenterology. 2008;135:24-31. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 200] [Article Influence: 11.8] [Reference Citation Analysis (0)] |
| 16. | Harrison R, Perry I, Haddadin W, McDonald S, Bryan R, Abrams K, Sampliner R, Talley NJ, Moayyedi P, Jankowski JA. Detection of intestinal metaplasia in Barrett’s esophagus: an observational comparator study suggests the need for a minimum of eight biopsies. Am J Gastroenterol. 2007;102:1154-1161. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 166] [Cited by in RCA: 158] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Ghuman S, Asghar K, Evans J, Kakhi S, Hawkes N. Real world surveillance of Barrett’s oesophagus-dose it make a difference using the Prague classification or following Seattle biopsy protocol. Gut. 2015;64:A418. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 18. | Singh A, Chak A. Advances in the management of Barrett’s esophagus and early esophageal adenocarcinoma. Gastroenterol Rep (Oxf). 2015;3:303-315. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Wani S, Rubenstein JH, Vieth M, Bergman J. Diagnosis and Management of Low-Grade Dysplasia in Barrett’s Esophagus: Expert Review From the Clinical Practice Updates Committee of the American Gastroenterological Association. Gastroenterology. 2016;151:822-835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 112] [Article Influence: 12.4] [Reference Citation Analysis (0)] |