Published online Sep 26, 2018. doi: 10.12998/wjcc.v6.i10.335
Peer-review started: May 28, 2018
First decision: July 3, 2018
Revised: July 23, 2018
Accepted: August 3, 2018
Article in press: August 4, 2018
Published online: September 26, 2018
Processing time: 121 Days and 5.1 Hours
Portal hypertension (PHT) is an important consequence of liver cirrhosis, which can lead to complications that adversely affect a patient’s quality of life and survival, such as upper gastrointestinal bleeding, ascites, and portosystemic encephalopathy. In recent years, advances in molecular biology have led to major discoveries in the pathological processes of PHT, including the signaling pathways that may be involved: PI3K-AKT-mTOR, RhoA/Rho-kinase, JAK2/STAT3, and farnesoid X receptor. However, the pathogenesis of PHT is complex and there are numerous pathways involved. Therefore, the targeting of signaling pathways for medical management is lagging. This article summarizes the progress that has been made in understanding the signaling pathways in PHT, and provides ideas for treatment of the disorder.
Core tip: Portal hypertension (PHT) is a syndrome of portal venous system hemodynamics in liver cirrhosis. Current therapeutic options are often insufficient to prevent progression of the disease. We therefore may find more effective clinical treatments by understanding the signal pathways involved in the disease. This paper is an up-to-date and thorough review of the signaling pathways that may be involved in the pathogenesis of PHT in liver cirrhosis.
- Citation: Xu W, Liu P, Mu YP. Research progress on signaling pathways in cirrhotic portal hypertension. World J Clin Cases 2018; 6(10): 335-343
- URL: https://www.wjgnet.com/2307-8960/full/v6/i10/335.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v6.i10.335
Portal hypertension (PHT), the main consequence of cirrhosis, can lead to complications, such as variceal hemorrhage, ascites, and portosystemic encephalo-pathy. These complications may cause both diminished quality of life and mortality, and also may necessitate liver transplantation[1-3]. PHT is characterized by abnormally elevated intrahepatic venous pressure, which is due to various etiological factors. Increased intrahepatic vascular resistance (IHVR) and increased portal venous blood flow are the major pathological processes in the development of PHT[4]. IHVR is mainly determined by liver fibrosis, intrahepatic vasoconstriction, intrahepatic angiogenesis, and abnormal blood flow. Narrowing of intrahepatic microvessels, caused by fibrosis, can increase resistance and be responsible, in part, for increased responsiveness of these venules to vasoconstrictive substances[5]. Angiogenesis, the formation of new vessels from preexisting vasculature, is an important pathophysiological feature of PHT that enhances IHVR[4,6]. Another feature of PHT is the development of hyper-dynamic splanchnic circulation, with an increased blood flow in splanchnic organs that drain into the portal vein and a consequent increase in portal venous inflow[7]. The increased splanchnic and portal blood flow further elevates portal pressure. Although the pathophysiology of PHT has been studied extensively, its precise mechanisms are undefined.
An improved understanding of the molecular mechanisms of PHT is crucial to developing effective treatment strategies. Despite the incomplete knowledge of PHT pathophysiology, certain molecular pathways have been identified. The current review reveals that PI3K-AKT-mTOR, RhoA/Rho-kinase, JAK2/STAT3, farnesoid X receptor (FXR) and other signaling pathways might be targetable in the treatment of PHT. We believe that this summary of the roles of the signaling pathways in PTH may help investigators find relevant targets for PHT treatment.
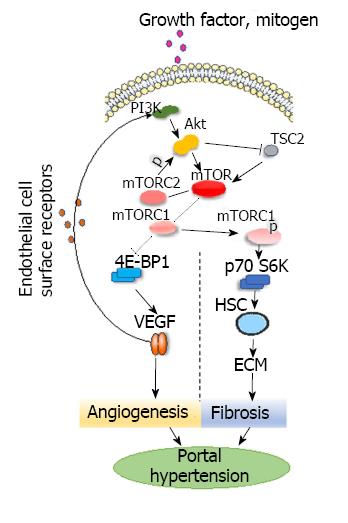
The kinase known as targets of rapamycin (TOR), belonging to the phosphatidylinositol kinase-related kinase, is an evolutionarily conserved protein kinase that was first found in yeast. The homologous substance of TOR in mammals is referred to collectively as mTOR[8]. mTOR is a ubiquitously expressed serine kinase that regulates cell growth and proliferation, and mTOR signaling plays a role in immunological processes, angiogenesis and fibrogenesis[9,10]. The mTOR signaling pathway is highly active in the evolution of PHT[11]. Mejias et al[9] found that rapamycin could reduce portal pressure by blocking mTOR and thus alleviate hyper-dynamic visceral circulation, an effect that may be due to rapamycin’s inhibition of lymphocyte proliferation, neovascularization, and fibrosis.
Mammals have two mTOR complexes: mTORC1 and mTORC2. mTORC1 is an important regulator of ribosome production and translation and has two downstream effectors, including the eukaryotic initiation factor 4E-binding protein-1 (4E-BP1) and ribosomal protein S6 kinase. mTORC1 integrates signaling from growth factor receptors, then activates the p70 ribosomal protein S6 kinase (p70S6K) by phosphorylation and inhibits the eukaryotic initiation factor 4E-BP1. Thus, mTORC1 forms two different signaling pathways to regulate mRNA translation and to control protein synthesis[12,13]. mTORC2 phosphorylates the serine/threonine protein kinase Akt/protein kinase B at serine residue Ser473[14] and participates in the regulation of phosphorylation and activation of cytoskeletal actin, protein kinase B (Akt/protein kinase B), protein kinase C, and glucocorticoid-induced protein kinase 1 in serum[8]. In vivo, growth factors, mitogen and other hormones lead to the activation of p70S6K by phosphorylation of mTOR through phosphatidylinositol 3-kinase-related kinase (PI3K)-Akt pathways, which upregulate the expression of cyclin D1, D3 and E to control cell-cycle progression[10]. One effect of this activity is increased proliferation of hepatic stellate cells (HSCs). Activation of HSCs increases the contractility of intrahepatic vessels, thereby increasing resistance to liver blood flow. p70S6K phosphorylation stimulates the production of the synthesis of collagen and other extracellular matrix components, predominately type I collagen[15]. It is generally accepted that the activation of HSCs leads to fibrosis, which is one of the important steps in the development of PHT[16]. It has been proposed that activation of mTOR promotes both HSC proliferation as well as the synthesis of extracellular matrix, which accelerates liver fibrosis and the development of PTH[15,16].
Akt, also called protein kinase B, is a threonine protein kinase akin to the PI3K protein family. One of the functions of Akt is direct phosphorylation of mTOR. Another is maintaining Rheb’s GTP-binding state by inactivating tuberous sclerosis complex 2 to enhance mTOR activity[17,18]. Akt is an important upstream mediator of mTOR and is regulated by mTORC2[14]. In previous studies[15,19], the Akt/mTOR signaling pathway was activated in bile duct ligation-induced cirrhotic rats and was implicated in the activation of HSCs. The Akt/mTOR signaling pathway is the major downstream effector of PI3K and regulates cell growth, proliferation, motility and apoptosis[14,17]. While AKT directly affects mTOR, mTORC1 reciprocally regulates the growth factor responsiveness of PI3K and Akt through feedback inhibition[20,21]. The direct phosphorylation of 4E-BP1 by mTORC1 reportedly initiates translation of hypoxia inducible factor-1α (HIF-1α), which promotes the expression of vascular endothelial growth factor (VEGF), thereby regulating angiogenesis in physiological and pathological conditions[22,23]. Under certain conditions, VEGF and endothelial cell surface receptors bind to activate the PI3K-Akt signaling pathway and further activate mTOR kinase, thereby enhancing portal pressure (Figure 1). It may be effective for inhibiting the development of PHT by inhibiting Akt or mTOR directly. However, the specific effect needs further investigation.
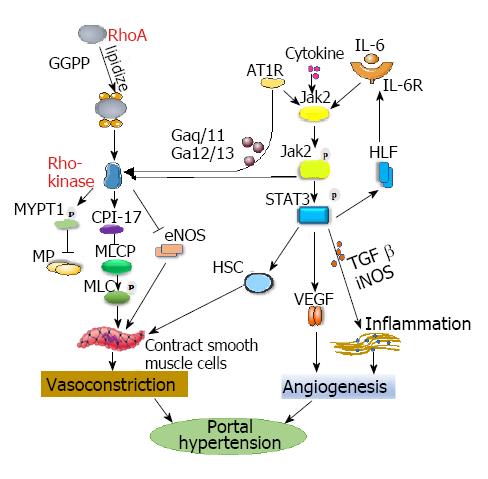
Activation of the RhoA/Rho-kinase signaling pathway, by participating in vasoconstriction and vasodilation responses[24-26], is one of the key mechanisms of PHT development.
The Ras homolog gene family member A (RhoA) is a member of the small molecular weight G proteins in the Ras superfamily. RhoA signaling participates in many cellular responses, including cell contraction, adhesion, proliferation and migration[27]. RhoA, a member of the GTP-binding protein Rho GTPase family, circulates between activated GTP-RhoA and stationary GDP-RhoA. RhoA-GDP and RhoA-GTP are interconverted by a dephosphorylation/phosphorylation process and then trigger or terminate a cellular cascade activation/reaction, acting as a “molecular switch”. Only RhoA activates Rho-kinase in the membrane-bound activated state and has downstream effects[28,29].
Geranylgeranyl pyrophosphate (GGPP), as a key substance in the transfer of RhoA to cell membranes, plays a role in the activation of the RhoA/Rho kinase signaling pathway. RhoA is linked to GGPP, a byproduct of cholesterol synthesis, to “lipidize” GGPP so it can be inserted into the cell membrane to form membrane-bound RhoA (a small GTPase protein on the cell surface) and activate the RhoA/Rho kinase pathway by binding to angiotensin[26,30,31]. Trebicka et al[32] found that statins, 3-hydroxy-3-methyl-glutaryl CoA reductase inhibitors, inhibited the expression of 3-hydroxy-3-methyl-glutaryl CoA reductase, which downregulated the expression of GGPP and then blocked the RhoA/Rho-kinase signaling pathway, leading to reduced activation of HSC. Liu et al[33] found that sodium ferulate can affect the activation of RhoA and the contraction of activated HSC by reducing the synthesis of GGPP in HSCs in liver cirrhosis. These actions reduce the intrahepatic resistance in cirrhotic rats. Zhang et al[34] found that a selective agonist of estrogen receptor β could reduce IHVR by reducing RhoA expression, thus inhibiting the myosin light chain activity and increasing the levels of endothelial nitric oxide synthase (eNOS), which leads to decreased portal vein pressure in cirrhotic ovariectomized rats.
Wei et al[35] demonstrated that sodium ferulate inhibits the hepatic RhoA/Rho-kinase signaling pathway and increases eNOS synthesis, eventually leading to reduced hepatic portal pressure in cirrhotic rats. This reaction indicates that the RhoA/Rho-kinase signaling pathway is involved in the formation of PHT. The activation of Rho kinase increases portal pressure in two ways: first, by inhibition of myosin light chain phosphatase, which produces a downstream effect that increases smooth muscle contractions[29,36]. Myosin phosphatase, myosin light chain, adducin, mono serine protein kinase, and protein kinase C-protein inhibitor protein (CPI-17) are substrates of Rho kinase. The most studied Rho kinase substrates involved in PHT formation are myosin phosphatase, myosin light chain and CPI-17. Activation of Rho kinase leads to the phosphorylation of myosin targeting subunit 1, which inactivates myosin phosphatase. Inactivation of myosin phosphatase did not lead to dephosphorylation of myosin light chain. This, in turn, leads to an increase in cytoplasmic myosin light chain phosphorylation and increased crosslinking of myosin, thereby promoting vasoconstriction. CPI-17 was combined with myosin light chain phosphatase to inhibit the activation of myosin light chain phosphatase and promote the contraction of smooth muscle cells[37]. The second way of increasing portal pressure is the downregulation of eNOS expression and reduction of its activity[38]. The activity of eNOS, which is another downstream target of the RhoA/Rho-kinase pathway[39] that is involved in regulating portal pressure, may be negatively-regulated by RhoA/Rho-eNOS activity; this effect causes the relaxation of vascular smooth muscle and plays a key role in maintaining the steady state of the vascular wall[40]. Rosado et al[41] found that terutroban, a thromboxane-A2/prostaglandin-endoperoxide receptor antagonist, reduced portal pressure by inhibiting Rho-kinase activity and enhancing eNOS-dependent vasodilatation in cirrhotic rats.
In addition, coupling of the angiotensin-type II receptor 1 (AT1R) to heterotrimeric G proteins (Gaq/11 and Ga12/13) allows stimulation and activation of the RhoA/Rho kinase pathway, which is involved in extracellular matrix production; these reactions are crucial in the development of fibrosis and PHT[42,43] (Figure 2).
Thus, the RhoA/Rho kinase signaling pathway is important in regulating IHVR and increasing portal pressure. It may be effective for inhibiting vasoconstriction by inhibiting the key mechanisms in the RhoA/Rho-kinase signaling pathway, such as phosphorylation of myosin light chain directly. However, the specific effect needs further investigation.
The janus kinase/signal transducers and activators of transcription (JAK/STAT) signaling pathway participates in numerous pathophysiological processes. Various cytokines produce corresponding tissue and cell-specific effects through combinations of four members of the JAK family and seven members of the STAT family. JAK2, the most conserved isoform of the JAK family, acts directly on downstream STAT3 in JAK/STAT signaling[44]. The JAK2/STAT3 pathway interacts with numerous cytokines that can be activated by angiotensin II, interferon-γ, transforming growth factor β (TGF-β), etc. Upon activation, STAT3 is phosphorylated to become p-STAT3, which can form homodimers or heterodimers, translocate to the nucleus, and bind to specific regulatory sequences on DNA[45]; these products then regulate the expression of VEGF, TGF-β, eNOS, and inducible nitric oxide synthase (iNOS), a process that is important in cell proliferation, fibrosis and angiogenesis[46-48]. It has been found that JAK2/STAT3 signaling is overactive in PHT and involved in its development[44,49].
Angiogenesis is considered one of the factors in the development of PHT. Pathological angiogenesis may lead to increased intrahepatic circulatory resistance, and subsequently cause PHT and its severe complications such as variceal bleeding. VEGF is one of the cytokines involved in the development of angiogenesis[50,51]. Wang et al[52] found that AG490, a specific antagonist of JAK2, decreased the formation of new blood vessels in the liver by inhibiting the expression of JAK2/STAT3 signaling, which suppressed the activation of HSCs and reduced the expression of VEGF. JAK2/STAT3 signaling may stimulate vascular hyperplasia and decrease vascular tone by increasing the expression of VEGF, thus promoting the development of PHT. Interleukin-17 activates HSCs by STAT3 signaling, and activation of HSCs plays a key role in the formation of PHT[53]. IL-6 also activates the STAT3 pathway. IL-6 binds to its receptor, activates JAK2, phosphorylates it, and then causes the phosphorylation of STAT3 in cells; these reactions result in the activation of HSCs and promotion of HLF expression, which can upregulate the activation of IL-6 and reactivate the STAT3 pathway. Thus, a loop is formed that constantly activates HSCs, ultimately causing hepatic vessel contractions that lead to increased portal pressure[54].
Endogenous angiogenesis and increased eNOS-derived nitric oxide levels in PHT have been considered important in the maintenance of PHT, and JAK2/STAT3 has been reported to promote eNOS protein expression[52,55].
Visceral inflammation is usually present in patients with PHT, especially in those with advanced PHT, and the inflammation can accentuate endothelial dysfunction and angiogenesis[56]. Relevant studies[52] have shown that enhanced JAK2/STAT3 signaling accelerates intestinal inflammation in PHT rats by upregulating TGF-β and iNOS expression. These findings suggest that JAK2/STAT3 participates in the pathogenesis of PHT by regulating factors such as VEGF, eNOS, TGF-β and iNOS.
Recent studies[49,57] have found a relationship between the JAK2/STAT3 signaling pathway and RhoA/Rho-kinase signaling. JAK2 was shown to establish a link between AT1R and the RhoA/Rho-kinase pathway in smooth muscle cells. AT1R stimulates JAK2 to phosphorylate and then induce Arhgef1, the nucleotide exchange factor responsible for activating RhoA, which activates Rho-kinase, eventually leading to vasoconstriction (Figure 2).
Activation of the above three signaling pathways mainly promotes the formation of PHT, while activation of the FXR pathway can reduce PHT.
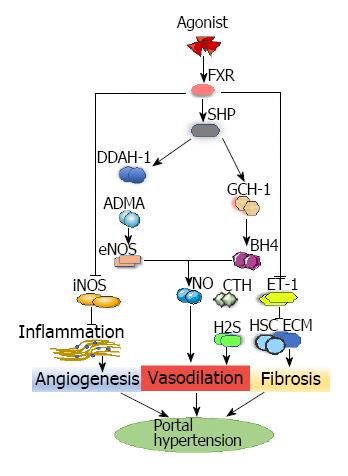
FXR is a bile acid-reactive transcription factor and member of the nuclear receptor superfamily (NR1H4)[58] that is highly expressed in the liver and small intestine. Like other nuclear receptor members, FXR has an N-terminal activation domain (AF1) that interacts with a cofactor, a conserved DNA-binding domain, and a unique ligand-binding domain, allowing receptor dimerization and the C-terminal activation domain (AF2) to regulate the interaction[59,60]. In recent years, it has been recognized that the FXR plays a key role in the metabolism of bile acids and intestinal flora, as bile acids and FXR closely interact[61,62]. In many liver diseases, FXR is involved in fibrosis, and in the gastrointestinal tract it has immunological activity and vascular function[63]. FXR is a major transcriptional regulator of genes involved in bile acid homeostasis and is a regulator of lipid and carbohydrate metabolism in the normal liver[64].
Studies have documented deficiency of the FXR system in rat cirrhosis models, and FXR agonists can improve PHT through various pathways by activating FXR, which is related to vascular regulation and PHT[64]. Small heterodimer partner, the direct target gene of FXR, is a downstream orphan nuclear receptor for FXR that inhibits many other nuclear receptors, including cholesterol 7 alpha-hydroxylase[65]; it is increased after stimulation of FXR agonist INT-747 in a cirrhosis model[64]. The beneficial effects of this process involve hemodynamic changes of intrahepatic endothelial dysfunction and the molecular repair of intrahepatic eNOS activity[64].
In cirrhotic PHT, a decrease in intrahepatic eNOS activity is key to the pathogenesis of increased IHVR[6], which is mainly caused by impaired hepatic vascular dilatation through the combination of reduced eNOS activity and nitric oxide bioavailability[66,67]. FXR affects blood vessel nitric oxide signaling by increasing eNOS concentrations[68]. In animal models of cirrhosis, obeticholic acid, a steroid FXR agonist and chenodeoxycholic acid derivative, restored intrahepatic eNOS levels and enhanced the expression of dimethylarginine dimethylamidohydrolase-1 (DDAH-1). Increases in DDAH-1 reduce the level of systemic asymmetric dimethylarginine (ADMA), thus upregulating the expression of eNOS, and then modulating nitric oxide production, which eventually results in decreased portal vein pressure[64,69]. ADMA is a competitive inhibitor of the eNOS substrate L-arginine and decreases eNOS phosphorylation of vascular endothelial cells in vitro and in vivo[70]. DDAH-1 is a key enzyme that metabolizes liver ADMA[69]. In addition, studies have found that alanine-glyoxylate aminotransferase-2 (AGXT2), which is present in mitochondria, is involved in the metabolism of ADMA. Rodionov et al[71] found that ADMA levels were significantly reduced in the liver and plasma of AGXT2-overexpressing mice. Caplin et al[72] found that the ADMA levels were significantly increased in the plasma of AGXT2 knockout mice. The FXR agonist PX20606 upregulates GTP cyclohydrolase-1, a key enzyme in the synthesis of cofactor tetrahydrobiopterin (BH4), resulting in increased amounts of BH4; sufficient concentrations of BH4 are essential for eNOS to catalyze nitric oxide. The enhancement of eNOS activity and BH4 has improved nitric oxide-mediated sinus endothelial function[68]. FXR agonism also decreases inflammatory responses, and the associated development of PHT, by reducing the expression of iNOS and cycloogenase 2[73].
The increase of internal vascular resistance caused by endothelial dysfunction is one of the factors in PHT formation. In some studies[68,74], endothelial dysfunction was mainly due to increased activity of vasoconstrictive factor (endothelin-1) and impaired nitric oxide signaling in sinusoidal endothelial cells. Endothelin-1 is a powerful vasoconstrictor in hepatic sinuses[75]. In liver damage, enhanced synthesis of endothelin-1 has activated HSCs, which promoted sine refactoring[6,68] and increased the amount of phosphorylated moesin, a marker of HSC contraction[75]. Endothelin-1 not only induces HSC proliferation and contraction, with consequent sinusoidal vasoconstriction, but also increases extracellular matrix synthesis[68]. FXR agonism ameliorated intrahepatic resistance[75] by decreasing the expression of endothelin-1[76], which inhibited endothelin-1-mediated contraction of hepatic stellate cell and increased the production of liver cystathionase-mediated hydrogen sulfide[68]. Cystathionase is a key enzyme for the local production of hydrogen sulfide, a potent nitric oxide-independent vasodilator[77] (Figure 3).
The above four signaling pathways have been extensively studied, however some novel signaling pathways need further study. Recent studies have shown that the increase in reactive oxygen leads to increased expression of Nuclear Factor-E2-related factor 2/Heme Oxygenase 1 (Nrf2/HO-1) in portal hypertensive rats. HO-1 is regulated by Nrf2 and can be used to induce hypovascular reactivity or as a vasodilator, which also results in increased expression of VEGF in the mesenteric artery of patients with PHT, which then forms the collateral portal vessels[78]. Therefore, reducing the portal pressure by inhibiting Nrf2/HO-1 signaling is effective. Zeng et al[79] found that Kruppel-like factor 2 inhibits the proliferation of sinusoidal endothelial cells and vascular formation by downregulating extracellular signal-regulated kinases 1/2 signaling, which inhibits the process of angiogenesis, and then ameliorates elevated portal pressure. Gao et al[80] found that combining celecoxib and octreotide not only significantly inhibited the expression of phospho-extracellular regulated kinase (p-ERK), HIF-1a, and VEGF, but also prevented HIF-1a from binding to VEGF by blocking the MAPK-ERK signaling pathway, which synergistically improves hepatic fibrosis and portal hypertonia in thioacetamide-induced cirrhotic rats by inhibiting both intrahepatic and extrahepatic angiogenesis. The mechanism responsible may be inactivation of the p-ERK-HIF-1α-VEGF signaling pathway.
In recent years, progress has been made in understanding how PHT develops and in the development of potential nonsurgical therapeutic approaches to PHT. The limitations of current PHT treatments are directed towards the outcomes of PHT, such as bleeding varices, and not towards the underlying causes. Several signaling pathways are involved in the pathogenesis of PHT, including PI3K-AKT-mTOR, RhoA/Rho kinase, JAK2/STAT3 and FXR. These pathways affect the development of PHT by regulating IHVR and portal vein blood flow. In addition, some newly discovered signaling pathways may be novel therapeutic targets, such as p-ERK-HIF-1α-VEGF signaling. Efforts directed toward modifying the pathways should be explored for the effective prevention and treatment of PHT, however the pathways are incompletely understood and deserve further investigation.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Gorrell MD, Gonzalez-Reimers E, Manenti A S- Editor: Dou Y L- Editor: Filipodia E- Editor: Wu YXJ
| 1. | Sanyal AJ, Bosch J, Blei A, Arroyo V. Portal hypertension and its complications. Gastroenterology. 2008;134:1715-1728. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 2. | Schuppan D, Afdhal NH. Liver cirrhosis. Lancet. 2008;371:838-851. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1686] [Cited by in RCA: 1565] [Article Influence: 92.1] [Reference Citation Analysis (0)] |
| 3. | Garcia-Tsao G. Portal hypertension. Curr Opin Gastroenterol. 2001;17:281-290. [PubMed] |
| 4. | Bosch J, Abraldes JG, Fernández M, García-Pagán JC. Hepatic endothelial dysfunction and abnormal angiogenesis: new targets in the treatment of portal hypertension. J Hepatol. 2010;53:558-567. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 152] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 5. | Zhou Q, Hennenberg M, Trebicka J, Jochem K, Leifeld L, Biecker E, Sauerbruch T, Heller J. Intrahepatic upregulation of RhoA and Rho-kinase signalling contributes to increased hepatic vascular resistance in rats with secondary biliary cirrhosis. Gut. 2006;55:1296-1305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 71] [Cited by in RCA: 70] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 6. | Fernandez M. Molecular pathophysiology of portal hypertension. Hepatology. 2015;61:1406-1415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 93] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 7. | Fernandez M, Mejias M, Garcia-Pras E, Mendez R, Garcia-Pagan JC, Bosch J. Reversal of portal hypertension and hyperdynamic splanchnic circulation by combined vascular endothelial growth factor and platelet-derived growth factor blockade in rats. Hepatology. 2007;46:1208-1217. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 129] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 8. | Jung CH, Ro SH, Cao J, Otto NM, Kim DH. mTOR regulation of autophagy. FEBS Lett. 2010;584:1287-1295. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1471] [Cited by in RCA: 1692] [Article Influence: 112.8] [Reference Citation Analysis (0)] |
| 9. | Mejias M, Garcia-Pras E, Gallego J, Mendez R, Bosch J, Fernandez M. Relevance of the mTOR signaling pathway in the pathophysiology of splenomegaly in rats with chronic portal hypertension. J Hepatol. 2010;52:529-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 81] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Neef M, Ledermann M, Saegesser H, Schneider V, Reichen J. Low-dose oral rapamycin treatment reduces fibrogenesis, improves liver function, and prolongs survival in rats with established liver cirrhosis. J Hepatol. 2006;45:786-796. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 115] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Patsenker E, Schneider V, Ledermann M, Saegesser H, Dorn C, Hellerbrand C, Stickel F. Potent antifibrotic activity of mTOR inhibitors sirolimus and everolimus but not of cyclosporine A and tacrolimus in experimental liver fibrosis. J Hepatol. 2011;55:388-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 103] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 12. | Sarbassov DD, Ali SM, Sabatini DM. Growing roles for the mTOR pathway. Curr Opin Cell Biol. 2005;17:596-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1166] [Cited by in RCA: 1229] [Article Influence: 61.5] [Reference Citation Analysis (0)] |
| 13. | Choo AY, Yoon SO, Kim SG, Roux PP, Blenis J. Rapamycin differentially inhibits S6Ks and 4E-BP1 to mediate cell-type-specific repression of mRNA translation. Proc Natl Acad Sci USA. 2008;105:17414-17419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 609] [Cited by in RCA: 673] [Article Influence: 39.6] [Reference Citation Analysis (0)] |
| 14. | Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098-1101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4837] [Cited by in RCA: 5256] [Article Influence: 262.8] [Reference Citation Analysis (0)] |
| 15. | Gäbele E, Reif S, Tsukada S, Bataller R, Yata Y, Morris T, Schrum LW, Brenner DA, Rippe RA. The role of p70S6K in hepatic stellate cell collagen gene expression and cell proliferation. J Biol Chem. 2005;280:13374-13382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 16. | Thoen LF, Guimarães EL, Dollé L, Mannaerts I, Najimi M, Sokal E, van Grunsven LA. A role for autophagy during hepatic stellate cell activation. J Hepatol. 2011;55:1353-1360. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 317] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 17. | Yang Q, Guan KL. Expanding mTOR signaling. Cell Res. 2007;17:666-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 390] [Cited by in RCA: 418] [Article Influence: 23.2] [Reference Citation Analysis (0)] |
| 18. | Chen Y, Klionsky DJ. The regulation of autophagy - unanswered questions. J Cell Sci. 2011;124:161-170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 439] [Cited by in RCA: 470] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 19. | Wang W, Yan J, Wang H, Shi M, Zhang M, Yang W, Peng C, Li H. Rapamycin ameliorates inflammation and fibrosis in the early phase of cirrhotic portal hypertension in rats through inhibition of mTORC1 but not mTORC2. PLoS One. 2014;9:e83908. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 20. | O’Reilly KE, Rojo F, She QB, Solit D, Mills GB, Smith D, Lane H, Hofmann F, Hicklin DJ, Ludwig DL. mTOR inhibition induces upstream receptor tyrosine kinase signaling and activates Akt. Cancer Res. 2006;66:1500-1508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1957] [Cited by in RCA: 2079] [Article Influence: 109.4] [Reference Citation Analysis (0)] |
| 21. | Zhang HH, Lipovsky AI, Dibble CC, Sahin M, Manning BD. S6K1 regulates GSK3 under conditions of mTOR-dependent feedback inhibition of Akt. Mol Cell. 2006;24:185-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 244] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 22. | Geerts AM, Vanheule E, Van Vlierberghe H, Leybaert L, Van Steenkiste C, De Vos M, Colle I. Rapamycin prevents mesenteric neo-angiogenesis and reduces splanchnic blood flow in portal hypertensive mice. Hepatol Res. 2008;38:1130-1139. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 23. | Karar J, Maity A. PI3K/AKT/mTOR Pathway in Angiogenesis. Front Mol Neurosci. 2011;4:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 712] [Cited by in RCA: 1068] [Article Influence: 76.3] [Reference Citation Analysis (0)] |
| 24. | Lauriol J, Keith K, Jaffré F, Couvillon A, Saci A, Goonasekera SA, McCarthy JR, Kessinger CW, Wang J, Ke Q. RhoA signaling in cardiomyocytes protects against stress-induced heart failure but facilitates cardiac fibrosis. Sci Signal. 2014;7:ra100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 25. | Sai X, Yonemura S, Ladher RK. Junctionally restricted RhoA activity is necessary for apical constriction during phase 2 inner ear placode invagination. Dev Biol. 2014;394:206-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 26. | Lee MH, Cho YS, Han YM. Simvastatin suppresses self-renewal of mouse embryonic stem cells by inhibiting RhoA geranylgeranylation. Stem Cells. 2007;25:1654-1663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 49] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 27. | Johnson LA, Rodansky ES, Haak AJ, Larsen SD, Neubig RR, Higgins PD. Novel Rho/MRTF/SRF inhibitors block matrix-stiffness and TGF-β-induced fibrogenesis in human colonic myofibroblasts. Inflamm Bowel Dis. 2014;20:154-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 167] [Article Influence: 15.2] [Reference Citation Analysis (0)] |
| 28. | Trebicka J, Hennenberg M, Laleman W, Shelest N, Biecker E, Schepke M, Nevens F, Sauerbruch T, Heller J. Atorvastatin lowers portal pressure in cirrhotic rats by inhibition of RhoA/Rho-kinase and activation of endothelial nitric oxide synthase. Hepatology. 2007;46:242-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 238] [Article Influence: 13.2] [Reference Citation Analysis (2)] |
| 29. | Bishop AL, Hall A. Rho GTPases and their effector proteins. Biochem J. 2000;348 Pt 2:241-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 413] [Cited by in RCA: 371] [Article Influence: 14.8] [Reference Citation Analysis (0)] |
| 30. | Charlton-Menys V, Durrington PN. Human cholesterol metabolism and therapeutic molecules. Exp Physiol. 2008;93:27-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 41] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 31. | Schmidmaier R, Baumann P, Simsek M, Dayyani F, Emmerich B, Meinhardt G. The HMG-CoA reductase inhibitor simvastatin overcomes cell adhesion-mediated drug resistance in multiple myeloma by geranylgeranylation of Rho protein and activation of Rho kinase. Blood. 2004;104:1825-1832. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 94] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Trebicka J, Schierwagen R. Statins, Rho GTPases and KLF2: new mechanistic insight into liver fibrosis and portal hypertension. Gut. 2015;64:1349-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 33. | Liu J, Peng L, Yang J, Wang M, Xu S, Liu J, Han P, He J, Tian D, Zhou Q. Sodium Ferulate Reduces Portal Pressure Through Inhibition of RhoA/Rho-Kinase and Activation of Endothelial Nitric Oxide Synthase in Cirrhotic Rats. Dig Dis Sci. 2015;60:2019-2029. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 34. | Zhang CG, Zhang B, Deng WS, Duan M, Chen W, Wu ZY. Role of estrogen receptor β selective agonist in ameliorating portal hypertension in rats with CCl4-induced liver cirrhosis. World J Gastroenterol. 2016;22:4484-4500. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 35. | Wei L, Yang J, Wang M, Xu SN, Liang HM, Zhou Q. Sodium ferulate lowers portal pressure in rats with secondary biliary cirrhosis through the RhoA/Rho-kinase signaling pathway: a preliminary study. Int J Mol Med. 2014;34:1257-1267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 36. | Wang Y, Zheng XR, Riddick N, Bryden M, Baur W, Zhang X, Surks HK. ROCK isoform regulation of myosin phosphatase and contractility in vascular smooth muscle cells. Circ Res. 2009;104:531-540. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 200] [Cited by in RCA: 200] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 37. | Antoniu SA. Targeting RhoA/ROCK pathway in pulmonary arterial hypertension. Expert Opin Ther Targets. 2012;16:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 38. | Ming XF, Viswambharan H, Barandier C, Ruffieux J, Kaibuchi K, Rusconi S, Yang Z. Rho GTPase/Rho kinase negatively regulates endothelial nitric oxide synthase phosphorylation through the inhibition of protein kinase B/Akt in human endothelial cells. Mol Cell Biol. 2002;22:8467-8477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 316] [Cited by in RCA: 333] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 39. | Anegawa G, Kawanaka H, Yoshida D, Konishi K, Yamaguchi S, Kinjo N, Taketomi A, Hashizume M, Shimokawa H, Maehara Y. Defective endothelial nitric oxide synthase signaling is mediated by rho-kinase activation in rats with secondary biliary cirrhosis. Hepatology. 2008;47:966-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 40] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 40. | Dudzinski DM, Michel T. Life history of eNOS: partners and pathways. Cardiovasc Res. 2007;75:247-260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 325] [Cited by in RCA: 310] [Article Influence: 17.2] [Reference Citation Analysis (0)] |
| 41. | Rosado E, Rodríguez-Vilarrupla A, Gracia-Sancho J, Tripathi D, García-Calderó H, Bosch J, García-Pagán JC. Terutroban, a TP-receptor antagonist, reduces portal pressure in cirrhotic rats. Hepatology. 2013;58:1424-1435. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 37] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Mehta PK, Griendling KK. Angiotensin II cell signaling: physiological and pathological effects in the cardiovascular system. Am J Physiol Cell Physiol. 2007;292:C82-C97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1315] [Cited by in RCA: 1464] [Article Influence: 77.1] [Reference Citation Analysis (0)] |
| 43. | Klein S, Van Beuge MM, Granzow M, Beljaars L, Schierwagen R, Kilic S, Heidari I, Huss S, Sauerbruch T, Poelstra K. HSC-specific inhibition of Rho-kinase reduces portal pressure in cirrhotic rats without major systemic effects. J Hepatol. 2012;57:1220-1227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 44. | Wang D, Yin J, Dong R, Zhao J, Wang Q, Wang N, Wang S, Du X, Lu J. Inhibition of Janus kinase-2 signalling pathway ameliorates portal hypertensive syndrome in partial portal hypertensive and liver cirrhosis rats. Dig Liver Dis. 2015;47:315-323. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 45. | Vera J, Rateitschak K, Lange F, Kossow C, Wolkenhauer O, Jaster R. Systems biology of JAK-STAT signalling in human malignancies. Prog Biophys Mol Biol. 2011;106:426-434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 40] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 46. | Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, Zhao J, Zhang HT. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol. 2014;44:1643-1651. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 237] [Article Influence: 21.5] [Reference Citation Analysis (0)] |
| 47. | Chong HC, Chan JS, Goh CQ, Gounko NV, Luo B, Wang X, Foo S, Wong MT, Choong C, Kersten S. Angiopoietin-like 4 stimulates STAT3-mediated iNOS expression and enhances angiogenesis to accelerate wound healing in diabetic mice. Mol Ther. 2014;22:1593-1604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 95] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 48. | Kim D, Lee IH, Kim S, Choi M, Kim H, Ahn S, Saw PE, Jeon H, Lee Y, Jon S. A specific STAT3-binding peptide exerts antiproliferative effects and antitumor activity by inhibiting STAT3 phosphorylation and signaling. Cancer Res. 2014;74:2144-2151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 49. | Granzow M, Schierwagen R, Klein S, Kowallick B, Huss S, Linhart M, Mazar IG, Görtzen J, Vogt A, Schildberg FA. Angiotensin-II type 1 receptor-mediated Janus kinase 2 activation induces liver fibrosis. Hepatology. 2014;60:334-348. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 108] [Article Influence: 9.8] [Reference Citation Analysis (0)] |
| 50. | Iwakiri Y, Shah V, Rockey DC. Vascular pathobiology in chronic liver disease and cirrhosis - current status and future directions. J Hepatol. 2014;61:912-924. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 203] [Cited by in RCA: 231] [Article Influence: 21.0] [Reference Citation Analysis (0)] |
| 51. | Hsu SJ, Lee FY, Wang SS, Hsin IF, Lin TY, Huang HC, Chang CC, Chuang CL, Ho HL, Lin HC. Caffeine ameliorates hemodynamic derangements and portosystemic collaterals in cirrhotic rats. Hepatology. 2015;61:1672-1684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 52. | Wang D, Wang Q, Yin J, Dong R, Wang Q, Du X, Lu J. Combined administration of propranolol + AG490 offers better effects on portal hypertensive rats with cirrhosis. J Gastroenterol Hepatol. 2016;31:1037-1044. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 15] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 53. | Meng F, Wang K, Aoyama T, Grivennikov SI, Paik Y, Scholten D, Cong M, Iwaisako K, Liu X, Zhang M. Interleukin-17 signaling in inflammatory, Kupffer cells, and hepatic stellate cells exacerbates liver fibrosis in mice. Gastroenterology. 2012;143:765-776.e3. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 536] [Cited by in RCA: 563] [Article Influence: 43.3] [Reference Citation Analysis (1)] |
| 54. | Xiang DM, Sun W, Ning BF, Zhou TF, Li XF, Zhong W, Cheng Z, Xia MY, Wang X, Deng X. The HLF/IL-6/STAT3 feedforward circuit drives hepatic stellate cell activation to promote liver fibrosis. Gut. 2017;pii:gutjnl-2016-313392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 55. | Schwabl P, Payer BA, Grahovac J, Klein S, Horvatits T, Mitterhauser M, Stift J, Boucher Y, Trebicka J, Trauner M. Pioglitazone decreases portosystemic shunting by modulating inflammation and angiogenesis in cirrhotic and non-cirrhotic portal hypertensive rats. J Hepatol. 2014;60:1135-1142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 56. | Reiberger T, Angermayr B, Schwabl P, Rohr-Udilova N, Mitterhauser M, Gangl A, Peck-Radosavljevic M. Sorafenib attenuates the portal hypertensive syndrome in partial portal vein ligated rats. J Hepatol. 2009;51:865-873. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 92] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 57. | Klein S, Rick J, Lehmann J, Schierwagen R, Schierwagen IG, Verbeke L, Hittatiya K, Uschner FE, Manekeller S, Strassburg CP. Janus-kinase-2 relates directly to portal hypertension and to complications in rodent and human cirrhosis. Gut. 2017;66:145-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 54] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 58. | Lee FY, Lee H, Hubbert ML, Edwards PA, Zhang Y. FXR, a multipurpose nuclear receptor. Trends Biochem Sci. 2006;31:572-580. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 277] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 59. | Thomas C, Pellicciari R, Pruzanski M, Auwerx J, Schoonjans K. Targeting bile-acid signalling for metabolic diseases. Nat Rev Drug Discov. 2008;7:678-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 869] [Cited by in RCA: 1044] [Article Influence: 61.4] [Reference Citation Analysis (0)] |
| 60. | Li Y, Jadhav K, Zhang Y. Bile acid receptors in non-alcoholic fatty liver disease. Biochem Pharmacol. 2013;86:1517-1524. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 87] [Cited by in RCA: 107] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 61. | Degirolamo C, Rainaldi S, Bovenga F, Murzilli S, Moschetta A. Microbiota modification with probiotics induces hepatic bile acid synthesis via downregulation of the Fxr-Fgf15 axis in mice. Cell Rep. 2014;7:12-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 223] [Cited by in RCA: 269] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 62. | Sayin SI, Wahlström A, Felin J, Jäntti S, Marschall HU, Bamberg K, Angelin B, Hyötyläinen T, Orešič M, Bäckhed F. Gut microbiota regulates bile acid metabolism by reducing the levels of tauro-beta-muricholic acid, a naturally occurring FXR antagonist. Cell Metab. 2013;17:225-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1367] [Cited by in RCA: 1683] [Article Influence: 140.3] [Reference Citation Analysis (0)] |
| 63. | Halilbasic E, Claudel T, Trauner M. Bile acid transporters and regulatory nuclear receptors in the liver and beyond. J Hepatol. 2013;58:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 264] [Cited by in RCA: 301] [Article Influence: 25.1] [Reference Citation Analysis (0)] |
| 64. | Verbeke L, Farre R, Trebicka J, Komuta M, Roskams T, Klein S, Elst IV, Windmolders P, Vanuytsel T, Nevens F. Obeticholic acid, a farnesoid X receptor agonist, improves portal hypertension by two distinct pathways in cirrhotic rats. Hepatology. 2014;59:2286-2298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 213] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 65. | Lu TT, Makishima M, Repa JJ, Schoonjans K, Kerr TA, Auwerx J, Mangelsdorf DJ. Molecular basis for feedback regulation of bile acid synthesis by nuclear receptors. Mol Cell. 2000;6:507-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1110] [Cited by in RCA: 1138] [Article Influence: 45.5] [Reference Citation Analysis (0)] |
| 66. | Van de Casteele M, Omasta A, Janssens S, Roskams T, Desmet V, Nevens F, Fevery J. In vivo gene transfer of endothelial nitric oxide synthase decreases portal pressure in anaesthetised carbon tetrachloride cirrhotic rats. Gut. 2002;51:440-445. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 64] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 67. | Laviña B, Gracia-Sancho J, Rodríguez-Vilarrupla A, Chu Y, Heistad DD, Bosch J, García-Pagán JC. Superoxide dismutase gene transfer reduces portal pressure in CCl4 cirrhotic rats with portal hypertension. Gut. 2009;58:118-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 68. | Schwabl P, Hambruch E, Seeland BA, Hayden H, Wagner M, Garnys L, Strobel B, Schubert TL, Riedl F, Mitteregger D. The FXR agonist PX20606 ameliorates portal hypertension by targeting vascular remodelling and sinusoidal dysfunction. J Hepatol. 2017;66:724-733. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 139] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 69. | Mookerjee RP, Mehta G, Balasubramaniyan V, Mohamed Fel Z, Davies N, Sharma V, Iwakiri Y, Jalan R. Hepatic dimethylarginine-dimethylaminohydrolase1 is reduced in cirrhosis and is a target for therapy in portal hypertension. J Hepatol. 2015;62:325-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 70. | Kajimoto H, Kai H, Aoki H, Yasuoka S, Anegawa T, Aoki Y, Ueda S, Okuda S, Imaizumi T. Inhibition of eNOS phosphorylation mediates endothelial dysfunction in renal failure: new effect of asymmetric dimethylarginine. Kidney Int. 2012;81:762-768. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 71. | Rodionov RN, Murry DJ, Vaulman SF, Stevens JW, Lentz SR. Human alanine-glyoxylate aminotransferase 2 lowers asymmetric dimethylarginine and protects from inhibition of nitric oxide production. J Biol Chem. 2010;285:5385-5391. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 72. | Caplin B, Wang Z, Slaviero A, Tomlinson J, Dowsett L, Delahaye M, Salama A; International Consortium for Blood Pressure Genome-Wide Association Studies, Wheeler DC, Leiper J. Alanine-glyoxylate aminotransferase-2 metabolizes endogenous methylarginines, regulates NO, and controls blood pressure. Arterioscler Thromb Vasc Biol. 2012;32:2892-2900. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 69] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 73. | Li YT, Swales KE, Thomas GJ, Warner TD, Bishop-Bailey D. Farnesoid x receptor ligands inhibit vascular smooth muscle cell inflammation and migration. Arterioscler Thromb Vasc Biol. 2007;27:2606-2611. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 133] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 74. | Gupta TK, Toruner M, Chung MK, Groszmann RJ. Endothelial dysfunction and decreased production of nitric oxide in the intrahepatic microcirculation of cirrhotic rats. Hepatology. 1998;28:926-931. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 261] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 75. | Li J, Kuruba R, Wilson A, Gao X, Zhang Y, Li S. Inhibition of endothelin-1-mediated contraction of hepatic stellate cells by FXR ligand. PLoS One. 2010;5:e13955. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 76. | He F, Li J, Mu Y, Kuruba R, Ma Z, Wilson A, Alber S, Jiang Y, Stevens T, Watkins S. Downregulation of endothelin-1 by farnesoid X receptor in vascular endothelial cells. Circ Res. 2006;98:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 77. | Zhao W, Zhang J, Lu Y, Wang R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001;20:6008-6016. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1511] [Cited by in RCA: 1564] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 78. | Qin J, He Y, Duan M, Luo M. Effects of Nuclear Factor-E2-related factor 2/Heme Oxygenase 1 on splanchnic hemodynamics in experimental cirrhosis with portal hypertension. Microvasc Res. 2017;111:12-19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 79. | Zeng XQ, Li N, Pan DY, Miao Q, Ma GF, Liu YM, Tseng YJ, Li F, Xu LL, Chen SY. Kruppel-like factor 2 inhibit the angiogenesis of cultured human liver sinusoidal endothelial cells through the ERK1/2 signaling pathway. Biochem Biophys Res Commun. 2015;464:1241-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 80. | Gao JH, Wen SL, Feng S, Yang WJ, Lu YY, Tong H, Liu R, Tang SH, Huang ZY, Tang YM. Celecoxib and octreotide synergistically ameliorate portal hypertension via inhibition of angiogenesis in cirrhotic rats. Angiogenesis. 2016;19:501-511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 42] [Article Influence: 4.7] [Reference Citation Analysis (0)] |