Published online May 16, 2017. doi: 10.12998/wjcc.v5.i5.187
Peer-review started: November 21, 2016
First decision: January 14, 2017
Revised: February 9, 2017
Accepted: March 12, 2017
Article in press: March 13, 2017
Published online: May 16, 2017
Processing time: 180 Days and 8.2 Hours
Elizabethkingia miricola (E. miricola) is a gram-negative non-fermentative bacterium which is rarely encountered. It is usually misidentified or considered as a contaminant in routine microbiology laboratories due to the limitations in conventional biochemical techniques. However, with the advent of the matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS), the identification of non-fermenters has become easy and this has led to enhanced understanding of the clinical significance of these uncommonly isolated microorganisms. The genus Elizabethkingia has only two species E. meningoseptica and E. miricola. Both of these organisms are known to be multi-drug resistant and therefore, their accurate identification and antimicrobial susceptibility testing are necessary prior to the initiation of appropriate therapy. In the world literature till date, only 3 cases of sepsis caused by E. miricola have been reported. We present the first case of E. miricola association with urinary tract infection.
Core tip: Non-fermenters except Pseudomonas and Acinetobacter are less commonly associated with urinary tract infection (UTI). But recently an upsurge in a number of reported cases has been noted due to the use of MALDI-TOF which is an easy and reliable identification technique. Till date in literature, there is no reported case of Elizabethkingia miricola (E. miricola) causing UTI, although its significance in blood and sputum samples of sepsis patients has been demonstrated earlier. This is the first case report showing a clinical association of E. miricola with symptomatic UTI and also demonstrating the multidrug resistance nature of this organism.
- Citation: Gupta P, Zaman K, Mohan B, Taneja N. Elizabethkingia miricola: A rare non-fermenter causing urinary tract infection. World J Clin Cases 2017; 5(5): 187-190
- URL: https://www.wjgnet.com/2307-8960/full/v5/i5/187.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i5.187
Urinary tract infections (UTI) are amongst the most common bacterial infections occurring in human beings during their lifetime[1]. The usual organisms responsible for UTI belong to the family Enterobacteriaceae and gram-positive bacteria like Staphylococcus and Enterococcus[2]. UTI caused by non-fermenters (NF) is being increasingly reported especially in the nosocomial settings, with Pseudomonas and Acinetobacter spp. being the most common agents. However, UTI due to other NFs like Alcaligenes, Flavobacterium, Oligella, Flavimonas, Agrobacter, Weeksiella are also on the rise[3]. Routine laboratory identification of NF is difficult and labour-intensive, which often misclassifies or misidentifies these agents and thereby may mask the exact clinical significance of these isolates. Nowadays, the identification of these NF has become easy by the advent of matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF-MS). We recently encountered a case of UTI caused by rare multidrug resistant non-fermenter E. miricola, which was identified by MALDI-TOF.
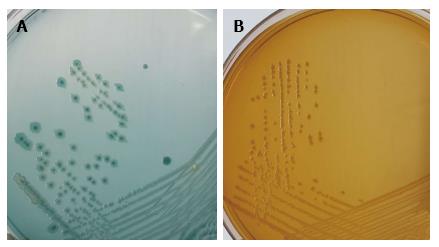
A 25-year-old female presented with complaints of increased bowel frequency, oliguria, fever and abdominal pain since one month. Detailed history revealed that the patient had difficulty in micturition for past two weeks. The routine laboratory investigations revealed a haemoglobin of 7.8 gm/dL, total leucocytes count 3200 cells/mm3, platelet count of 70000 cells/mm3. Renal function tests revealed normal sodium concentration (139 mEq/L), hyperkalemia (8.2 mEq/L), hyperuricemia (74 mg/dL) and elevated creatinine levels (7.5 mg/dL). Coagulation profile was normal. Ultrasonography (USG) revealed bilateral hydroureteronephrosis with normal renal parenchyma and features of vesicoureteric reflux. The midstream urine sample was subjected to microbiological testing. The wet mount microscopic examination showed 1-2 RBCs, numerous pus cells and bacteria per high-power field[4]. The semi-quantitative culture done on the cysteine lysine electrolyte deficient agar showed significant bacterial growth (colony count > 105 CFU/mL). The colonies were non-lactose fermenting, translucent, greenish blue, smooth having entire edges and became mucoid on prolonged incubation. Subculture on MacConkey agar showed pale, translucent, glistening colonies with entire edges (Figure 1). Gram staining showed 0.5 μm × 2 μm gram-negative bacilli, with no spores and no capsule. The isolate was also subjected to conventional identification using a battery of biochemical tests. The isolate was catalase positive, oxidase positive, produced indole, was non-nitrate reducing, mannitol fermenting, esculin and gelatinase hydrolysis positive. Urease was produced and this test helped to distinguish it from E. meningoseptica. The isolate was confirmed as Elizabethkingia miricola (E. miricola) (identification score of 2.29) by using MALDI-TOF-MS (BrukerDaltonics, Bremen, Germany). The antimicrobial susceptibility was carried out using Kirby-Bauer disc diffusion method and the antibiotics tested were chosen from the available literature as there are no CLSI guidelines available till now[5,6]. The isolate was sensitive to gentamicin, ceftriaxone, aztreonam, piperacillin-tazobactam and imipenem, and resistant to ampicillin, ciprofloxacin, levofloxacin, vancomycin and colistin. The patient was started on piperacillin-tazobactam and responded well to the treatment. The patient improved clinically and the follow-up urine culture after two weeks of therapy was sterile.
E. miricola was first isolated from Mir space station, Russia and hence named as E. miricola[7]. Previously, it was classified into genus Chryseobacterium but later in 2005, the genus was changed to Elizabethkingia on the basis of the comparative analytical studies involving DNA hybridization and sequencing of the 16S rRNA region[8]. E. miricola is a gram-negative (0.5 μm × 1-2.5 μm), non-motile, non-spore-forming bacterium. It grows well on blood and MacConkey agar producing non-fermenting sticky colonies. Biochemical reactions show indole positive, citrate positive, produce acid from D-glucose, D-fructose, D-lactose, trehalose, D-mannitol, D-maltose, but not from D-xylose, L-arabinose, D-cellobiose, sucrose and raffinose. It can be differentiated from Chryseobacterium because of the absence of yellow pigment in culture. Urease production is the test used to differentiate E. miricola from E. meningoseptica[8]. Till date, E. miricola has been isolated from blood and sputum and has been found to be responsible for sepsis. The first case of E. miricola was reported in 2008 in an adult with mantle cell carcinoma, who underwent stem cell transplant[5]. In this case, E. miricola was isolated from sputum and blood and the identification was confirmed using 16S rRNA sequencing. Later on, E. miricola was isolated from the blood sample of a young female with alcoholic pancreatitis[6]. More recently, E. miricola has been isolated from a patient with severe sepsis and pulmonary abscess[9]. In both the above cases, the isolate was identified by MALDI-TOF. In the present case, E. miricola was isolated from the urine sample of a young female with clinical features of UTI and bilateral hydroureteronephrosis. The clinical presentation pointed towards differential diagnosis like pyelonephritis, renal abscess, renal infarction, venous obstruction or ATN. However, the USG findings of bilateral hydroureteronephrosis and sterile blood culture pointed towards localised urinary tract infection.
E. miricola has been found to be multidrug resistant similar to E. meningoseptica which is known to harbor β-lactamases showing resistant to β-lactams and carbapenems[10]. The E. miricola isolates have been found to be resistant to many antibiotics. Previous studies have shown resistance to ampicillin, ceftazidime, imipenem, gentamicin, cotrimoxazole, colistin and with variable susceptibility to ciprofloxacin, vancomycin and rifampicin[5,6,11]. It is interesting to note that, E. miricola isolates in previous studies were sensitive to levofloxacin, but in our case, the isolate was resistant to both ciprofloxacin and levofloxacin. Limited clinical reports, varied susceptibility profiles, lack of antimicrobial susceptibility breakpoint and no defined consensus for the empiric treatment regimen makes it difficult to treat such rare organisms.
We present the first case report of human UTI caused by rare multidrug resistant E. miricola. The present case emphasizes the clinical importance of rare non-fermenters like E. miricola in human infections especially in case of UTI. The knowledge of newer species and their antimicrobial susceptibility profile will help in formulating appropriate antibiotic treatment regimens to tackle such rarely encountered bacteria.
A 25-year-old female complaining of difficulty in micturition, oliguria fever with abdominal pain.
Urinary tract infections (UTI) with bilateral hydroureteronephrosis.
Chronic pyelonephritis.
The routine laboratory investigations revealed anemia, leucopenia, hyperkalemia, hyperuricaemia and elevated creatinine levels. Urine culture had significant bacterial growth (colony count >105 CFU/mL) of Elizabethkingia miricola (E. miricola).
Bilateral hydroureteronephrosis.
Bilateral hydroureteronephrosis with urinary tract infection.
Piperacillin-tazobactam.
E. miricola has been reported to cause sepsis and pulmonary infection.
Rare non-fermenters can cause UTI and prompt identification is required to guide proper antimicrobial therapy. CLSI/EUCAST guidelines need to be developed.
Interesting case of unusual bacterial cause of UTI with a severe clinical scenario.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: India
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P- Reviewer: Simone G, Woo HH S- Editor: Song XX L- Editor: A E- Editor: Lu YJ
| 1. | Hausler SM. Urinary tract infections. Topley and Wilson’s Microbiology and Microbial infections. 9th edition. London: Edward Arnold 1998; . |
| 2. | Warren JW. Clinical presentations and epidemiology of urinary tract infection. Urinary tract infections molecular pathogenesis and clinical management. Washington DC: American Society for Microbiology Press 1996; 3-27. |
| 3. | KLS , Rao GG, Kukkamalla AM. Prevalence of Non-fermenters In Urinary Tract Infections In A Tertiary Care Hospital. Webmed Central Microbiology. 2011;2:WMC001464. |
| 4. | Wilson ML, Gaido L. Laboratory diagnosis of urinary tract infections in adult patients. Clin Infect Dis. 2004;38:1150-1158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 375] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 5. | Green O, Murray P, Gea-Banacloche JC. Sepsis caused by Elizabethkingia miricola successfully treated with tigecycline and levofloxacin. Diagn Microbiol Infect Dis. 2008;62:430-432. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 6. | Rossati A, Kroumova V, Bargiacchi O, Brustia D, Luigi Garavelli P. Elizabethkingia miricola bacteriemia in a young woman with acute alcoholic pancreatitis. Presse Med. 2015;44:1071-1072. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 7. | Li Y, Kawamura Y, Fujiwara N, Naka T, Liu H, Huang X, Kobayashi K, Ezaki T. Chryseobacterium miricola sp. nov., a novel species isolated from condensation water of space station Mir. Syst Appl Microbiol. 2003;26:523-528. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 81] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 8. | Kim KK, Kim MK, Lim JH, Park HY, Lee ST. Transfer of Chryseobacterium meningosepticum and Chryseobacterium miricola to Elizabethkingia gen. nov. as Elizabethkingia meningoseptica comb. nov. and Elizabethkingia miricola comb. nov. Int J Syst Evol Microbiol. 2005;55:1287-1293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 194] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 9. | Gonzalez C, Coolen-Allou N, Allyn J, Estève JB, Belmonte O, Allou N. [Severe sepsis and pulmonary abscess with bacteremia due to Elizabethkingia miricola]. Med Mal Infect. 2016;46:49-51. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 14] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 10. | Jung SH, Lee B, Mirrakhimov AE, Hussain N. Septic shock caused by Elizabethkingia meningoseptica: a case report and review of literature. BMJ Case Rep. 2013;2013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 11. | Hoque SN, Graham J, Kaufmann ME, Tabaqchali S. Chryseobacterium (Flavobacterium) meningosepticum outbreak associated with colonization of water taps in a neonatal intensive care unit. J Hosp Infect. 2001;47:188-192. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 87] [Article Influence: 3.6] [Reference Citation Analysis (0)] |