Published online Sep 26, 2018. doi: 10.12998/wjcc.v6.i10.393
Peer-review started: May 18, 2018
First decision: June 14, 2018
Revised: June 19, 2018
Accepted: June 28, 2018
Article in press: June 28, 2018
Published online: September 26, 2018
Processing time: 131 Days and 19.5 Hours
Gardner’s syndrome (GS) is a rare syndrome with autosomal dominant inheritance, which is characterized by multiple intestinal polyps, dental anomalies, desmoid tumors, and soft tissue tumors. All gastrointestinal symptoms seen in GS are associated with the underlying familial adenomatosis polyposis and abdominal desmoid tumors, with the most common symptoms being anemia, lower gastrointestinal bleeding, abdominal pain, diarrhea, obstruction, and mucous defecation. To our best knowledge, no case of GS that has presented with gastrointestinal perforation and bleeding has ever been reported in the English language medical literature. A 37-year-old male who had been diagnosed with GS five years earlier was referred to our clinic for lower gastrointestinal bleeding. Despite the absence of a bleeding focus on conventional angiography, the patient was operated on with laparotomy, due to the persistence of both signs and symptoms of mild peritonitis. On the laparotomy, the patient was noted to have areas of perforation in the duodenum, splenic flexura, and mid-rectum. The third and fourth part of the duodenum, the proximal 15 cm segment of the jejunum, a 10 cm segment of the terminal ileum, the whole colon, and the upper and middle rectum were resected, and duodeno-jejunal side-to-side anastomosis and terminal ileostomy were performed. The histopathological analysis of the large mass measuring 30 cm × 20 cm was reported as a desmoid tumor. The pathological examination of the tumor foci detected in the colonic specimen revealed poorly differentiated adenosquamous carcinoma.
Core tip: Gardner’s syndrome (GS) is a rare syndrome with autosomal dominant inheritance, which is characterized by multiple intestinal polyps, dental anomalies, desmoid tumors, and soft tissue tumors. All gastrointestinal symptoms that are seen in GS are associated with the underlying familial adenomatosis polyposis and abdominal desmoid tumors, with the most common symptoms being anemia, lower gastrointestinal bleeding, abdominal pain, diarrhea, obstruction, and mucous defecation. To our best knowledge, no case of GS that has presented with gastrointestinal perforation and bleeding has ever been reported in the English language medical literature. Herein, we report a complicated GS case that we managed for multiple intestinal perforation and massive gastrointestinal bleeding.
- Citation: Akbulut S, Koc C, Dirican A. Unusual complication in patient with Gardner’s syndrome: Coexistence of triple gastrointestinal perforation and lower gastrointestinal bleeding: A case report and review of literature. World J Clin Cases 2018; 6(10): 393-397
- URL: https://www.wjgnet.com/2307-8960/full/v6/i10/393.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i10.393
Gardner’s syndrome (GS) is a rare, autosomal dominant disorder characterized by gastrointestinal (colonic polyps, gastric polyps, duodenal polyps, and desmoid tumors) and extragastrointestinal (osteomas, dental abnormalities, epidermal cysts, fibromas, lipomas, pilomatricomas, congenital hypertrophies of retinal pigment epithelium, adrenal adenomas, and nasal angiofibromas) manifestations[1,2]. GS was first considered to be a variant of familial adenomatosis polyps (FAP). However, the detection of adenomatous polyposis coli (APC) gene mutations in families with GS, as well as the extracolonic manifestations of GS in patients with FAP, have shown that GS and FAP are relatively similar to one another. The gastrointestinal symptoms seen in GS are usually closely related to the underlying FAP and abdominal desmoid tumors. The most common gastrointestinal symptoms are nonspecific abdominal pain, weight loss, palpable abdominal masses, diarrhea, obstruction, mucous defecation, and rare lower gastrointestinal bleeding[1-3]. To our knowledge, no GS case presenting with gastrointestinal perforation and massive lower gastrointestinal bleeding has ever been reported in the English language medical literature. In this study, we report a complicated GS case that we managed for multiple intestinal perforation and massive gastrointestinal bleeding.
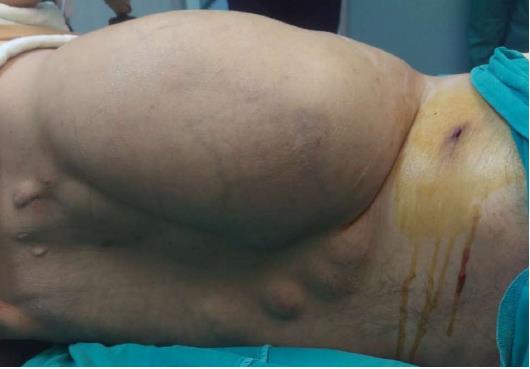
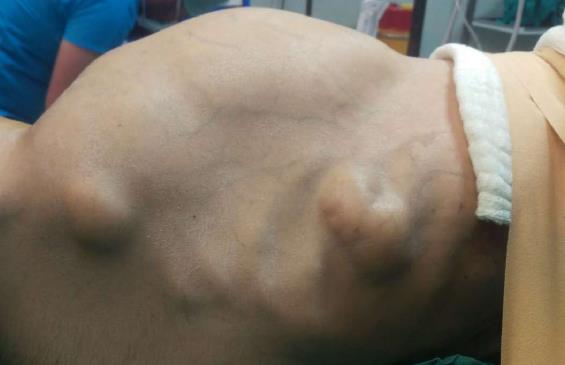
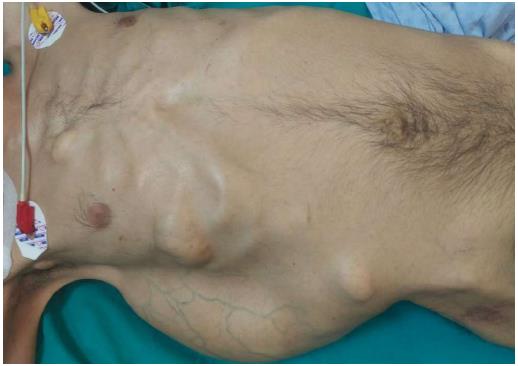
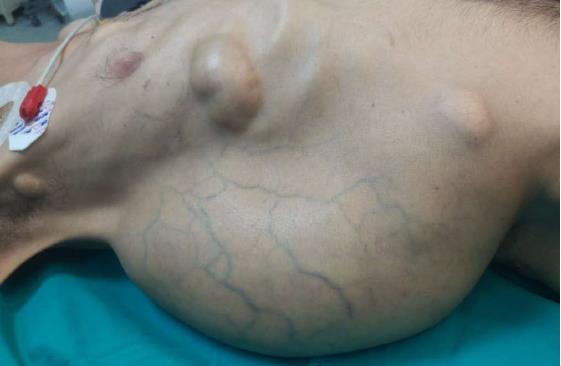
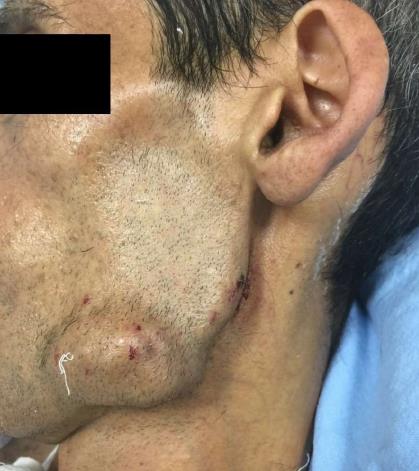
A 37-year-old man (BMI = 19 kg/m2) who had been diagnosed with GS five years earlier was referred to our center with lower gastrointestinal bleeding. The patient’s male sibling had died of colonic cancer, but we had no information as to whether he also had GS. This patient had been transfused with 12 units of whole blood suspension before being referred to our center, and had a final hemoglobin level of 8 g/dL, a creatinine value of 2.9 mg/dL, an albumin level of 2.1 g/dL and a total bilirubin level of 6.3 mg/dL. An endoscopic examination failed to detect any abnormalities, with the exception of duodenal polyposis. Colonoscopy was attempted, but no higher than the rectosigmoid region was reached due to bleeding. Upon physical examination, the patient was noted to have a mass with a size of 30 cm × 20 cm, which originated from the right flank region and was partially fixed to the abdominal wall. There were also multiple lesions (likely lipomas) with a size range between 3 cm - 7 cm on the abdomen, thoracic wall, and axilla (Figures 1-4). There was a mass lesion compatible with an osteoma in the left mandible (Figure 5). Conventional celiac and mesenteric angiographies that were performed to detect the hemorrhagic focus showed no extravasation that was indicative of bleeding. The patient’s hemodynamic and biochemical parameters improved, but since he had persistent signs of mild periotinits, a decision was made to proceed with surgery. Upon the request of the patient, the mass lesions on the abdominal and thoracic walls were the first to be excised. A midline incision was then performed, and approximately 500 cc of seropurulent fluid was drained. Upon exploration, there were perforated areas in the fourth part of the duodenum, the splenic flexura of the colon, and the middle part of the rectum. The perforations in the splenic flexura and middle part of the rectum were consistent with tumor perforation. Firstly, the resection included the proximal 15 cm section of the jejunum, as well as the third and fourth parts of the duodenum. Then, a side-to-side anastomosis was performed between the proximal jejunum and the second part of the duodenum. There were polypoid lesions detected in the mucosa from both of the anastomosed small intestinal segments. A tube was inserted from the distal jejunum to the proximal part of the anastomosis in order to protect the jejuno-duodenal anastomosis. After carrying out a total abdominal colectomy, which included 10 cm of distal ileum, the whole colon and the upper/middle part of the rectum, the distal rectal stump was closed and an end ileostomy was created. Additionally, multiple masses consistent with metastases were detected in both lobes of the liver. The patient developed a postoperative pneumothorax and a chest tube was inserted. The patient then developed a severe lung infection during the intensive care unit follow-up, and attempts to extubate him failed. A tracheostomy was therefore opened. Despite having no gastrointestinal problems, the patient died due to pulmonary complications. Histopathological examination revealed the presence of three foci of poorly-differentiated adenosquamous carcinoma in the duodenal serosa, the largest of which was 10 mm in diameter. These cancer foci were thought to have developed following diffuse lymphovascular invasion of the tumor into the splenic flexura. There were a total of 260 polypoid lesions in the duodenal mucosa, the largest of which measured 10 mm in diameter. Two foci of poorly-differentiated adenosquamous carcinoma (Pan-Cytokeratin: strongly positive, Cytokeratin 5/6: 40% positive, Ki67: 80% positive, Cytokeratin 20: negative, p53: negative, Synaptophysin: negative, CgA: negative) were present in the colon, the diameters of which were 80 mm and 30 mm. A total of 172 polypoid lesions were detected in the colonic and rectal mucosa, the largest of which had a diameter of 10 mm. Tumors were detected in both areas of colonic perforation. In the colon specimen, a tumor was detected in 5 of the 40 lymph nodes. The small lesions excised from the abdominal wall were reported to be epidermoid cysts. Pathological examination of the large mass that was excised from the right flank region was revealed to be a desmoid tumor (S100: negative, Desmin: focal positive, α-smooth muscle actin: positive, CD117: negative, DOG-1: negative, Ki67: 4%-5% positive).
In 1951, Gardner reported a significant association between colonic polyps with high malignant potential and some bone and soft tissue tumors[1]. In 1952, Gardner and his colleagues reported that the co-occurrence of multiple bone tumors and colonic polyps is an autosomal dominant condition[1]. In 1953, Gardner and his colleagues defined this syndrome, characterized by hereditary colonic polyposis that was associated with bone and soft tissue tumors, as GS[1]. The gastrointestinal components of GS are colorectal polyps, gastric polyps, small bowel polyps, and mesenteric desmoid tumors. The extragastrointestinal components of GS include osteomas, dental anomalies (unerupted teeth, supernumerary teeth, dentigerous cysts, and odontomas), skin and subcutaneous lesions (epidermal cysts, fibromas, lipomas, pilomatricomas), desmoid tumors of the abdominal wall, congenital hypertrophy of the retinal pigment epithelium, adrenal adenomas, and nasal angiofibromas. The incidence of cancers affecting extracolonic sites, such as the duodenum, periampullary region, thyroid, pancreas, stomach, central nervous system, liver, small bowel distal to the duodenum, and adrenal gland, is one or several folds higher than that of the general population.
In 80% of patients with GS, a hereditary mutation is found in the APC gene (5q21-5q22), a tumor suppressor gene located in the 21st and 22nd loci of the long arm of the fifth chromosome[1,2]. Twenty percent of GS cases occur as a result of sporadic, uninherited mutations in the APC gene. To date, around 1400 mutations have been detected in the APC gene[1]. Hence, the diversity of both gastrointestinal and extragastrointestinal manifestations of GS results from the variability of APC mutation penetration. The number of colonic polyps depends on the location of the APC mutation. Mutations occurring in the center of the APC gene result in about 5000 colonic polyps. When mutations affect the proximal or distal parts of the gene, however, approximately 1000 polyps develop in the colon. When mutations are found in the extreme ends of the APC gene, on the other hand, about 100 polyps develop, which is defined as attenuated FAP.
While GS and FAP incidences range between 0.62 and 2.38 per million people, their prevalence ranges between 0.88 and 46.5 per million people[4-6]. GS occurs equally in both sexes. The symptoms mostly appear in the second decade of life[1]. Bone and soft tissue tumors usually appear 10 years earlier than colonic polyps[1]. While polyps begin to appear at puberty, GS is not diagnosed until the third decade of life in the majority of affected people[1]. All untreated people with malignant GS transformations develop tumors in the fourth decade of life[1].
The gold standard for the diagnosis of GS and FAP is identifying the APC gene mutation[1]. This test is also very effective in revealing gene mutations in the relatives of GS patients[1]. However, the detection of either multiple polyps in a colonoscopy or bone and soft tissue tumors during a physical examination are sufficient for diagnosis without a genetic diagnostic test[1]. The different diagnoses of GS include Peutz–Jeghers syndrome, Juvenile polyposis, multiple hamartoma syndrome, Basal-cell nevus syndrome and Cronkite-Canada syndrome[2]. All of these syndromes involve gastrointestinal polypoid lesions that develop at varying rates. Importantly, however, these syndromes lack extraintestinal signs, such as osteomas, epidermal inclusion cysts, and multiple impacted permanent teeth[2].
We believe that the case presented here will contribute several important aspects to the literature. Firstly, colorectal cancer shows an aggressive behavior. Despite being just under 40 years old, the patient suffered from an aggressive cancer that caused multiple perforations, duodenal invasion, and liver metastases. In our opinion, the role of squamous differentiation is an important contributor to this aggressive behavior. Adenosquamous cancers constitute 0.025% to 0.2% of all colorectal cancers, although the corresponding rate of FAP is quite low[7,8]. The rates of both local and distant metastasis are moderately higher for colorectal adenosquamous cancers vs. adenocarcinomas. As extrapolated from the case presented here, colorectal adenosquamous cancers are highly aggressive cancers[7,8]. To the best of our knowledge, no study in the literature has ever reported the development of colorectal adenosquamous cancer in patients with GS. This is because all of the polyps that develop in FAP and GS are adenomatous polyps, whereas all of the tumors that develop from these are adenocarcinomas. The second important contribution of this case is the mode of GS presentation. The gastrointestinal signs and symptoms of GS are almost always related to the underlying FAP and desmoid tumors. Hence, the most common symptoms are anemia, abdominal pain, intestinal obstruction, and hemorrhage. To the best of our knowledge, there has been no case of GS reported that has presented with massive lower gastrointetsinal bleeding and multiple intestinal perforations. In the present case, we believe that the bleeding developed secondary to perforations. The detection of abundant blood in the perirectal pouch where the perforation opens supports this claim.
In conclusion, although GS is a rare condition, it should be taken seriously, as it may cause colorectal cancers until the age of 40. Therefore, close follow-up of GS patients and prophylactic surgery during early stages of GS are the most appropriate approaches. Patients who are diagnosed with upper gastrointestinal polyps should be closely examined by endoscopy. The case presented here clearly demonstrates that serious life-threatening complications like tumor perforation and massive bleeding may develop in neglected GS cases.
A 37-year old cachectic male patient was referred to our center with lower gastrointestinal bleeding.
Gardner’s syndrome (GS), lower gastrointestinal bleeding due to gastrointestinal polyposis.
Bleeding due to colorectal cancer, bleeding due to colonic polyposis.
Hemoglobin: 8 g/dL, creatinine: 2.9 mg/dL, albumin: 2.1 g/dL, total bilirubin: 6.3 mg/dL.
Conventional celiac and mesenteric angiographies that were performed to detect the hemorrhagic focus showed no extravasation that was indicative of bleeding.
Poorly-differentiated adenosquamous carcinoma of the colon, colorectal polyposis, duodenal polyposis, desmoid tumor.
Resection of the distal duodenum and proximal jejunum, latero-lateral duodenojejunal anastomosis, total abdominal colectomy with end ileostomy, distal rectal stump closure, resection of the desmoid tumor-like lesions.
To the best of our knowledge, no case of GS has ever been reported in the English language medical literature that presents with gastrointestinal perforation and bleeding.
Although GS is a rare condition, it should be taken seriously since it may cause colorectal cancers until the age of 40. Therefore, close follow-up of GS patients and prophylactic surgery during early stages of GS are the most appropriate approaches. Patients who are diagnosed with upper gastrointestinal polyps should be closely examined by endoscopy. The case presented here clearly demonstrates that serious life-threatening complications like tumor perforation and massive bleeding may develop in neglected GS cases.
CARE Checklist (2013) statement: The authors have read the guidelines of the CARE Checklist (2013) and the manuscript adheres to CARE Checklist guidelines.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Gurkan A, Zerem E S- Editor: Ji FF L- Editor: Filipodia E- Editor: Song H
| 1. | Fotiadis C, Tsekouras DK, Antonakis P, Sfiniadakis J, Genetzakis M, Zografos GC. Gardner’s syndrome: a case report and review of the literature. World J Gastroenterol. 2005;11:5408-5411. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 52] [Cited by in RCA: 51] [Article Influence: 2.6] [Reference Citation Analysis (1)] |
| 2. | Bhargava P, Guttal K, Khan S, Shekhawat C. Polyps of malignancy: Gardner’s syndrome. J Indian Acad Oral Med Radiol. 2016;28:470-473. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 3. | Agrawal D, Newaskar V, Shrivastava S, Nayak PA. External manifestations of Gardner’s syndrome as the presenting clinical entity. BMJ Case Rep. 2014;2014:pii: bcr2013200293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Varesco L. Familial adenomatous polyposis: genetics and epidemiology. Tech Coloproctol. 2004;8 Suppl 2:s305-s308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 14] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 5. | Järvinen HJ. Epidemiology of familial adenomatous polyposis in Finland: impact of family screening on the colorectal cancer rate and survival. Gut. 1992;33:357-360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 100] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 6. | Bülow S. Results of national registration of familial adenomatous polyposis. Gut. 2003;52:742-746. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 140] [Article Influence: 6.4] [Reference Citation Analysis (1)] |
| 7. | Kang DB, Oh JT, Jo HJ, Park WC. Primary adenosquamous carcinoma of the colon. J Korean Surg Soc. 2011;80 Suppl 1:S31-S35. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Sunkara T, Caughey ME, Makkar P, John F, Gaduputi V. Adenosquamous Carcinoma of the Colon. Case Rep Gastroenterol. 2018;11:791-796. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |