Published online Nov 6, 2018. doi: 10.12998/wjcc.v6.i13.650
Peer-review started: July 19, 2018
First decision: August 25, 2018
Revised: September 13, 2018
Accepted: October 11, 2018
Article in press: October 11, 2018
Published online: November 6, 2018
Processing time: 110 Days and 2.4 Hours
To determine the therapeutic effect of photodynamic therapy (PDT) for middle-advanced stage upper gastrointestinal carcinomas.
We searched PubMed, EMBASE, the Cochrane Library, China National Knowledge Infrastructure, China Science and Technology Journal Database, and Wanfang Database from inception to April 2018 for randomized controlled studies. These studies compared PDT with other palliative therapies (radiotherapy, chemotherapy, or Nd:YAG laser) and compared PDT, radiotherapy, or chemotherapy alone with PDT combined with chemotherapy/radiotherapy. In our meta-analysis, both fixed and random effects models were used to estimate the risk ratio (RR) for dichotomous outcomes (the response rate and one-year survival rate).
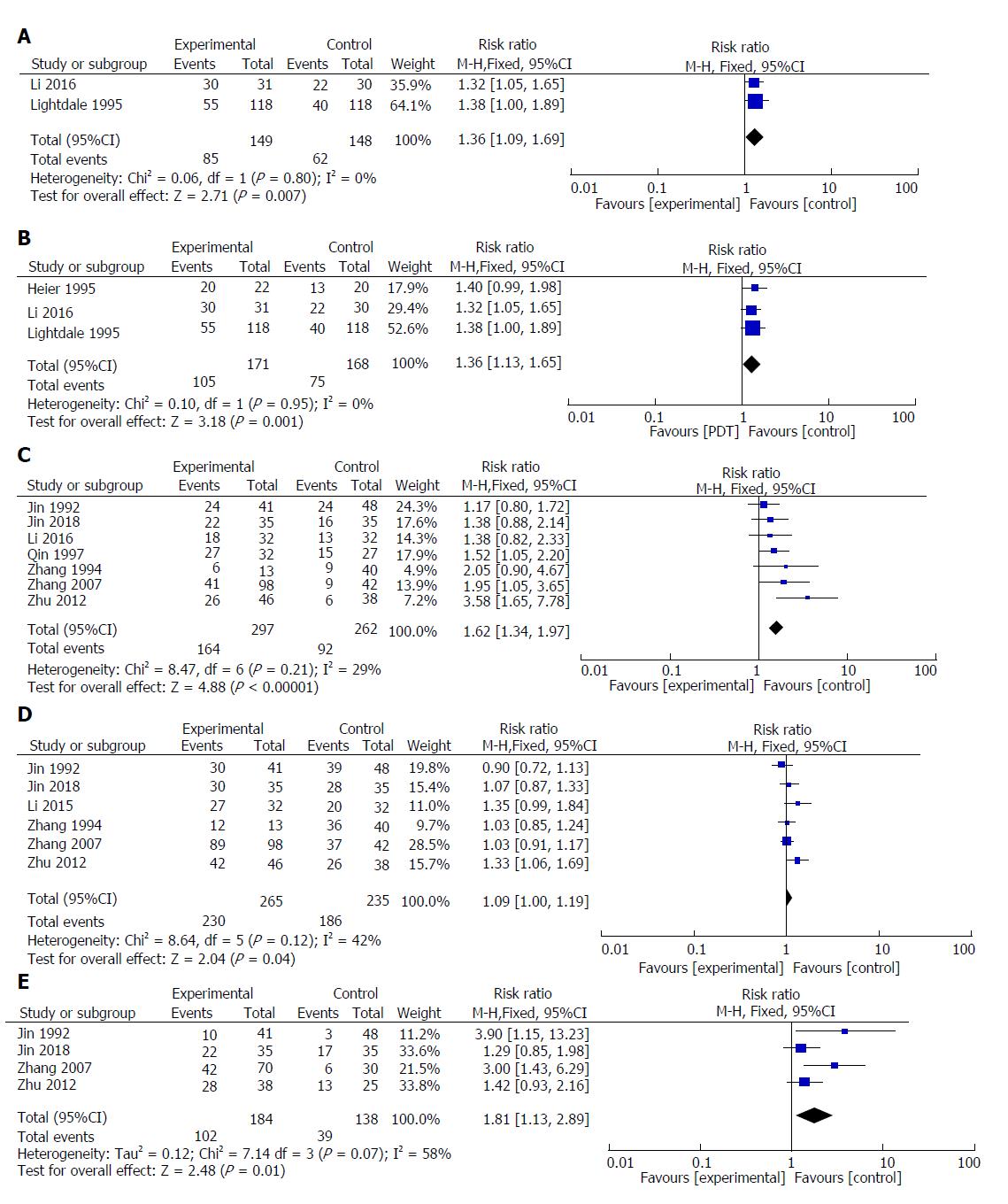
Ten random controlled clinical studies with 953 patients were included in the analysis. The effective rate for PDT was better than that of radiotherapy or Nd:YAG laser for the treatment of middle-advanced upper gastrointestinal carcinomas [RR = 1.36; 95% confidence interval (CI): 1.13-1.65; P = 0.001]. In addition, PDT combined with chemotherapy had significantly better efficacy and a higher one-year survival rate than PDT or chemotherapy alone (significant remission rate, RR = 1.62; 95%CI: 1.34-1.97; P < 0.00001; one-year survival rate, RR = 1.81; 95%CI: 1.13-2.89; P = 0.01).
PDT is a useful method for the treatment of middle-advanced stage upper gastrointestinal carcinomas. PDT combined with chemotherapy or radiotherapy can enhance its efficacy and prolong survival time.
Core tip: Photodynamic therapy (PDT) is minimally invasive compared with chemotherapy and radiotherapy for the treatment of cancers. Limited data exist regarding the efficacy of PDT alone or combined with radiotherapy and chemotherapy for the treatment of middle-advanced stage upper gastrointestinal carcinomas. Therefore, we performed a systematic review and meta-analysis of ten available randomized controlled clinical trials that addressed the efficacy of PDT alone or combined with radiotherapy and chemotherapy in patients with middle-advanced stage upper gastrointestinal carcinomas. Our analysis showed that PDT may be suitable for single or combined treatment of middle-advanced stage upper gastrointestinal carcinomas, especially for elderly patients, those with severe diseases, those with severe complications, and those unwilling to undergo surgery.
- Citation: Chen B, Xiong L, Chen WD, Zhao XH, He J, Zheng YW, Kong FH, Liu X, Zhang ZJ, Miao XY. Photodynamic therapy for middle-advanced stage upper gastrointestinal carcinomas: A systematic review and meta-analysis. World J Clin Cases 2018; 6(13): 650-658
- URL: https://www.wjgnet.com/2307-8960/full/v6/i13/650.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i13.650
Upper gastrointestinal carcinomas, including esophageal and gastric cancer, constitute major health problems worldwide[1]. Gastric cancer is the fifth most common malignancy and is the third leading cause of cancer death. In 2012, there were approximately 95200 new cases of gastric cancer in the world. Esophageal carcinoma is the eighth most common cancer worldwide. In 2012, the number of new cases of esophageal cancer was approximately 456000, and the number of deaths was approximately 400000. The five-year survival rate ranged between 13% and 18%[2].
At present, the standard treatment for upper gastrointestinal carcinomas is surgical resection. However, many patients are diagnosed in middle-advanced stage or are elderly, and most of them have lost their best chance of surgery. These patients can undergo palliative treatments only to relieve esophageal obstruction symptoms to improve their quality of life[3]. Palliatively, the principal regimens for middleadvanced stage upper gastrointestinal carcinomas include chemotherapy, radiotherapy, and endoscopic ablation[4]. Specially, endoscopic ablation treatment includes photodynamic therapy (PDT)[5], Nd:YAG laser[6], radiofrequency ablation[7], and argon plasma coagulation[8].
Chemotherapy and radiotherapy are curative treatment options for upper gastrointestinal carcinomas. Chemotherapy has a mitigating effect on a small group of patients whose disease is in the advanced stage. However, low response rates, severe toxic side-effects, and drug resistance have limited further use of chemotherapy[9]. Radiotherapy can cause extensive damage to adjacent organs, thereby reducing its clinical value[10]. Therefore, it is urgent to develop new antitumor treatments to overcome these limitations.
In recent decades, PDT has become an alternative to radiotherapy and chemotherapy for a variety of diseases including cancer because of its minimally invasive nature[11]. It utilizes a specific light wavelength to activate a photosensitizer in cells, thereby generating reactive oxygen species to damage cancer cells[12]. By virtue of the fact that PDT is noninvasive, highly selective, and lowly toxic, it can reduce damage to surrounding tissues of the intestine and decrease the risk of intestinal leakage. In addition, it has high patient acceptance/compliance and is a relatively simple procedure [13]. Therefore, PDT has played an increasingly important role in the treatment of upper gastrointestinal tumors and precancerous diseases. PDT can also be used in combination with chemotherapy or radiation to improve efficacy and reduce side-effects[14].
Recently, several randomized controlled trials (RCTs) have verified the efficacy of PDT alone or PDT combined with radiotherapy/chemotherapy for treatment of middle-advanced stage upper gastrointestinal carcinomas. Therefore, we undertook a systematic review and meta-analysis of RCTs regarding the combination of PDT with chemotherapy or radiotherapy for the treatment of middle-advanced stage upper gastrointestinal carcinomas, with particular reference to survival rate and tumor response rate (the significant remission rate and effective rate).
PRISMA statement guidelines were followed for the calculation and reporting of meta-analysis data[15]. A literature retrieval was carried out in PubMed, EMBASE, the Cochrane Library, China National Knowledge Infrastructure, China Science and Technology Journal Database, and Wanfang Database from inception to May 2018 using the following key words: "upper gastrointestinal carcinoma”, “gastric carcinoma”, “stomach cancer”, “cardia carcinoma”, “esophageal carcinoma”, “esophagocardiac carcinoma”, “photodynamic therapy”, “endoscopic therapy”, “ablation”, “radiotherapy”, “brachytherapy”, “irradiation”, “brachyradiotherapy”, “chemotherapy”, “randomized controlled trial”, and “clinical studies”. All analyses were based on previously published studies. Therefore, no ethics approval or patient consent was required.
Studies that met the following criteria were included: adults with middle-advanced stage upper gastrointestinal carcinomas confirmed by endoscopy or pathologic diagnosis; the intervention arm was PDT alone or PDT combined with chemotherapy/radiotherapy; and the control arm was palliative treatment (radiotherapy, chemotherapy, or Nd:YAG laser). RCTs had access to the full text. The following studies were excluded: those with no control group; those in which the study outcomes did not include complete or available efficacy data; case reports, abstracts, letters, comments, reviews without original data; and studies that presented insufficient data.
Two reviewers read the structured table to extract data from relevant articles. Any disagreement between the two reviewers was resolved by discussion with a third reviewer. For duplicated articles, we extracted relevant data from the most recent article. We extracted first author, publication year, study design, participant number, average age, proportion of men and women, type of upper gastrointestinal carcinomas, intervention methods, and primary outcomes.
The Cochrane System Evaluation Manual Intervention was used to assess the quality of randomized controlled clinical studies. Funnel plots were constructed to assess the risk of publication bias.
Tumor response rate (the significant remission rate and effective rate) was evaluated one month after PDT. The criteria for PDT established at the national conference held in 1984 were as follows: complete remission (CR), tumor disappearance and no histologic examination of cancer cells for at least one month; partial remission (PR), extensive tumor necrosis and tumor volume regressed for at least 1 mo; mild remission (MR), the tumor volume decreased less than 50% for at least one month; and no remission (NR), no obvious necrosis and tumor volume decreased slightly or even increased. The significant remission rate included CR and PR. The effective rate included CR, PR, and MR. The meta-analysis was performed using Review Manager (version 5.3.0) software. We calculated risk ratio (RR) and 95% confidence interval (CI) values for binary variables. In addition, a P-value < 0.05 was considered significant. Heterogeneity tests were performed using the chi-square statistic (P-value < 0.10 considered significant) and I2 tests. If P < 0.10 or I2 > 50%, indicating that the heterogeneity was large, a random effects model was used. If P > 0.10 or I2 < 50%, indicating that the heterogeneity was small, a fixed effects model was used.
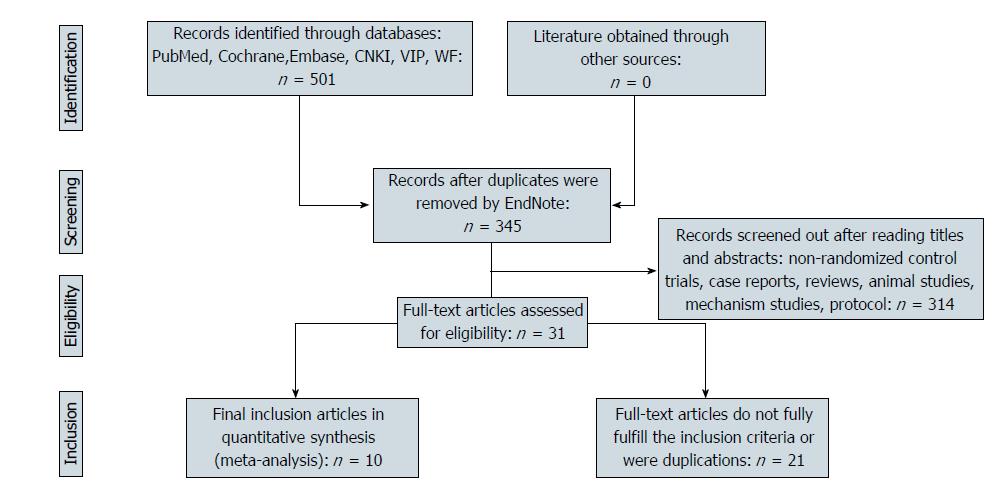
A flow chart of the study selection process and exclusion criteria is shown in Figure 1. A total of 501 studies were identified based on the search criteria. We filtered by title, abstract, and full text. Eventually, ten studies met the inclusion and exclusion criteria[16-25]. The total number of patients was 953; of these patients, 523 underwent PDT alone or PDT combined with other palliative therapies (radiotherapy, chemotherapy), and 430 were in the control group. The characteristics of all included studies are shown in Table 1.
| Study | Study design | Time of case inclusion | Cancer type | Age (yr) | Gender(M/F) | Sample size (E/C) | Intervention | Main outcomes | |
| Experiment | Control | ||||||||
| Jin et al[16] | RCT | Sep. 1982-July 1990 | Advanced cardiac cancer | (34-91) | 139/5 | 144 (96/48) | PDT1 + CHT2 | CHT2 | SR, ER, MST, OSR |
| Zhang et al[17] | RCT | 1990-1994 | Advanced upper gastrointestinal cancer | 56.8 (29-77) | 42/41 | 53 (13/40) | PDT1 + CHT3 | PDT1 | SR, ER, MST |
| Heier et al[18] | RCT | NA | Esophageal cancer | 69 (42-87) | 26/16 | 42 (22/20) | PDT4 | Nd:YAG5 | ER, AEs, KPS, EG |
| Lightdale et al[19] | RCT | Sep. 1988-Aug. 1992 | Esophageal cancer | 70 | 169/67 | 236 (118/118) | PDT6 | Nd:YAG7 | SR, ER, AEs, SP |
| Qin et al[20] | RCT | June 1993-May 1995 | Middle-advanced stage esophageal cancer | 58.6 (43-73) | 44/15 | 59 (32/27) | PDT8 + RAT9 | RAT9 | SR |
| Zhang et al[21] | RCT | NA | Advanced esophagocardiac cancer | 60.8 (40-81) | 111/29 | 140 (98/42) | PDT10 + CHT3 | PDT10 | SR, ER, OSR |
| Zhou et al[22] | RCT | Dec. 2007-June 2009 | Middle-advanced stage cardiac cancer | 59.5 (38-82) | 63/21 | 84 (46/38) | PDT11 + CHT12 | CHT12 | SR, ER, OSR |
| Li et al[23] | RCT | Jan. 2013-Oct. 2014 | Middle-advanced stage gastric cancer | NA | 45/19 | 64 (32/32) | PDT1 + CHT13 | PDT1 | SR, ER, AEs |
| Li et al[24] | RCT | Feb. 2013- Feb. 2014 | Advanced esophageal cancer | (53-83) | 40/22 | 61 (31/30) | PDT1 | RAT14 | SR, ER, AEs |
| Jin et al[25] | RCT | Sep. 2014- Sep. 2015 | Advanced esophageal cancer | (47-76) | 41/29 | 70 (35/35) | PDT1 + CHT15 | CHT15 | SR, ER, AEs, OSR |
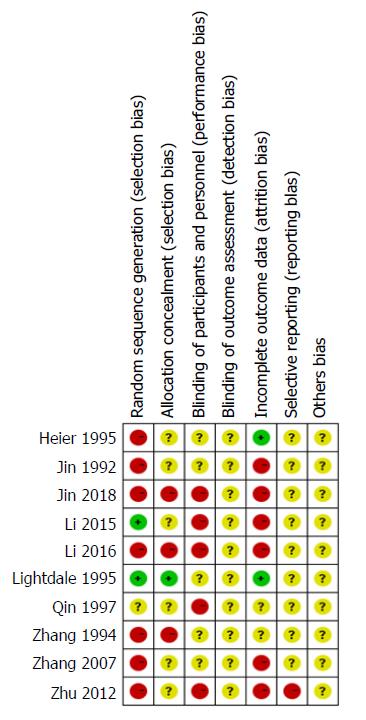
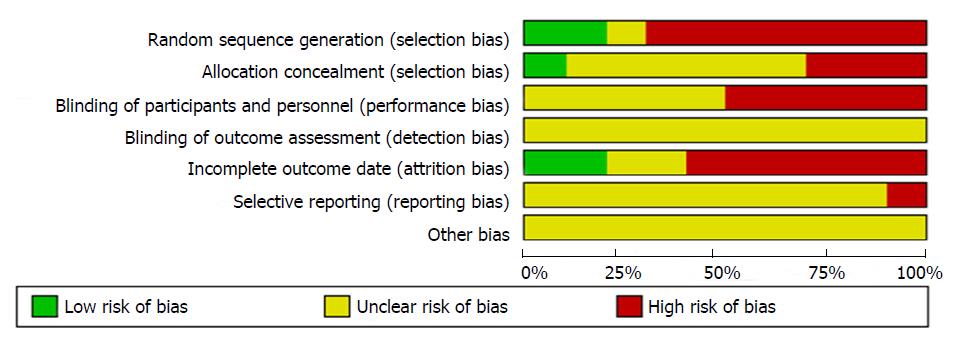
We used the standards of the Cochrane System Evaluation Manual Intervention for this assessment[26]. For the included RCTs, assessment of the bias risk involved the following parameters: sequence generation, allocation concealment, blinding, incomplete outcome data, selective reporting bias, and other potential sources of bias. Detailed results of the assessments are shown in Figures 2 and 3.
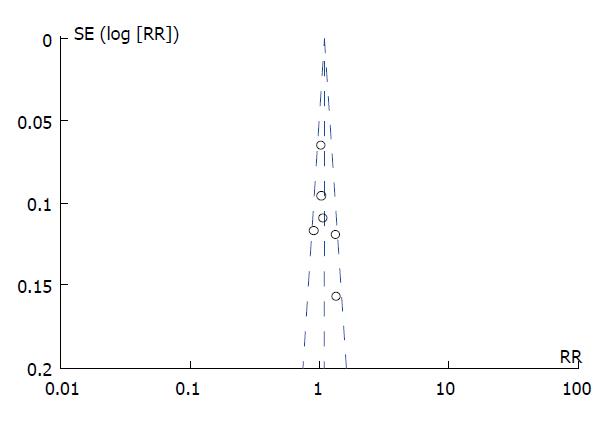
Potential publication bias was assessed by visual inspection of the funnel plots (Figure 4), which suggested no obvious evidence of publication bias.
Two studies reported the significant remission rates of PDT and other palliative treatments (Nd:YAG laser or radiotherapy). The result of meta-analysis showed that the value of the significant remission rate (CR + PR) increased with PDT therapy (RR = 1.36; 95%CI: 1.09-1.69; P = 0.007; I2 = 0%). Therefore, we used a fixed model (Figure 5A).
Three studies reported the effective rates of PDT and other palliative treatments (Nd:YAG laser or radiotherapy). The result of meta-analysis showed that the value of the effective rate (CR + PR + MR) increased with PDT therapy (RR = 1.36; 95%CI: 1.13-1.65; P = 0.001; I2 = 0%). Therefore, we used a fixed model (Figure 5B).
Seven included studies reported the significant remission rates of PDT combined with chemotherapy/radiotherapy and PDT/chemotherapy/ radiotherapy alone. The result of meta-analysis showed that the value of the significant remission rate increased in PDT combined with chemotherapy or radiotherapy (RR = 1.62; 95%CI: 1.34-1.97; P < 0.00001; I2 = 29%). Therefore, we used a fixed model (Figure 5C).
Six included studies reported the effective rates of PDT combined with chemotherapy/radiotherapy and PDT/chemotherapy/radiotherapy alone. The result of meta-analysis showed that the value of the effective rate increased with PDT combined with chemotherapy (RR = 1.09; 95%CI: 1.00-1.19; P = 0.04; I2 = 42%). Therefore, we used a fixed model (Figure 5D).
Four included studies reported one-year survival rates for PDT combined with chemotherapy and PDT or chemotherapy alone. The results of meta-analysis showed that the value of the one-year survival rate increased with PDT combined with chemotherapy (RR = 1.81; 95%CI: 1.13-2.89; P = 0.01; I2 = 58%). Therefore, we used a random model (Figure 5E).
Upper gastrointestinal carcinomas are the most common malignant tumors of the digestive tract. Because the early symptoms of patients are atypical, most of these diseases are already in the middle-advanced stage at diagnosis[27]. Currently, the primary method for radical treatment of upper gastrointestinal carcinomas is surgical resection. However, due to complicated patient conditions, old age, and high surgical risk, many patients are reluctant to undergo surgery[28]. In recent years, increasing numbers of domestic and foreign scholars have used PDT alone or in combination with other palliative therapies to treat middle-advanced stage upper gastrointestinal carcinomas. Although the total number of cases is small, the treatment of cancer has achieved positive results.
Lightdale et al[19] reported that PDT was an effective method for treating esophageal cancer and that its efficacy was superior to that of Nd: YAG laser. Furthermore, this treatment can prolong the survival time of patients. The PDT procedure is also simple and more accepted by patients. Li et al[24] reported that PDT alleviated the dysphagia associated with esophageal cancer. Dysphagia improved significantly during this period and the curative effect was better than that of local radiotherapy. Our study showed that PDT was suitable for middle-advanced stage upper gastrointestinal carcinomas, and that it increased the significant remission rate (CR + PR) and the effective rate (CR + PR + MR) over that of Nd:YAG laser or radiotherapy alone, which is in agreement with results from studies by Lightdale and Li[19,24].
PDT has several potential advantages over traditional palliative treatments. Unlike chemotherapy and radiotherapy, neither tumor DNA nor rapidly dividing tumor cells are targeted by PDT, therefore, PDT rarely induces secondary tumorigenesis. PDT can also be reused in the same location without increasing its toxicity[29]. Unlike Nd:YAG laser, PDT selectively kills cancer cells without damaging the surrounding tissue, extending the healing time of wounds, or increasing the formation of scars after treatment, thereby reducing the possibility of posttreatment obstruction[30].
However, due to the limited ability of the laser to penetrate tissue, PDT has little effect on deep invasive lesions[31]. PDT combined with chemotherapy or radiotherapy can improve overall efficacy. Zhang et al[21] reported that PDT combined with local chemotherapy was a safe and effective way to treat advanced esophageal cancer. Local administration of 5-FU improved PDT efficacy and prolonged survival time. Qin et al[20] reported that PDT combined with local radiotherapy was an effective treatment for advanced esophageal cancer. Local radiotherapy also improved radiological outcomes and extended survival time. Our study showed that PDT combined with radiotherapy or chemotherapy significantly improved the significant remission rate, the effective rate, and the one-year survival rate for middle-advanced stage upper gastrointestinal carcinomas over rates achieved with single therapy, which is consistent with the work of Zhang and Qin.
The primary mechanisms of the coordinated effect of PDT and chemotherapy are the following: first, PDT and chemotherapeutic drugs act on tumor cells in different cell cycles; second, chemotherapeutic drugs increase the uptake of photosensitizers by increasing the permeability of cell membrane to photosensitizers; third, chemotherapeutic drugs damage the repair function of cells, thereby enhancing photodynamic killing capacity[32,33]. The synergistic mechanism of PDT combined with radiotherapy is mainly because some anti-PDT tumor cells are sensitive to radiotherapy, and some anti-radiotherapy tumor cells are sensitive to PDT. In addition, it has been reported that some photosensitizers can also be used as radiosensitizers[34,35].
To the best of our knowledge, this study is the first systematic review and meta-analysis of RCTs to analyze the effect of PDT alone or PDT combined with chemotherapy or radiotherapy for the treatment of middle-advanced stage upper gastrointestinal carcinomas. Nevertheless, there were some deficiencies in the current meta-analysis. First, the number of studies included in this analysis was relatively small, and most of the studies were published a long time ago. Second, the evaluation of the literature indicated that the quality of the literature was generally low. Third, RCTs were conducted in different ways, including tumor characteristics, PDT treatment options, differences in chemotherapy or radiotherapy, and outcome assessment. These differences may lead to heterogeneity and may have an impact on our results. Therefore, it is necessary to carry out more and better RCTs to provide more rigorous evidence for the therapeutic effects of various interventions and to guide clinical practice.
In summary, the results of this meta-analysis provide evidence that PDT is an effective treatment for middle-advanced stage upper gastrointestinal carcinomas. Other palliative treatments (chemotherapy/radiotherapy/Nd:YAG laser) were less effective than PDT. Compared with PDT/radiotherapy/ chemotherapy alone, PDT combined with radiotherapy/chemotherapy gave significantly better tumor response rates and a higher one-year survival rate. Our results suggest that PDT may be suitable for single or combined treatment of middle-advanced stage upper gastrointestinal carcinomas, especially for elderly patients, those with severe diseases, those with severe complications, and those unwilling to undergo surgery.
The standard treatment for upper gastrointestinal cancers is surgical resection. However, many patients are diagnosed in middle-advanced stage or are elderly, and most of them have lost their best chance of surgery. These patients can only receive palliative treatments to relieve esophageal obstruction symptoms. Photodynamic therapy (PDT) can significantly improve the symptoms of patients with middle-advanced stage upper gastrointestinal carcinomas. PDT can also be used in combination with chemotherapy or radiation to improve efficacy and reduce side-effects.
The main aim of the present study was to clarify the effect of PDT alone or in combination with radiotherapy/chemotherapy for the treatment of middle-advanced stage upper gastrointestinal carcinomas.
This was the first comprehensive article on this topic to take into account all the available evidence. We quantified the effect of PDT in addition to radiotherapy/chemotherapy for middle-advanced stage upper gastrointestinal carcinomas.
A meta-analysis was performed according to the guidelines of the PRISMA protocol. PubMed, Cochrane Library, EMBASE, Cochrane Library, China National Knowledge Infrastructure, and China Science and Technology Journal Database databases were comprehensively searched for studies analyzing the effect of PDT alone or combined with radiotherapy/chemotherapy for middle-advanced stage upper gastrointestinal carcinomas. The risks of bias and quality of the individual studies were assessed using funnel plots and the Cochrane System Evaluation Manual Intervention. Both fixed and random effects models were used to estimate the risk ratios for dichotomous outcomes.
The statistical analysis involved 953 patients with middle-advanced stage upper gastrointestinal carcinomas. The results showed that the effective rate of PDT was better than that of radiotherapy or Nd:YAG laser for the treatment of middle-advanced stage upper gastrointestinal carcinomas. Furthermore, PDT combined with chemotherapy gave significantly better efficacy and higher one-year survival rates than PDT or chemotherapy alone.
PDT may be suitable for single or combined treatment of middle-advanced stage upper gastrointestinal carcinomas, especially for patients in old age, with severe diseases, severe complications, or who are unwilling to undergo surgery.
Further research would be essential to understand the effect of PDT alone or combined with radiotherapy/chemotherapy in the treatment of middle-advanced stage upper gastrointestinal carcinomas. A multicenter, double-blind, randomized controlled trial could provide a better answer.
Conflict-of-interest statement: No potential conflicts of interest relevant to this article are reported.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Aseni P, Einama T S- Editor: Ma RY L- Editor: Wang TQ E- Editor: Wu YXJ
| 1. | Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893-2917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11128] [Cited by in RCA: 11831] [Article Influence: 845.1] [Reference Citation Analysis (4)] |
| 2. | Ferlay J, Soerjomataram I, Ervik M, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F. Cancer Incidence and Mortality Worldwide: IARC Cancer Base No.11. Int J Cancer. 2014;136:E359-E386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20108] [Cited by in RCA: 20491] [Article Influence: 2049.1] [Reference Citation Analysis (20)] |
| 3. | Reed CE, Marsh WH, Carlson LS, Seymore CH, Kratz JM. Prospective, randomized trial of palliative treatment for unresectable cancer of the esophagus. Ann Thorac Surg. 1991;51:552-5; discussion 556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 34] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Lambert R. Palliation of carcinoma of the esophagus: is there a hope for cure? Am J Gastroenterol. 1994;89:S27-S40. [PubMed] |
| 5. | Gossner L, May A, Sroka R, Stolte M, Hahn EG, Ell C. Photodynamic destruction of high grade dysplasia and early carcinoma of the esophagus after the oral administration of 5-aminolevulinic acid. Cancer. 1999;86:1921-1928. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 6. | Swain CP, Bown SG, Edwards DA, Kirkham JS, Salmon PR, Clark CG. Laser recanalization of obstructing foregut cancer. Br J Surg. 1984;71:112-115. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 31] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Sharma V, Mahantshetty U, Dinshaw KA, Deshpande R, Sharma S. Palliation of advanced/recurrent esophageal carcinoma with high-dose-rate brachytherapy. Int J Radiat Oncol Biol Phys. 2002;52:310-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 69] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 8. | Akhtar K, Byrne JP, Bancewicz J, Attwood SE. Argon beam plasma coagulation in the management of cancers of the esophagus and stomach. Surg Endosc. 2000;14:1127-1130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 50] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 9. | Chabner BA, Roberts TG Jr. Timeline: Chemotherapy and the war on cancer. Nat Rev Cancer. 2005;5:65-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1320] [Cited by in RCA: 1373] [Article Influence: 68.7] [Reference Citation Analysis (0)] |
| 10. | Thorsen LB, Thomsen MS, Berg M, Jensen I, Josipovic M, Overgaard M, Overgaard J, Skogholt P, Offersen BV; Danish Breast Cancer Cooperative Group Radiotherapy committee. CT-planned internal mammary node radiotherapy in the DBCG-IMN study: benefit versus potentially harmful effects. Acta Oncol. 2014;53:1027-1034. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 67] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Moan J, Peng Q. An outline of the hundred-year history of PDT. Anticancer Res. 2003;23:3591-3600. [PubMed] |
| 12. | Dougherty TJ. Photodynamic therapy. Clin Chest Med. 1985;6:219-236. [PubMed] |
| 13. | Brown SB, Brown EA, Walker I. The present and future role of photodynamic therapy in cancer treatment. Lancet Oncol. 2004;5:497-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1298] [Cited by in RCA: 1261] [Article Influence: 60.0] [Reference Citation Analysis (0)] |
| 14. | Dolmans DE, Fukumura D, Jain RK. Photodynamic therapy for cancer. Nat Rev Cancer. 2003;3:380-387. [PubMed] [DOI] [Full Text] |
| 15. | Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP, Clarke M, Devereaux PJ, Kleijnen J, Moher D. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13930] [Cited by in RCA: 13305] [Article Influence: 831.6] [Reference Citation Analysis (0)] |
| 16. | Jin ML, Yang BQ, Zhang W, Ren P. Combined treatment with photodynamic therapy and chemotherapy for advanced cardiac cancers. J Photochem Photobiol B. 1992;12:101-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 23] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 17. | Zhang NZ, Li JH. Photodynamic therapy combined with local chemotherapy for upper gastrointestinal tumors. Zhongguo Jiguang Yixue Zazhi. 1994;3:91-93. [DOI] [Full Text] |
| 18. | Heier SK, Rothman KA, Heier LM, Rosenthal WS. Photodynamic therapy for obstructing esophageal cancer: light dosimetry and randomized comparison with Nd:YAG laser therapy. Gastroenterology. 1995;109:63-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 109] [Cited by in RCA: 86] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 19. | Lightdale CJ, Heier SK, Marcon NE, McCaughan JS Jr, Gerdes H, Overholt BF, Sivak MV Jr, Stiegmann GV, Nava HR. Photodynamic therapy with porfimer sodium versus thermal ablation therapy with Nd:YAG laser for palliation of esophageal cancer: a multicenter randomized trial. Gastrointest Endosc. 1995;42:507-512. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 193] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 20. | Qin YC, Han DS. Short-term efficacy of photodynamic therapy combined with radiotherapy for esophageal cancer. Shiyong Zhongliu Zazhi. 1997;109-111. |
| 21. | Zhang NZ, Zhu Y, Pan W, Ma WQ, Shao AL. Photodynamic therapy combined with local chemotherapy for the treatment of advanced esophagocardiac carcinoma. Photodiagnosis Photodyn Ther. 2007;4:60-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 22. | Zhu L. Efficacy of endoscopic photodynamic therapy combined with local chemotherapy for esophageal and cardiac carcinoma. Zhongguo Shequ Yisheng. 2012;14:29-29. [DOI] [Full Text] |
| 23. | Li Q, Wang KS. Efficacy and adverse reactions of photodynamic therapy combined with S-1 for elderly patients with gastric cancer. Zhongguo Zhongliu Linchuang Yu Kangfu. 2015;959-961. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 24. | Li JS, Zeng QX. Efficacy of endoscopic photodynamic therapy for esophageal cancer. Taishan Yixueyuan Xuebao. 2016;37:1415-1416. [DOI] [Full Text] |
| 25. | Jin Y, Kang C, Guo ZL, Chen XW, Liu J. Efficacy of photodynamic therapy combined with nedaplatin and 5-Fu in the treatment of patients with cisplatin-resistant advanced esophageal cancer. Yixue Linchuang Yanjiu. 2018;35:155-157. [DOI] [Full Text] |
| 26. | Higgins J. Cochrane handbook for systematic reviews of interventions. Version 5.1.0 [updated March 2011]. The Cochrane Collaboration 2011. S38. |
| 27. | Shao LF, Gao ZG, Yang NP, Wei GQ, Wang YD, Cheng CP. Results of surgical treatment in 6,123 cases of carcinoma of the esophagus and gastric cardia. J Surg Oncol. 1989;42:170-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Savage AP, Baigrie RJ, Cobb RA, Barr H, Kettlewell MG. Palliation of malignant dysphagia by laser therapy. Dis Esophagus. 1997;10:243-246. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 29. | Simone CB, Busch TM, Cengel KA. Radiotherapy and Photodynamic Therapy for Malignant Pleural Mesothelioma. In: Testa JR. Asbestos and Mesothelioma. Springer International Publishing AG 2017; 295-312. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 30. | Wang H, Xu Y, Shi J, Gao X, Geng L. Photodynamic therapy in the treatment of basal cell carcinoma: a systematic review and meta-analysis. Photodermatol Photoimmunol Photomed. 2015;31:44-53. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 31. | Moghissi K, Dixon K. Photodynamic therapy (PDT) in esophageal cancer: a surgical view of its indications based on 14 years experience. Technol Cancer Res Treat. 2003;2:319-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 32. | Harbord M, Dawes RF, Barr H, Giovannini M, Viens P, Eysselein V, Mishra L, Orenberg EK, Bown SG. Palliation of patients with dysphagia due to advanced esophageal cancer by endoscopic injection of cisplatin/epinephrine injectable gel. Gastrointest Endosc. 2002;56:644-651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 33. | Edell ES, Cortese DA. Combined effects of hematoporphyrin derivative phototherapy and adriamycin in a murine tumor model. Lasers Surg Med. 1988;8:413-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 26] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 34. | Berg K, Luksiene Z, Moan J, Ma L. Combined treatment of ionizing radiation and photosensitization by 5-aminolevulinic acid-induced protoporphyrin IX. Radiat Res. 1995;142:340-346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 35. | Luksiene Z, Berg K, Moan J. Combination of photodynamic therapy and x-irradiation: a study on 5-aminolevulinic acid (ALA) radiomodifying properties. Proc. SPIE. 1995;2325:306-311. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.1] [Reference Citation Analysis (0)] |