Published online Oct 26, 2018. doi: 10.12998/wjcc.v6.i12.521
Peer-review started: July 19, 2018
First decision: August 8, 2018
Revised: August 17, 2018
Accepted: October 9, 2018
Article in press: October 9, 2018
Published online: October 26, 2018
Processing time: 99 Days and 23.2 Hours
To explore the effect of alanine aminotransferase (ALT) on the performance of non-invasive fibrosis tests in chronic hepatitis B (CHB) patients.
A total of 599 treatment-naive and biopsy-proven CHB patients were included in the study. The cohort was divided into the following three groups: Normal ALT (ALT ≤ 40), slightly elevated ALT (40 < ALT ≤ 80) and elevated ALT (ALT > 80). The diagnostic performance of five common non-invasive fibrosis tests for liver fibrosis (stages S2-4), including the aspartate aminotransferase (AST)-to-platelet (PLT) ratio index (APRI), fibrosis index based on 4 factors (FIB-4), King’s score, Forns index and gamma-glutamyl transpeptidase (GGT)-to-PLT ratio (GPR), were evaluated for each group.
Higher ALT levels were associated with higher non-invasive fibrosis test scores. Patients with the same fibrosis stage but higher ALT levels showed higher non-invasive test scores. The areas under the receiver operating characteristics curves (AUROCs) of the non-invasive tests for prediction of ≥ S2 were higher for patients with ALT ≤ 40 U/L (range 0.705-0.755) and 40 < ALT ≤ 80 U/L (range 0.726-0.79) than for patients with ALT > 80 U/L (range 0.604-0.701). The AUROCs for predicting ≥ S3 and S4 were higher in patients with ALT ≤ 40 U/L (range 0.736-0.814 for ≥ S3, 0.79-0.833 for S4) than in patients with 40 < ALT ≤ 80 U/L (range 0.732-0.754 for ≥ S3, range 0.626-0.723 for S4) and ALT > 80 U/L (range 0.7-0.784 for ≥ S3, range 0.662-0.719 for S4). The diagnostic accuracy of the non-invasive tests decreased in a stepwise manner with the increase in ALT.
ALT has a significant effect on the diagnostic performance of non-invasive fibrosis tests. The ALT level should be considered before performing these non-invasive tests.
Core tip: Because of their high applicability and good interlaboratory reproducibility, many convenient non-invasive fibrosis tests have been established. To explore the effect of alanine aminotransferase (ALT) on the performance of non-invasive fibrosis tests in chronic hepatitis B (CHB) patients, we retrospectively analyzed 599 treatment-naive and biopsy-proven CHB patients at our hospital. The diagnostic accuracy of the non-invasive tests decreased in a stepwise manner with the increase in ALT. ALT has a significant effect on the diagnostic performance of non-invasive fibrosis tests.
- Citation: Wang L, Fan YX, Dou XG. Declining diagnostic accuracy of non-invasive fibrosis tests is associated with elevated alanine aminotransferase in chronic hepatitis B. World J Clin Cases 2018; 6(12): 521-530
- URL: https://www.wjgnet.com/2307-8960/full/v6/i12/521.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i12.521
Liver fibrosis is one of the main characteristics of chronic hepatitis B (CHB). Accurate assessment of liver fibrosis is vital for predicting disease progression and choosing optimal treatment timing. Liver biopsy (LB) is the current gold standard for evaluating the liver fibrosis stage. However, repeatedly utilizing LB in patients is very difficult because of its invasiveness and complications[1]. Therefore, non-invasive methods have been developed to assess liver fibrosis when the risk-benefit trade-off does not favor a LB[2].
According to the EASL-ALEH Clinical Practice Guidelines, non-invasive methods were recently divided into serum biomarkers and liver stiffness (LS) measurements[3]. Because of their high applicability (> 95%) and good interlaboratory reproducibility, serum biomarkers are widely used in clinical practice[3]. Over the past few years, many convenient non-invasive fibrosis tests have been established, including the aspartate aminotransferase (AST)-to-platelet (PLT) ratio index (APRI)[4] and the fibrosis index based on 4 factors (FIB-4)[5]. Moreover, the APRI and FIB-4 were recommended by the WHO for evaluation of liver fibrosis in resource-limited regions[6]. However, most non-invasive fibrosis tests have been developed primarily based on chronic hepatitis C patients, and their use in assessing liver fibrosis in CHB patients is controversial[7-9]. Therefore, more data should be collected regarding the use of these established non-invasive tests to predict liver fibrosis in CHB patients. Recently, Lemoine et al[10] constructed the gamma-glutamyl transpeptidase (GGT)-to-PLT ratio (GPR) model based on CHB patients, but its diagnostic performance assessing liver fibrosis varied among different studies[11,12].
Alanine aminotransferase (ALT) is considered an accurate and valuable routine indicator of liver inflammation. Many studies have reported the influence of serum ALT levels on LS[13,14]. According to the EASL-ALEH Clinical Practice Guidelines for CHB[3], LS measurements should be interpreted in patients with an elevated ALT level and should not be used in patients with ALT levels more than 10 times the upper limit of normal (ULN). However, no report has investigated the effects of changes in CHB patients’ serum ALT levels on the diagnostic performances of these non-invasive tests. For these reasons, the aim of this study was to explore the effect of ALT on the diagnostic performance of non-invasive fibrosis tests in CHB patients.
The study included treatment-naive CHB patients who underwent LB from 2012-2017 in the Shengjing Hospital affiliated with China Medical University. CHB was defined as hepatitis B surface antigen-positive lasting at least six months. The exclusion criteria were as follows: (1) coinfection with human immunodeficiency virus; (2) other liver disease (other viral hepatitis, autoimmune liver disease, drug hepatitis and liver failure); (3) significant alcohol intake (> 20 g/d for women and > 30 g/d for men) for more than 5 years; (4) tumor; (5) liver transplantation; and (6) hematological diseases.
The study was approved by the Medical Ethics Committee of Shengjing Hospital of China Medical University and was performed in accordance with the guidelines of the 1975 Declaration of Helsinki. Written informed consent for the LB was obtained from all patients.
Laboratory data, including the blood test results (PLT), liver biochemistry (ALT; aspartate aminotransferase, AST; alkaline phosphatase, ALP; γ-glutamyltransferase, GGT and cholesterol), HBV serological marker levels (HBsAg; hepatitis B envelope antigen, HBeAg; antibodies against HBsAg, anti-HBs; antibodies against HBeAg, anti-HBe and antibodies against HBcAb, anti-HBc), blood coagulation (international normalized ratio, INR) and HBV DNA levels, were recorded for all included patients.
All liver specimens were a minimum of 10 mm with at least five portal tracts. A histopathological assessment was performed by two expert pathologists who were unaware of the patient’s clinical characteristics. According to Scheuer’s classification score, liver fibrosis was classified from 0 to 4[15].
Non-invasive prediction methods and calculation formulas were applied for the CHB patients according to the original reported formulas with the original cut off values for the APRI, FIB-4, Forns index[16], GPR and King’s score[17]. The formulas of these non-invasive tests are as follows:
APRI = AST(/ULN)/ PLT (109/L) × 100
FIB-4 = Age (year) × AST(ULN)/{PLT (109/L) × [ALT(ULN)]1/2}
GPR = GGT(/ULN)/ PLT (109/L) × 100
King’s score = Age × AST × INR/PLT
Forns index = 7.811 - 3.131 × ln(PLT count) + 0.781 × ln(GGT) + 3.467 × ln(age) - 0.014 × (cholesterol)
Continuous and abnormal variables are expressed as the median (interquartile range, IQR), and categorical data are expressed as the frequency and percentage; the data were compared using the Kruskal-Wallis test. Spearman’s rank correlation was used to analyze the liver fibrosis stages and the non-invasive test results. The diagnostic performances of these non-invasive tests were assessed by receiver operating characteristic (ROC) curve analysis. The diagnostic performances of each test for the assessment of significant fibrosis, advanced fibrosis and cirrhosis are described as the areas under the receiver operating characteristics curve (AUROCs) with 95% confidence intervals (95%CIs) and the diagnostic accuracy. All P-values were 2-sided, and any value of P < 0.05 was considered statistically significant. The data analysis was performed using SPSS, version 22.0 (SPSS Inc., Chicago, IL, United States) and the GraphPad Software, version 7.0 (GraphPad Prism Inc., San Diego, CA, United States).
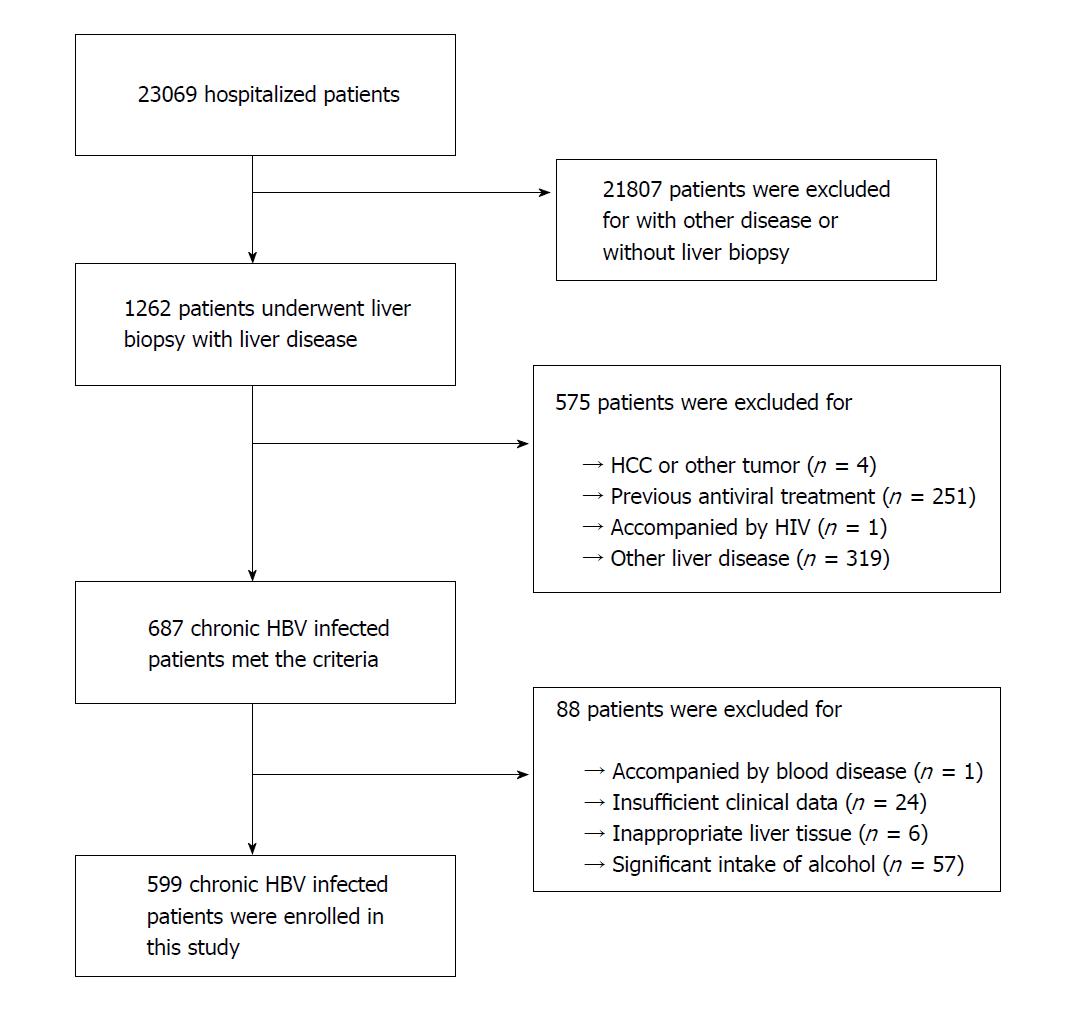
From January 2012 to July 2017, 1262 biopsy-proven patients with liver disease were assessed in the study. Among them, 575 patients were excluded according to the exclusion criteria, and 88 patients were excluded because of insufficient liver tissue and clinical data. Finally, 599 CHB patients were included in the cohort (Figure 1). The median (IQR) age of the patients was 37 (29-44) years, and 349 (58.3%) patients were male. In all, 96 (16%) patients had significant fibrosis (≥ S2), 54 (9%) had advanced fibrosis (≥ S3), and 38 (6.3%) had cirrhosis (S4). The clinical parameters and stages of fibrosis are shown in Table 1.
| Characteristics | Total group(n = 599) | ALT ≤ 40 group (n = 272) | 40 < ALT ≤ 80group (n = 190) | ALT > 80 group(n = 137) | P-value |
| Age (yr) | 37 (29, 44) | 39 (31.25, 46) | 37.5 (28, 43.25) | 34 (28, 40.5) | < 0.001 |
| Male (%) | 349 (58.3) | 131 (48.2) | 130 (68.4) | 88 (64.2) | < 0.001 |
| ALT (U/L) | 44 (28, 76) | 26 (31, 33) | 56 (48, 66) | 127 (95, 218.5) | < 0.001 |
| AST (U/L) | 32 (24, 48) | 24 (20, 28) | 36 (31, 42) | 70 (55, 115.5) | < 0.001 |
| GGT (U/L) | 25 (17, 41) | 19 (14, 25) | 30 (20, 41.25) | 48 (30, 78) | < 0.001 |
| Cholesterol (mmol/L) | 4.31 (3.84, 4.96) | 4.35 (3.86, 4.95) | 4.24 (3.75, 4.95) | 4.34 (3.84, 4.99) | 0.732 |
| PLT (109/L) | 182 (150, 217) | 184 (155, 223) | 177.5 (145.5, 212) | 180 (149, 215) | 0.215 |
| INR | 1 (1, 1.1) | 1 (1, 1.1) | 1 (0.99, 1.1) | 1 (0.94, 1.1) | 0.4 |
| HBV DNA (log10IU/mL) | 7.03 (4.24, 8.23) | 4.77 (3.32, 7.78) | 7.64 (5.72, 8.23) | 8.03 (6.81, 8.23) | < 0.001 |
| S (%) | |||||
| S0 | 264 (44.1) | 128 (47.1) | 80 (42.1) | 56 (40.9) | |
| S1 | 147 (24.5) | 73 (26.8) | 53 (27.9) | 21 (15.3) | |
| S2 | 96 (16) | 35 (12.9) | 29 (15.3) | 32 (23.4) | |
| S3 | 54 (9) | 19 (7.0) | 18 (9.5) | 17 (12.4) | |
| S4 | 38 (6.3) | 17 (6.3) | 10 (5.3) | 11 (8.0) | |
| Stage S0-1 fibrosis | 411 (68.6) | 201 (73.9) | 133 (70) | 77 (56.2) | 0.001 |
| Stage S2-4 fibrosis | 188 (31.4) | 71 (26.1) | 57 (30) | 60 (43.8) | |
| APRI | 0.53 (0.38,0.86) | 0.38 (0.3, 0.49) | 0.61 (0.44,0.8) | 1.16 (0.88, 1.93) | < 0.001 |
| FIB-4 | 1.02 (0.72,1.47) | 1.02 (0.72,1.37) | 0.96 (0.66,1.55) | 1.22 (0.82, 1.79) | < 0.001 |
| GPR | 0.22 (0.15,0.38) | 0.16 (0.11, 0.23) | 0.24 (0.17,0.38) | 0.42 (0.25, 0.68) | < 0.001 |
| King’s score | 6.77 (4.31,11.49) | 4.89 (3.54,7.03) | 7.18 (4.58,11.58) | 13.04 (9.81, 27.15) | < 0.001 |
| Forns index | 6.48 (5.44,7.51) | 6.33 (5.37,7.37) | 6.57 (5.51,7.69) | 6.65 (5.57, 7.57) | 0.081 |
To detect the effect of ALT on non-invasive fibrosis tests, the patients were divided into the following three groups: normal ALT (ALT ≤ 40), slightly elevated ALT (40 < ALT ≤ 80) and elevated ALT (ALT > 80). The baselines for these three groups are shown in Table 1.
The ALT levels were significantly correlated with AST (r = 0.878), GGT (r = 0.565), HBV DNA (r = 0.363) and HBsAg (r = 0.137) (P < 0.05 for all). Significant negative associations were also found between the ALT level and age (r = -0.206) and male sex (r = -0.195) (P < 0.05 for all). Other clinical factors, including cholesterol, INR and PLT, had no association with the ALT level (P > 0.05 for all).
In addition, the ALT levels were positively correlated with the fibrosis stage (r = 0.141), APRI (r = 0.762), GPR (r = 0.545), King’s score (r = 0.615), FIB-4 (r = 0.125) and Forns index (r = 0.107) (P < 0.05 for all). Increasing ALT levels were significantly associated with an increased fibrosis stage (P < 0.05).
The patients with ALT > 80 had the highest fibrosis scores, whereas the lowest fibrosis scores were observed for patients with ALT ≤ 40. In general, CHB patients with higher ALT levels had significantly higher fibrosis scores on the non-invasive tests (P < 0.05 for all) except for the Forns index (P = 0.081) (Table 1). Moreover, the patients with higher ALT levels showed significantly higher fibrosis scores on the non-invasive tests than those with lower ALT levels at the same stage of liver fibrosis (P < 0.05 for all) except for Forns index and FIB-4 at S1, S2 and S4 (Table 2).
| Characteristics | APRI | FIB-4 | GPR | King’s score | Forns index |
| S0 (n) | |||||
| ALT ≤ 40 (128) 40 < ALT ≤ 80 (80) ALT > 80 (56) P-value | 0.37 (0.26-0.44) 0.51 (0.39-0.68) 1.02 (0.85-1.5) < 0.001 | 0.88 (0.65-1.26) 0.77 (0.57-1.15) 0.97 (0.74-1.52) 0.019 | 0.15 (0.1-0.2) 0.22 (0.14-0.31) 0.29 (0.22-0.48) < 0.001 | 4.3 (3.09-5.89) 5.91 (4.1-8.08) 11.62 (7.99-18.36) < 0.001 | 6.06 (5.04-7.03) 6.16 (4.91-7.04) 6.27 (5.36-7.26) 0.5 |
| S1 (n) | |||||
| ALT ≤ 40 (73) 40 < ALT ≤ 80 (53) ALT > 80 (21) P-value | 0.36 (0.3-0.44) 0.52 (0.42-0.72) 0.94 (0.65-1.68) < 0.001 | 0.92 (0.7-1.29) 0.82 (0.58-1.28) 1.1 (0.85-1.36) 0.177 | 0.13 (0.1-0.19) 0.23 (0.19-0.32) 0.55 (0.23-0.89) < 0.001 | 4.59 (3.54-6.42) 6.55 (4.11-9.67) 11.7 (8.58-21.25) < 0.001 | 5.9 (5.24-6.99) 6.04 (5.34-7.32) 6.95 (6.06-7.54) 0.098 |
| S2 (n) | |||||
| ALT ≤ 40 (35) 40 < ALT ≤ 80 (29) ALT > 80 (32) P-value | 0.44 (0.37-0.58) 0.76 (0.66-0.93) 1.32 (0.87-2.15) < 0.001 | 1.2 (0.8-1.54) 1.34 (0.98-1.83) 1.24 (0.82-1.73) 0.472 | 0.2 (0.12-0.34) 0.32 (0.21-0.54) 0.45 (0.25-1.09) < 0.001 | 5.88 (4.26-9.25) 10.9 (7.6-13.85) 12.42 (9.95-28.94) < 0.001 | 6.79 (5.97-8.06) 7.55 (6.41-8.1) 6.5 (5.41-7.67) 0.111 |
| S3 (n) | |||||
| ALT ≤ 40 (19) 40 < ALT ≤ 80 (18) ALT > 80 (17) P-value | 0.49 (0.38-0.74) 0.81 (0.73-0.98) 1.95 (1.35-5.32) < 0.001 | 1.2 (0.9-1.62) 1.57 (1.18-2.11) 2.23 (1.47-2.81) 0.023 | 0.34 (0.17-0.44) 0.46 (0.22-0.57) 0.89 (0.46-1.57) 0.001 | 7.28 (4.44-9.28) 11.8 (9.59-16.76) 28.37 (18.46-48.99) < 0.001 | 7.48 (6.4-8.01) 7.99 (7.01-8.79) 7.38 (6.32-9.15) 0.538 |
| S4 (n) | |||||
| ALT ≤ 40 (17) 40 < ALT ≤ 80 (10) ALT > 80 (11) P-value | 0.57 (0.49-0.73) 0.67 (0.6-0.85) 1.95 (1.09-3.37) < 0.001 | 1.78 (1.24-2.49) 1.31 (1.09-1.45) 1.82 (0.92-3.3) 0.168 | 0.35 (0.18-0.58) 0.4 (0.29-0.5) 0.55 (0.46-0.94) 0.019 | 9.48 (6.15-14.1) 8.96 (8.26-11.96) 27.56 (12.86-45.31) 0.001 | 8.4 (6.35-9.3) 7.67 (6.71-8.15) 7.48 (5.77-9.11) 0.617 |
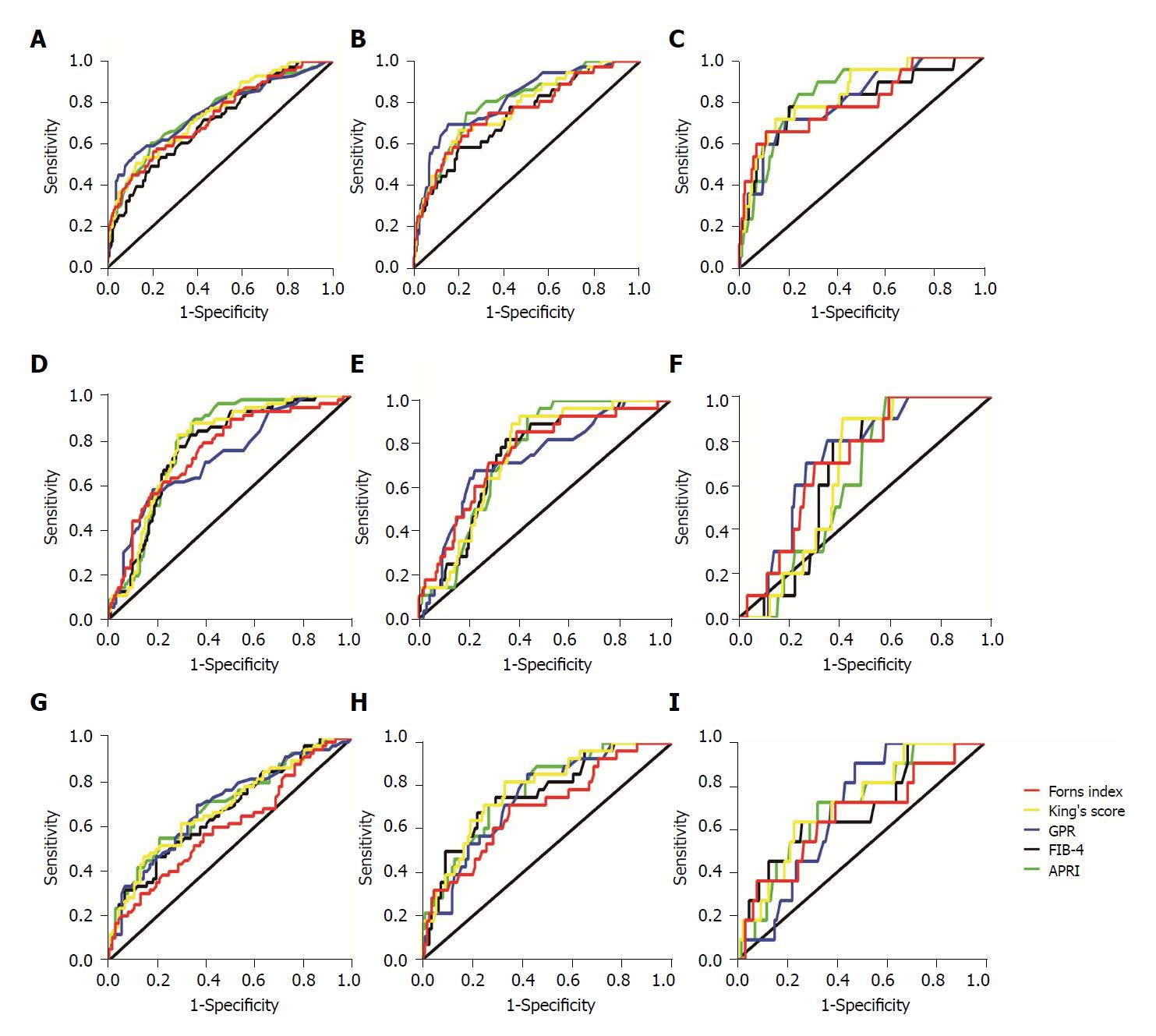
A summary of the diagnostic performances of these non-invasive tests, including the AUROCs, 95%CIs and P-values in the different ALT groups for the prediction of significant fibrosis, advanced fibrosis and cirrhosis, are shown in Table 3 and Figure 2.
| Tests | ALT ≤ 40 | 40 < ALT ≤ 80 | ALT > 80 | ||||||
| ≥ S2 | ≥ S3 | S4 | ≥ S2 | ≥ S3 | S4 | ≥ S2 | ≥ S3 | S4 | |
| APRI | |||||||||
| AUROC 95%CI P-value Diagnostic accuracy (optimized cut off) | 0.746 0.676-0.815 < 0.001 75.74% (≤ 0.5) 73.90% (> 1.5) | 0.795 0.716-0.873 < 0.001 - | 0.833 0.744-0.922 < 0.001 93.01% (< 1.0) 93.75% (≥ 2.0) | 0.79 0.727-0.854 < 0.001 62.63% (≤ 0.5) 72.63% (> 1.5) | 0.751 0.678-0.824 < 0.001 - | 0.626 0.519-0.732 0.182 83.68% (< 1.0) 94.21% (≥ 2.0) | 0.701 0.612-0.789 < 0.001 45.99% (≤ 0.5) 66.42% (> 1.5) | 0.778 0.688-0.868 < 0.001 - | 0.71 0.568-0.851 0.021 42.34% (< 1.0) 77.37% (≥ 2.0) |
| FIB-4 | |||||||||
| AUROC 95%CI P-value Diagnostic accuracy (optimized cut off) | 0.705 0.635-0.774 < 0.001 - | 0.736 0.647-0.825 < 0.001 78.68% (< 1.45) 87.13% (> 3.25) | 0.792 0.665-0.919 < 0.001 - | 0.767 0.699-0.836 < 0.001 - | 0.747 0.666-0.829 < 0.001 72.63% (< 1.45) 85.79% (> 3.25) | 0.663 0.563-0.763 0.083 - | 0.672 0.582-0.762 0.001 - | 0.765 0.668-0.863 < 0.001 72.26% (< 1.45) 81.02% (> 3.25) | 0.698 0.533-0.862 0.03 - |
| GPR | |||||||||
| AUROC 95%CI P-value Diagnostic accuracy (optimized cut off) | 0.755 0.684-0.826 < 0.001 81.62% (≥ 0.32) | 0.814 0.737-0.892 < 0.001 87.13% (≥ 0.32) | 0.799 0.69-0.907 < 0.001 93.01% (≥ 0.56) | 0.726 0.648-0.803 < 0.001 71.05% (≥ 0.32) | 0.732 0.633-0.83 < 0.001 70.53% (≥ 0.32) | 0.723 0.607-0.839 0.018 84.74% (≥ 0.56) | 0.696 0.607-00.785 < 0.001 61.31% (≥ 0.32) | 0.748 0.654-0.841 < 0.001 52.55% (≥ 0.32) | 0.693 0.58-0.807 0.034 65.69% (≥ 0.56) |
| King’s score | |||||||||
| AUROC 95%CI P-value Diagnostic accuracy (optimized cut off) | 0.75 0.685-0.816 < 0.001 77.21% (≥ 12.3) | 0.773 0.689-0.857 < 0.001 - | 0.831 0.733-0.929 < 0.001 94.44% (> 16.7) | 0.781 0.715-0.847 < 0.001 72.11% (≥ 12.3) | 0.751 0.672-0.83 < 0.001 - | 0.659 0.56-0.759 0.09 87.37% (> 16.7) | 0.69 0.601-0.779 0.001 43.80% (≥ 12.3) | 0.784 0.694-0.874 < 0.001 - | 0.719 0.577-0.862 0.016 61.31% (> 16.7) |
| Forns index | |||||||||
| AUROC 95%CI P-value Diagnostic accuracy (optimized cut off) | 0.731 0.661-0.8 < 0.001 33.09% (< 4.2) 68.75% (> 6.9) | 0.758 0.668-0.849 < 0.001 - | 0.79 0.665-0.915 < 0.001 - | 0.752 0.677-0.827 < 0.001 35.79% (< 4.2) 66.84% (> 6.9) | 0.754 0.66-0.848 < 0.001 - | 0.708 0.587-0.829 0.027 - | 0.604 0.508-0.7 0.038 46.72% (< 4.2) 58.39% (> 6.9) | 0.7 0.588-0.81 0.001 - | 0.662 0.485-0.838 0.076 - |
Generally, the AUROCs of the non-invasive tests for the prediction of ≥ S2 in CHB patients with ALT ≤ 40 U/L (range 0.705-0.755) and 40 < ALT ≤ 80 U/L (range 0.726-0.79) were higher than those in patients with ALT > 80 U/L (range 0.604-0.701). For the ≥ S3 and S4 predictions, the AUROCs of the non-invasive tests in CHB patients with ALT ≤ 40 U/L (range 0.736-0.814 for ≥ S3, range 0.79-0.833 for S4) were higher than those in patients with 40 < ALT ≤ 80 U/L (range 0.732-0.754 for ≥ S3, range 0.626-0.723 for S4) and ALT > 80 U/L (range 0.7-0.784 for ≥ S3, range 0.662-0.719 for S4) (Table 3).
However, evaluating the effect of ALT on non-invasive fibrosis tests based only on one AUROC from one stage is difficult, and a more comprehensive and systematic assessment is greatly need. Thus, a grading system based on AUROCs to evaluate the effect of ALT on the prediction of liver fibrosis (≥ S2, ≥ S3 and S4) at different ALT levels was developed (Table 4). According to the AUROC grading system, non-invasive fibrosis tests were grade A in CHB patients with ALT ≤ 40 U/L and grade B in patients with 40 < ALT ≤ 80 U/L and ALT > 80 U/L. Although the non-invasive tests in patients with 40 < ALT ≤ 80 U/L and ALT > 80 U/L were both grade B, the grading points in the patients with 40 < ALT ≤ 80 U/L were higher than those in the patients with ALT > 80 U/L (Table 4). Above all, the grading points of the AUROCs decreased in a stepwise manner with the increase in the serum ALT level.
| ALT groups | AUROC | Points | Grade | ||||
| ≥ 0.8 | 0.750-0.800 | 0.700-0.750 | 0.650-0.70 | < 0.65 | |||
| ALT ≤ 40 | GPR (≥ S3) | APRI (≥ S3) | APRI (≥ S2) | - | - | 22 | A |
| APRI (S4) | FIB-4 (S4) | FIB-4 (≥ S2) | |||||
| King’s score (S4) | GPR (≥ S2) | FIB-4 (≥ S3) | |||||
| GPR (S4) | Forns index (≥ S2) | ||||||
| King’s score (≥ S2) | |||||||
| King’s score (≥ S3) | |||||||
| Forns index (≥ S3) | |||||||
| Forns index (S4) | |||||||
| 40 < ALT ≤ 80 | - | APRI (≥ S2) | FIB-4 (≥ S3) | FIB-4 (S4) | APRI (S4) | 16.5 | B |
| APRI (≥ S3) | GPR (≥ S2) | King’s score (S4) | |||||
| FIB-4 (≥ S2) | GPR (≥ S3) | ||||||
| King’s score (≥ S2) | GPR (S4) | ||||||
| King’s score (≥ S3) | Forns index (S4) | ||||||
| Forns index (≥ S2) | |||||||
| Forns index (≥ S3) | |||||||
| ALT > 80 | - | APRI (≥ S3) | APRI (≥ S2) | FIB-4 (≥ S2) | Forns index (≥ S2) | 12.5 | B |
| King’s score (≥ S3) | APRI (S4) | FIB-4 (S4) | |||||
| FIB-4 (≥ S3) | GPR (≥ S3) | GPR (≥ S2) | |||||
| King’s score (S4) | GPR (S4) | ||||||
| Forns index (≥ S3) | King’s score (≥ S2) | ||||||
| Forns index (S4) | |||||||
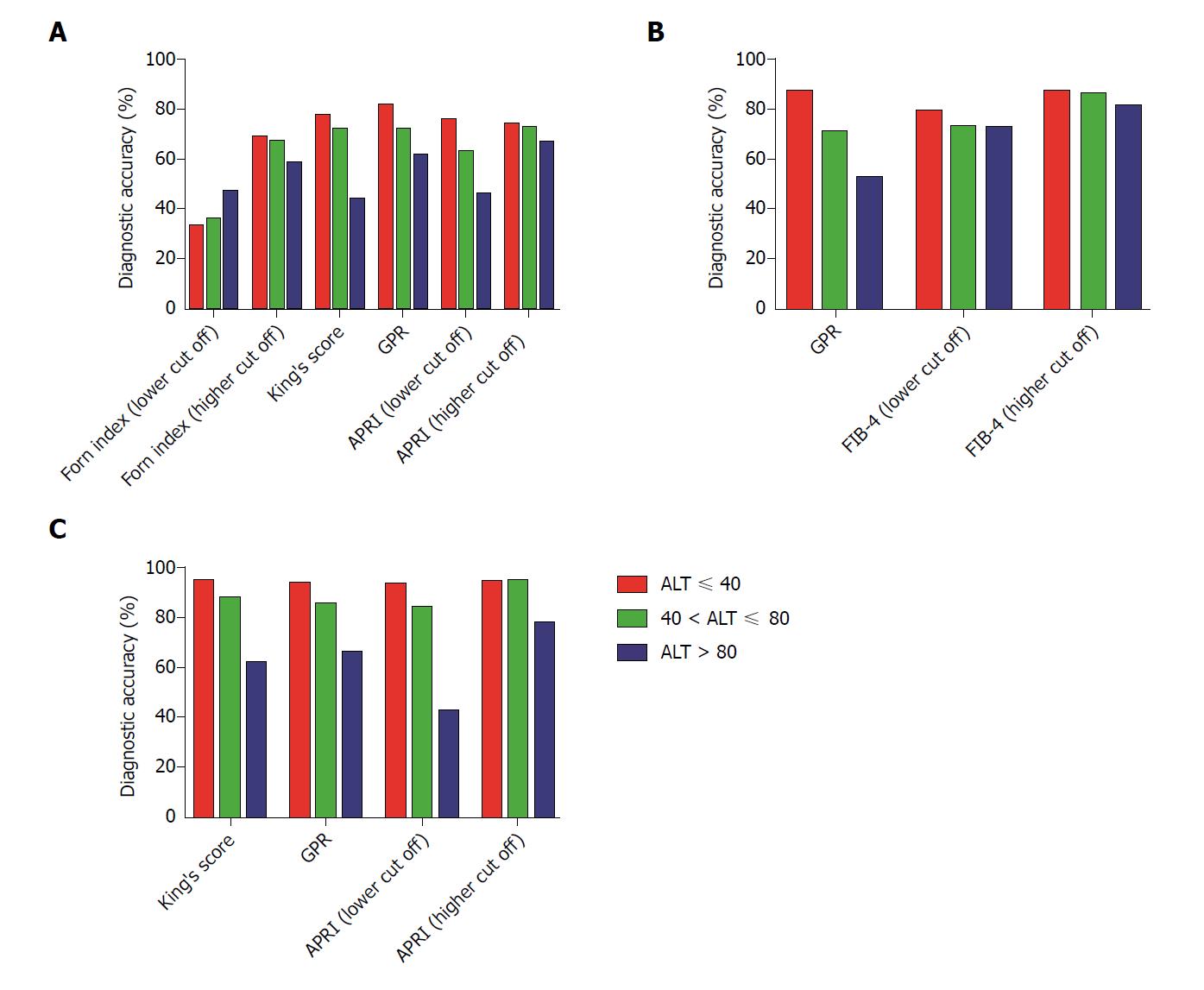
The relationship between the diagnostic accuracy and serum ALT was also evaluated using the cut off value as the original threshold. For the ≥ S2 prediction, the diagnostic accuracies of the APRI (lower cut off value 0.5), APRI (higher cut off value 1.5), Forns index (lower cut off value 4.2), Forns index (higher cut off value 6.9), GPR and King’s score were 75.74%, 73.90%, 33.09%, 68.75%, 81.62% and 77.21%, respectively, for patients with ALT ≤ 40 U/L; 62.63%,72.63%, 35.79%, 66.84%, 71.05% and 72.11%, respectively, for patients with 40 < ALT ≤ 80 U/L; and 45.99%, 66.42%, 46.72%, 58.39%, 61.31% and 43.80%, respectively, for patients with ALT > 80 U/L.
For the ≥ S3 prediction, the diagnostic accuracies of GPR, FIB-4 (lower cut off value 1.45) and FIB-4 (higher cut off value 3.25) were 87.13%, 78.68% and 87.13% for patients with ALT ≤ 40 U/L, 70.53%, 72.63% and 85.79%, respectively, for patients with 40 < ALT ≤ 80 U/L and were 52.55%, 72.26% and 81.02%, respectively, for patients with ALT > 80 U/L.
For the S4 prediction, the diagnostic accuracies of APRI (lower cut off value 1.0), APRI (higher cut off value 2.0) GPR and King’s score wered 93.01%, 93.75%, 93.01% and 94.44%, respectively, for patients with ALT ≤ 40 U/L; 83.68%, 94.21%, 84.74% and 87.37%, respectively, for patients with 40 < ALT ≤ 80 U/L; and 42.34%, 77.37%, 65.69% and 61.31%, respectively, for patients with ALT > 80 U/L.
The above results indicate that the non-invasive tests for the prediction of liver fibrosis (≥ S2, ≥ S3 and S4) exhibited the highest diagnostic accuracy for patients in the ALT ≤ 40 U/L group and the lowest diagnostic accuracy for patients in the ALT > 80 U/L group, except for the Forns index (using the lower cut off values) for the ≥ S2 prediction and APRI (using the higher cut off values) for the S4 prediction (Table 3 and Figure 3). The diagnostic accuracy of the non-invasive tests decreased in a stepwise manner with the increase in the serum ALT level.
In this study, we evaluated the diagnostic performances of five convenient non-invasive tests for liver fibrosis among a large cohort of treatment-naive CHB patients with different ALT levels. To the best of our knowledge, this study is the first to thoroughly analyze the effect of ALT on the diagnostic performance and accuracy of non-invasive tests in CHB patients. The results showed that elevated serum ALT levels negatively affected the diagnostic performance of the non-invasive fibrosis tests. These non-invasive fibrosis tests showed a better diagnostic accuracy in CHB patients with normal ALT levels than in patients with abnormal ALT levels.
One important finding of our study was that CHB patients with higher ALT levels showed significantly higher fibrosis scores in the non-invasive tests even at the same liver fibrosis stage. The result demonstrated that non-invasive tests could overestimate liver fibrosis in some CHB patients with elevated ALT. In other words, patients who had elevated ALT with no or mild fibrosis would be considered to have significant fibrosis, advanced fibrosis or cirrhosis. For example, the GPR, which had the highest AUROCs for the non-invasive test to predict cirrhosis in the study, showed that 6/272 (2.21%) CHB patients with normal ALT, 19/190 (10%) patients with slightly elevated ALT and 41/137 (29.9%) patients with elevated ALT would be misdiagnosed (i.e., approximately 10% of CHB patients with slightly elevated ALT and 30% of patients with elevated ALT would be misclassified as having cirrhosis). ALT flares often occur during the progression of CHB, especially for patients with acute or active hepatitis. Thus, the effect of ALT should be considered when assessing liver fibrosis using non-invasive tests.
To detect the effect of ALT on non-invasive tests, we compared the diagnostic performance and accuracy of the non-invasive tests in different ALT groups. In this study, most AUROCs (11/15) for prediction of ≥ S2, ≥ S3 or S4 by the non-invasive tests were greater than 0.75 in patients with normal ALT but less than 0.75 (12/15) in patients with elevated ALT. Thus, the tests showed a slightly higher performance for the CHB patients with normal ALT than for the patients with elevated ALT.
To further examine the effect of ALT on the non-invasive tests, an overall analysis of AUROCs should be conducted in patients with different ALT levels. However, the performance of AUROCs varies among different fibrosis tests, etiologies and cohorts[10,11]. Therefore, evaluating the diagnostic performance of non-invasive fibrosis tests using one AUROC from one stage is difficult. A simple, comprehensive and systematic assessment is more reasonable and acceptable; thus, the AUROC grading system for the prediction of liver fibrosis was developed. In this study, we comprehensively evaluated the effect of ALT on five common non-invasive fibrosis tests based on the AUROC grading system. The ability of the AUROCs to predict liver fibrosis decreased with the increase in the ALT level. Additionally, studies have reported that elevated ALT can influence the diagnostic performances of non-invasive fibrosis tests, although most of these studies have focused on ≥ S2 or S4[18-20]. In our study, the effect of ALT on the AUROCs of the non-invasive fibrosis tests among three fibrosis stages (≥ S2, ≥ S3 and S4) were all included. The AUROCs for prediction of ≥ S2, ≥ S3 and S4 were more stable in patients with ALT ≤ 40 U/L.
Similarly, the best diagnostic accuracy of the non-invasive tests was found in patients with a normal ALT level, whereas the accuracy was lowest in patients with elevated ALT. The diagnostic accuracy of these non-invasive tests decreased in a stepwise manner with the increase in the ALT level. In patients with normal ALT, the AUROCs of these non-invasive tests for the diagnosis of cirrhosis ranged from 0.79 to 0.833 with a diagnostic accuracy range from 93.01% to 94.44% when the cut off value was the original threshold. However, for CHB patients with elevated ALT, the AUROCs of these non-invasive tests for the diagnosis of cirrhosis ranged from 0.662 to 0.719 with a diagnostic accuracy range from 42.34% to 77.37% when the cut off value was the original threshold. From the above results, we can infer that the diagnostic performances of the non-invasive tests were less reliable in CHB patients with elevated ALT levels.
The phenomenon may contribute to liver inflammation. Liver fibrosis is the process by which damaged hepatocytes are repaired and is a product of dysregulated inflammation in chronic viral hepatitis[21]. Liver inflammation can influence LS[14]. Moreover, systemic inflammation can serve as a degenerating factor for intrahepatic hypertension in cirrhosis[22]. The serum ALT level is the most direct and valuable marker that reflects liver inflammation in the clinic. To some extent, more severe liver inflammation is associated with greater ALT elevation[23], which has more impact on the non-invasive tests. This phenomenon may be the main reason why the non-invasive tests showed better diagnostic performances in patients with normal ALT.
This study had several limitations. First, this study was a single-center retrospective study. A multicenter study will be performed in the future. Second, we compared only some of the more convenient non-invasive diagnostic tests. Other non-invasive diagnostic tests and LS measurements will be evaluated in future studies.
In conclusion, the ALT level has a significant effect on the diagnostic performance and accuracy of non-invasive fibrosis tests. The ALT level should be considered before performing these non-invasive tests.
Because of their high applicability and good interlaboratory reproducibility, many convenient non-invasive fibrosis tests have been established. Many studies have reported the influence of alanine aminotransferase (ALT) levels on liver stiffness (LS) measurements. However, no report has investigated the effects of changes in the serum ALT levels of chronic hepatitis B (CHB) patients on the diagnostic performances of these non-invasive tests.
To explore the effect of serum ALT on the diagnostic performances of non-invasive fibrosis tests in CHB patients.
A total of 599 treatment-naive and biopsy-proven CHB patients were included in the study. The cohort was divided into the following three groups: normal ALT (ALT ≤ 40), slightly elevated ALT (40 < ALT ≤ 80) and elevated ALT (ALT > 80). The diagnostic performances of five common non-invasive fibrosis tests for liver fibrosis (stages S2-4), including the aminotransferase (AST)-to-platelet (PLT) ratio index (APRI), fibrosis index based on 4 factors (FIB-4), King’s score, Forns index and gamma-glutamyl transpeptidase (GGT)-to-PLT ratio (GPR), were evaluated for each group.
Higher ALT levels were associated with higher non-invasive test scores for the prediction of liver fibrosis. Patients with the same fibrosis stage but higher ALT levels showed higher non-invasive test scores. The areas under the receiver operating characteristics curves (AUROCs) of the non-invasive tests for the ≥ S2 prediction were higher in patients with ALT ≤ 40 U/L (range 0.705-0.755) and 40 < ALT ≤ 80 U/L (range 0.726-0.79) than in patients with ALT > 80 U/L (range 0.604-0.701). The AUROCs for the ≥ S3 and S4 predictions were higher in patients with ALT ≤ 40 U/L (range 0.736-0.814 for ≥ S3, 0.79-0.833 for S4) than in patients with 40 < ALT ≤ 80 U/L (range 0.732-0.754 for ≥ S3, range 0.626-0.723 for S4) and ALT > 80 U/L (range 0.7-0.784 for ≥ S3, range 0.662-0.719 for S4). The diagnostic accuracy of the non-invasive fibrosis tests decreased in a stepwise manner with the increase ALT level.
ALT has a significant effect on the diagnostic performances of non-invasive fibrosis tests.
The ALT level should be considered before performing these non-invasive tests.
The authors thank their colleagues at the Department of Infectious Diseases, Shengjing Hospital of China Medical University, for their assistance with the completion of this study.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen GX, Hann HW, Inoue K S- Editor: Ji FF L- Editor: A E- Editor: Tan WW
| 1. | Rockey DC, Caldwell SH, Goodman ZD, Nelson RC, Smith AD; American Association for the Study of Liver Diseases. Liver biopsy. Hepatology. 2009;49:1017-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1449] [Cited by in RCA: 1580] [Article Influence: 98.8] [Reference Citation Analysis (1)] |
| 2. | Trépo C, Chan HL, Lok A. Hepatitis B virus infection. Lancet. 2014;384:2053-2063. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1004] [Cited by in RCA: 1157] [Article Influence: 105.2] [Reference Citation Analysis (0)] |
| 3. | European Association for Study of Liver; Asociacion Latinoamericana para el Estudio del Higado. EASL-ALEH Clinical Practice Guidelines: Non-invasive tests for evaluation of liver disease severity and prognosis. J Hepatol. 2015;63:237-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1177] [Cited by in RCA: 1332] [Article Influence: 133.2] [Reference Citation Analysis (0)] |
| 4. | Wai CT, Greenson JK, Fontana RJ, Kalbfleisch JD, Marrero JA, Conjeevaram HS, Lok AS. A simple noninvasive index can predict both significant fibrosis and cirrhosis in patients with chronic hepatitis C. Hepatology. 2003;38:518-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2762] [Cited by in RCA: 3245] [Article Influence: 147.5] [Reference Citation Analysis (0)] |
| 5. | Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: an inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32-36. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1288] [Cited by in RCA: 1609] [Article Influence: 89.4] [Reference Citation Analysis (0)] |
| 6. | World Health Organization. Guidelines for the prevention, care and treatment of persons with chronic hepatitis B infection. Geneva: World Health Organization 2015; Available from: http://www.who.int/hiv/pub/hepatitis/hepatitis-b-guidelines/en/. |
| 7. | Kim WR, Berg T, Asselah T, Flisiak R, Fung S, Gordon SC, Janssen HL, Lampertico P, Lau D, Bornstein JD. Evaluation of APRI and FIB-4 scoring systems for non-invasive assessment of hepatic fibrosis in chronic hepatitis B patients. J Hepatol. 2016;64:773-780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 170] [Cited by in RCA: 224] [Article Influence: 24.9] [Reference Citation Analysis (0)] |
| 8. | Teshale E, Lu M, Rupp LB, Holmberg SD, Moorman AC, Spradling P, Vijayadeva V, Boscarino JA, Schmidt MA, Gordon SC; CHeCS Investigators. APRI and FIB-4 are good predictors of the stage of liver fibrosis in chronic hepatitis B: the Chronic Hepatitis Cohort Study (CHeCS). J Viral Hepat. 2014;21:917-920. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 82] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 9. | Li Q, Ren X, Lu C, Li W, Huang Y, Chen L. Evaluation of APRI and FIB-4 for noninvasive assessment of significant fibrosis and cirrhosis in HBeAg-negative CHB patients with ALT ≤ 2 ULN: A retrospective cohort study. Medicine (Baltimore). 2017;96:e6336. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 10. | Lemoine M, Shimakawa Y, Nayagam S, Khalil M, Suso P, Lloyd J, Goldin R, Njai HF, Ndow G, Taal M. The gamma-glutamyl transpeptidase to platelet ratio (GPR) predicts significant liver fibrosis and cirrhosis in patients with chronic HBV infection in West Africa. Gut. 2016;65:1369-1376. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 210] [Cited by in RCA: 276] [Article Influence: 30.7] [Reference Citation Analysis (1)] |
| 11. | Schiavon LL, Narciso-Schiavon JL, Ferraz MLG, Silva AEB, Carvalho-Filho RJ. The γ-glutamyl transpeptidase to platelet ratio (GPR) in HBV patients: just adding up? Gut. 2017;66:1169-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 12. | Huang R, Wang G, Tian C, Liu Y, Jia B, Wang J, Yang Y, Li Y, Sun Z, Yan X. Gamma-glutamyl-transpeptidase to platelet ratio is not superior to APRI,FIB-4 and RPR for diagnosing liver fibrosis in CHB patients in China. Sci Rep. 2017;7:8543. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 13. | Wong GL, Wong VW, Choi PC, Chan AW, Chum RH, Chan HK, Lau KK, Chim AM, Yiu KK, Chan FK. Assessment of fibrosis by transient elastography compared with liver biopsy and morphometry in chronic liver diseases. Clin Gastroenterol Hepatol. 2008;6:1027-1035. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 14. | Raizner A, Shillingford N, Mitchell PD, Harney S, Raza R, Serino J, Jonas MM, Lee CK. Hepatic Inflammation May Influence Liver Stiffness Measurements by Transient Elastography in Children and Young Adults. J Pediatr Gastroenterol Nutr. 2017;64:512-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 15. | Scheuer PJ. Classification of chronic viral hepatitis: a need for reassessment. J Hepatol. 1991;13:372-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1130] [Cited by in RCA: 1198] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
| 16. | Forns X, Ampurdanès S, Llovet JM, Aponte J, Quintó L, Martínez-Bauer E, Bruguera M, Sánchez-Tapias JM, Rodés J. Identification of chronic hepatitis C patients without hepatic fibrosis by a simple predictive model. Hepatology. 2002;36:986-992. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 672] [Cited by in RCA: 721] [Article Influence: 31.3] [Reference Citation Analysis (0)] |
| 17. | Cross TJ, Rizzi P, Berry PA, Bruce M, Portmann B, Harrison PM. King’s Score: an accurate marker of cirrhosis in chronic hepatitis C. Eur J Gastroenterol Hepatol. 2009;21:730-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 18. | Jia J, Hou J, Ding H, Chen G, Xie Q, Wang Y, Zeng M, Zhao J, Wang T, Hu X. Transient elastography compared to serum markers to predict liver fibrosis in a cohort of Chinese patients with chronic hepatitis B. J Gastroenterol Hepatol. 2015;30:756-762. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 19. | Fung J, Lai CL, Cheng C, Wu R, Wong DK, Yuen MF. Mild-to-moderate elevation of alanine aminotransferase increases liver stiffness measurement by transient elastography in patients with chronic hepatitis B. Am J Gastroenterol. 2011;106:492-496. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 52] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 20. | Li Q, Chen L, Zhou Y. Diagnostic accuracy of liver stiffness measurement in chronic hepatitis B patients with normal or mildly elevated alanine transaminase levels. Sci Rep. 2018;8:5224. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 21. | Koyama Y, Brenner DA. Liver inflammation and fibrosis. J Clin Invest. 2017;127:55-64. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 548] [Cited by in RCA: 908] [Article Influence: 113.5] [Reference Citation Analysis (0)] |
| 22. | Mehta G, Mookerjee RP, Sharma V, Jalan R. Systemic inflammation is associated with increased intrahepatic resistance and mortality in alcohol-related acute-on-chronic liver failure. Liver Int. 2015;35:724-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 84] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 23. | Kim WR, Flamm SL, Di Bisceglie AM, Bodenheimer HC; Public Policy Committee of the American Association for the Study of Liver Disease. Serum activity of alanine aminotransferase (ALT) as an indicator of health and disease. Hepatology. 2008;47:1363-1370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 521] [Cited by in RCA: 585] [Article Influence: 34.4] [Reference Citation Analysis (0)] |