Published online Apr 16, 2017. doi: 10.12998/wjcc.v5.i4.134
Peer-review started: November 9, 2016
First decision: November 30, 2016
Revised: January 8, 2017
Accepted: January 16, 2017
Article in press: January 18, 2017
Published online: April 16, 2017
Processing time: 158 Days and 1.1 Hours
To determine the prevalence of esophageal squamous papillomas (ESPs) in a tertiary teaching hospital and to assess for any clinical associations, including relations with esophageal squamous cell carcinomas (SCCs).
Data from a total of 6962 upper gastrointestinal endoscopies over a five year period were retrospectively obtained and analysed.
ESP was found in sixteen patients (0.23%). Eight (50%) patients had a high body mass index, seven (44%) had history of cigarette smoking. Reflux esophagitis was found in four (25%) patients. All ESPs were solitary with a mean endoscopic size of 3.8 mm and located in the mid to lower esophagus. Human papilloma virus (HPV) was tested in three (19%) patients and was negative. Esophageal SCC was found in seven patients (0.10%) during the same period. None of the specimens were tested for HPV, and none had associated papillomatous changes.
ESP is an uncommon tumour with unclear clinical associations and malignant potential.
Core tip: Esophageal squamous papilloma is a rare endoscopic finding with uncertain clinicopathological associations. They are usually asymptomatic and their aetiology is unknown. A high body mass index and a history of cigarette smoking, both risk factors for gastroesophageal reflux disease, were the most prevalent patient characteristic in our cohort with esophageal squamous papillomas (ESPs), however no definite associations can be established. None of the esophageal squamous cell carcinomas during the same study period progressed from ESP. Long-term longitudinal studies would be valuable to clarify clinical associations and the malignant potential of ESPs in order to establish appropriate management and surveillance strategies.
- Citation: Jideh B, Weltman M, Wu Y, Chan CHY. Esophageal squamous papilloma lacks clear clinicopathological associations. World J Clin Cases 2017; 5(4): 134-139
- URL: https://www.wjgnet.com/2307-8960/full/v5/i4/134.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i4.134
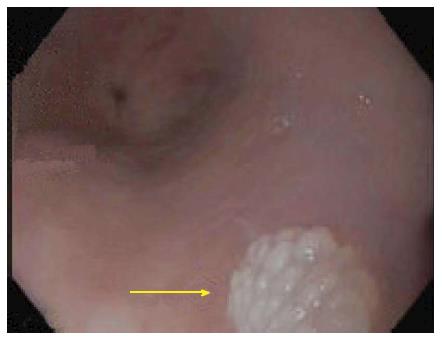
Esophageal squamous papilloma (ESP) is a rare tumour of the esophagus with a reported prevalence of 0.01% to 0.45%[1-5]. Lesions rarely cause symptoms, and are usually an incidental finding on endoscopy. Typical endoscopic appearance is that of a small, less than 5mm sessile wart-like fleshy nodule (Figure 1) located predominantly in the middle to lower esophagus[1,2]. Larger lesions with a more raised, erythematous appearance have also been described[6]. The aetiology has not yet been established; proposed factors include chronic gastroesophageal reflux disease (GERD), human papilloma virus (HPV) and mucosal trauma[5,7-13]. The clinical associations and malignant potential of these lesions is unknown. Currently, there is no consensus on appropriate management and surveillance strategies for ESPs. In this study we aimed to identify the prevalence of ESPs in an Australian tertiary hospital cohort and to assess for possible clinical associations. We also attempted to assess its association with esophageal squamous cell carcinoma (SCC).
All patients between June 2010 and March 2015 with ESP and esophageal SCC at a tertiary teaching hospital (Nepean Hospital) were retrospectively identified using the electronic pathology department database. Over this period a total of 6962 upper gastrointestinal endoscopies were performed. Patients were identified and their medical records and endoscopic reports were reviewed and analysed. The clinical information assessed included age, gender, body mass index (BMI), cigarette smoking history and use of acid suppression therapy [proton pump inhibitor (PPI)]. The endoscopic findings comprised the location, size and number of lesions; presence of a hiatus hernia, and presence of reflux esophagitis. Results of HPV testing were noted when performed; histology reports for patients with esophageal SCC during the same period were carefully perused for any papillomatous changes.
Among the 6962 upper gastrointestinal endoscopies performed over the study period, sixteen patients were found to have ESPs giving a prevalence of 0.23%. Patient characteristics are summarised in Table 1.The patients with ESPs comprised of ten (62.5%) females with mean age of 52 ± 16 (SD) years (range 33-83 years). Eight (50%) patients were overweight or obese having BMIs between 25-39; seven (44%) patients were cigarette smokers; three (19%) patients were using regular acid suppression therapy (PPIs). The indications for the endoscopic procedure varied with two of the sixteen patients having the procedure for investigation of GERD and one patient for dysphagia. One patient had evidence of a hiatus hernia, which was small. Reflux esophagitis was found in four (25%) of the sixteen patients. All patients had solitary papillomas; mean endoscopic size of lesions was 3.8 ± 3.2 (SD) mm (range 1-12 mm) and the mean size of histological specimens was 2.9 ± 1.5 (SD) mm (range 1-7 mm). All the papillomas were found in the middle to lower esophagus. Seven (44%) of them were biopsied; seven (44%) were removed with a polypectomy snare and details of the remaining two were not documented. Patients that had lesions biopsied did not have a repeat gastroscopy within the same study period for definitive resection of the lesion. Two (13%) patients had repeat endoscopies following endoscopic snare resection within the same study period and there was no evidence of papilloma recurrence. Helicobacter pylori was not evident on microscopy in any of the patients. HPV testing was performed on only three patient and all were negative.
| Patient No. | Age (yr) | Sex(M/F) | Cigarette smoking (Y/N) | BMI | PPI use (Y/N) | Indication for endoscopy | Location of ESP from incisors | No. of ESPs | Endoscopic size of ESP (mm) | Histological size of ESP specimen (mm) | Hiatus hernia (Y/N) | Reflux esophagitis (Y/N) | Helicobacter pylori (positive/negative) | HPV test (positive/negative) | Method of ESP resection/sampling |
| 1 | 57 | F | Y | 20 | N | Upper GI bleed | Distal | 1 | Small | 2 | N | N | Negative | n/a | n/a |
| 2 | 83 | F | N | 19 | Y | Dyshpagia | 20 | 1 | 3 | 3 | N | Y | Negative | n/a | Snare polypectomy |
| 3 | 66 | M | N | 24 | N | Abdominal pain | 32 | 1 | Small | 4 | N | N | Negative | n/a | Snare polypectomy |
| 4 | 74 | M | N | 36 | N | Abdominal pain | 39 | 1 | Small | 5 | N | N | n/a | n/a | Hot biopsy |
| 5 | 60 | F | N | 34 | Y | GERD | 33 | 1 | 4 | 3 | Y (3 cm) | Y | Negative | n/a | Snare polypectomy |
| 6 | 39 | M | N | 30 | N | GERD | 38 | 1 | 3 | 3 | N | Y | Negative | Negative | Snare polypectomy |
| 7 | 39 | F | N | 24 | N | Bloating | 28 | 1 | 3 | 2 | N | N | Negative | n/a | Biopsy |
| 8 | 33 | F | N | 21 | N | Anaemia | 27 | 1 | Small | 3 | N | Y | Negative | n/a | Snare polypectomy |
| 9 | 49 | M | Y | 30 | N | Abdominal pain, family history CRC | Distal | 1 | Small | 3 | N | N | Negative | n/a | n/a |
| 10 | 37 | F | N | 24 | N | Family history of gastric cancer | 32 | 1 | 1 | 1 | N | N | Negative | Negative | Biopsy |
| 11 | 54 | F | Y | 23 | N | Variceal screen | 35 | 1 | 2 | 4 | N | N | n/a | n/a | Biopsy |
| 12 | 39 | M | Y | 34 | N | Abdominal pain | 39 | 1 | 4 | 2 | N | N | Negative | n/a | Biopsy |
| 13 | 81 | F | Y | 28 | N | Diarrhoea | 25 | 1 | Small | 2 | N | N | Negative | n/a | Biopsy |
| 14 | 45 | M | Y | 31 | Y | Abdominal pain | Upper third | 1 | 2 | 2 | N | N | Negative | n/a | Snare polypectomy |
| 15 | 38 | F | Y | n/a | N | Bloating, abdominal pain | 25 | 1 | n/a | 1 | n/a | n/a | Negative | n/a | Biopsy |
| 16 | 41 | F | N | 27 | N | Abdominal pain | 35 | 1 | 12 | 7 | N | N | Negative | Negative | Snare polypectomy |
Seven patients were observed to have esophageal SCC in the same period, giving a prevalence of 0.10%. Patient characteristics are summarised in Table 2. The group comprised of five (71%) females and with mean age of 71 ± 15 (SD) years (range 50-92 years). Two (29%) patients were overweight, one (14%) patient was underweight with a BMI of 18, and the remaining four (57%) patients had BMIs within healthy range. Three (43%) patients were cigarette smokers. HPV was not tested on any of the specimens. There were no reported papillomatous changes on histological examination.
| Patient No. | Age (yr) | Sex (M/F) | Cigarette smoking (Y/N) | BMI | Indication for endoscopy | Location of SCC from incisors (cm) | HPV test (positive/negative) | Papillomatous changes on histopathology | Management of SCC |
| 1 | 50 | M | Y | 21 | Dysphagia, B/G achalasia | 40 | n/a | No | Ivor-Lewis esophagectomy |
| 2 | 92 | F | N | 29 | Dysphagia | 32 | n/a | No | Palliation |
| 3 | 62 | F | Y | 25 | n/a | Middle | n/a | No | Ivor-Lewis esophagectomy |
| 4 | 86 | F | N | 20 | Dysphagia | 15 | n/a | No | Radiation therapy, Palliation |
| 5 | 76 | F | N | 20 | n/a | Middle | n/a | No | Neoadjuvant Chemo-Radiation, Ivor-Lewis esophagectomy |
| 6 | 59 | F | N | 18 | n/a | Middle | n/a | No | Ivor-Lewis esophagectomy, Chemotherapy, Palliation |
| 7 | 72 | M | Y | 20 | Dyspnoea. B/G achalasia | Distal | n/a | No | Radiation therapy, PEG tube feeding, Palliation |
In our study the prevalence of ESPs was 0.23% which is consistent with previously published studies[1-5]. The majority of the patients were middle-aged also similar to previous studies in the literature. The female predominance in our cohort is an inconsistent observation compared to previous reports on ESPs[1,7,8,14]. Although GERD has been postulated to be a factor in the aetiology of ESP[5,9,15], only two (12.5%) of our study patients underwent upper endoscopy for GERD. However, we cannot ascertain with any certainty that the other patients did not have GERD. This is supported by the finding of reflux esophagitis in two (12.5%) patients who had the procedure for an indication other than GERD (one for the investigation of anaemia and the other for dysphagia, Table 1).
A high BMI was the most prevalent of the assessed patient characteristics in our study with 50% of patients having a BMI in the overweight-obese range. An association between BMI and ESPs has not been previously demonstrated. However, an elevated BMI is an established risk factor for GERD[16]. The second most prevalent clinical characteristic in the studied patients was a history of cigarette smoking found in seven (44%) patients. Cigarette smoking was not found to be associated with ESP in a previous study[14], but similar to a high BMI, cigarette smoking is a risk factor for the development of GERD[17]. Hiatus hernia is another risk factor for GERD which was observed in one (6.25%) patient in our cohort and it was small-sized.
The mean size and location of ESPs were consistent with previous observations[1,2]. They were all solitary and appeared as rounded well delineated sessile wart-like lesions (Figure 1) as traditionally described. Multiple lesions have been observed in some studies[18-20].
ESPs were not all removed with therapeutic intent, which is the general recommendation, despite the ambiguity about their malignant potential[21]. Histological diagnosis remains important due to the endoscopic resemblance to other pathologies including glycogenic acanthosis, verrucoid border of SCC, and verrucous carcinoma[2,21]. Case reports of alternative ablative techniques including radiofrequency ablation have been described[22]. Recurrence after definitive endoscopic removal is thought to be low[2]. This was true for the two patients in our series that had repeat gastroscopies within the same study period and no evidence of papilloma recurrent was found. It is unclear whether other lesions not endoscopically removed were not followed due to lack of well-established management and surveillance guidelines.
Three patients in our cohort had testing for HPV (serotype 16) in the ESP specimen and the results were all negative. Although HPV infection is a proposed aetiological factor since the demonstration of HPV antigens in ESPs[23], the extent of the contribution is controversial and most reported lesions, similar to our study, are found in the absence of HPV[2,13,14,24,25]. Helicobacter pylori has not been proposed to have any association in any of the previous ESP studies, and in our cohort the bacterium was not detected on microscopy in any of the patients.
The prevalence of esophageal SCC in this study was 0.10%. Most patients (71%) were females and generally older than the cohort with ESPs years. The risk of esophageal SCC, unlike esophageal adenocarcinomas, is not generally increased with obesity[26] and this was true in our cohort with five (71%) patients having BMIs within healthy range. Cigarette smoking is an established risk factor for esophageal SCC and in our group three (43%) patients had a history of cigarette smoking.
HPV was not tested in any of the esophageal SCC specimens in our cohort, neither were any papillomatous changes reported. Whilst HPV infection and papilloma formation are considered a precursor in cervical and oropharyngeal squamous carcinoma[27,28], the relation between HPV and esophageal SCC is controversial with conflicting results across multiple studies. Several systematic reviews and meta-analyses have addressed this relation, two of the most recent by Li et al[29] and who Petrick et al[30] concluded that further studies are needed to clarify the association.
This study has several limitations. The study is a retrospective assessment of results which can lead to the possibility of inaccurate and incomplete data. It was performed in a single, tertiary-care institution which can introduce a selection bias. Most patients with ESP did not have follow-up gastroscopies to assess for ESP clearance or recurrence. Finally, the analysis of results is largely descriptive given the low prevalence and small absolute numbers of patients with ESPs making it difficult to draw conclusions on any clinical associations.
In summary, ESPs remains a rare endoscopic finding with uncertain clinicopathological associations. They are usually asymptomatic and their aetiology is unknown. Whilst a high BMI and a history of cigarette smoking, both risk factors for GERD, were the most prevalent patient characteristic in our cohort with ESP, no definite associations can be established. None of the esophageal SCCs during the same study period progressed from ESP. Long-term longitudinal studies would be valuable to clarify clinical associations and the malignant potential of ESPs in order to establish appropriate management and surveillance strategies.
Esophageal squamous papilloma (ESP) is a rare tumour with a reported prevalence of 0.01% to 0.45%. It is usually asymptomatic and discovered incidentally on upper endoscopy. The aetiology, clinical associations along with its malignant potential are unknown. The aim of this study was to determine the prevalence of ESPs in a tertiary teaching hospital and to assess for any clinical associations, including relations with esophageal squamous cell carcinomas (SCCs).
There are limited studies on ESPs. Gastroesophageal reflux disease (GERD), human papilloma virus (HPV) and mucosal trauma are proposed aetiological factors. No studies have assessed associations between ESPs and SCCs.
This study identified certain clinical features to be prevalent in patients with ESP including high body mass index and cigarette smoking, which have not been previously described. Also, the SCCs in the study period did not seem to progress from ESPs which may suggest ESP are benign.
This study contributes to the body of hypotheses surrounding ESP. Large longitudinal studies are required to help clarity clinicopathological associations of ESPs and their malignancy potential in order to establish appropriate management and surveillance strategies.
The authors aimed to identify the prevalence of ESPs in an Australian tertiary hospital cohort and to assess for possible clinical associations and to assess its association with esophageal SCC whose large data from a total of 6962 upper gastrointestinal endoscopies. Well written, well balanced.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Australia
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D, D
Grade E (Poor): 0
P- Reviewer: Hokama A, Hoff DAL, Lin Q, Watanabe M S- Editor: Ji FF L- Editor: A E- Editor: Li D
| 1. | Takeshita K, Murata S, Mitsufuji S, Wakabayashi N, Kataoka K, Tsuchihashi Y, Okanoue T. Clinicopathological characteristics of esophageal squamous papillomas in Japanese patients--with comparison of findings from Western countries. Acta Histochem Cytochem. 2006;39:23-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 2. | Mosca S, Manes G, Monaco R, Bellomo PF, Bottino V, Balzano A. Squamous papilloma of the esophagus: long-term follow up. J Gastroenterol Hepatol. 2001;16:857-861. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 63] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 3. | Orlowska J, Jarosz D, Gugulski A, Pachlewski J, Butruk E. Squamous cell papillomas of the esophagus: report of 20 cases and literature review. Am J Gastroenterol. 1994;89:434-437. [PubMed] |
| 4. | Sablich R, Benedetti G, Bignucolo S, Serraino D. Squamous cell papilloma of the esophagus. Report on 35 endoscopic cases. Endoscopy. 1988;20:5-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 5. | Franzin G, Musola R, Zamboni G, Nicolis A, Manfrini C, Fratton A. Squamous papillomas of the esophagus. Gastrointest Endosc. 1983;29:104-106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Behrens A. Endoscopic Imaging of a Large Esophageal Papilloma. VJGIEN. 2013;1:29-30. [RCA] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 7. | Carr NJ, Bratthauer GL, Lichy JH, Taubenberger JK, Monihan JM, Sobin LH. Squamous cell papillomas of the esophagus: a study of 23 lesions for human papillomavirus by in situ hybridization and the polymerase chain reaction. Hum Pathol. 1994;25:536-540. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 29] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 8. | Carr NJ, Monihan JM, Sobin LH. Squamous cell papilloma of the esophagus: a clinicopathologic and follow-up study of 25 cases. Am J Gastroenterol. 1994;89:245-248. [PubMed] |
| 9. | Fernández-Rodríguez CM, Badia-Figuerola N, Ruiz del Arbol L, Fernández-Seara J, Dominguez F, Avilés-Ruiz JF. Squamous papilloma of the esophagus: report of six cases with long-term follow-up in four patients. Am J Gastroenterol. 1986;81:1059-1062. [PubMed] |
| 10. | Parnell SA, Peppercorn MA, Antonioli DA, Cohen MA, Joffe N. Squamous cell papilloma of the esophagus. Report of a case after peptic esophagitis and repeated bougienage with review of the literature. Gastroenterology. 1978;74:910-913. [PubMed] |
| 11. | Polit SA. Squamous cell papillomas of the esophagus. J Pediatr Gastroenterol Nutr. 1990;11:285-287. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 12. | Politoske EJ. Squamous papilloma of the esophagus associated with the human papillomavirus. Gastroenterology. 1992;102:668-673. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 41] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Poljak M, Cerar A, Orlowska J. p53 protein expression in esophageal squamous cell papillomas: a study of 36 lesions. Scand J Gastroenterol. 1996;31:10-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 14. | Talamini G, Capelli P, Zamboni G, Mastromauro M, Pasetto M, Castagnini A, Angelini G, Bassi C, Scarpa A. Alcohol, smoking and papillomavirus infection as risk factors for esophageal squamous-cell papilloma and esophageal squamous-cell carcinoma in Italy. Int J Cancer. 2000;86:874-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 15. | Odze R, Antonioli D, Shocket D, Noble-Topham S, Goldman H, Upton M. Esophageal squamous papillomas. A clinicopathologic study of 38 lesions and analysis for human papillomavirus by the polymerase chain reaction. Am J Surg Pathol. 1993;17:803-812. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 50] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 16. | Cook MB, Greenwood DC, Hardie LJ, Wild CP, Forman D. A systematic review and meta-analysis of the risk of increasing adiposity on Barrett’s esophagus. Am J Gastroenterol. 2008;103:292-300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 92] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 17. | Kahrilas PJ, Gupta RR. Mechanisms of acid reflux associated with cigarette smoking. Gut. 1990;31:4-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 134] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 18. | Brinson RR, Schuman BM, Mills LR, Thigpen S, Freedman S. Multiple squamous papillomas of the esophagus associated with Goltz syndrome. Am J Gastroenterol. 1987;82:1177-1179. [PubMed] |
| 19. | Darani M, Villa F. Multiple squamous papillomas of the esophagus diagnosed by endoscopy. JAMA. 1976;236:2655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 20. | Sandvik AK, Aase S, Kveberg KH, Dalen A, Folvik M, Naess O. Papillomatosis of the esophagus. J Clin Gastroenterol. 1996;22:35-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 25] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Lavergne D, de Villiers EM. Papillomavirus in esophageal papillomas and carcinomas. Int J Cancer. 1999;80:681-684. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 22. | Del Genio G, Del Genio F, Schettino P, Limongelli P, Tolone S, Brusciano L, Avellino M, Vitiello C, Docimo G, Pezzullo A. Esophageal papilloma: Flexible endoscopic ablation by radiofrequency. World J Gastrointest Endosc. 2015;7:290-294. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 6] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 23. | Syrjänen K, Pyrhönen S, Aukee S, Koskela E. Squamous cell papilloma of the esophagus: a tumour probably caused by human papilloma virus (HPV). Diagn Histopathol. 1982;5:291-296. [PubMed] |
| 24. | Chang F, Janatuinen E, Pikkarainen P, Syrjänen S, Syrjänen K. Esophageal squamous cell papillomas. Failure to detect human papillomavirus DNA by in situ hybridization and polymerase chain reaction. Scand J Gastroenterol. 1991;26:535-543. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 28] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 25. | Loke SL, Ma L, Wong M, Srivastava G, Lo I, Bird CC. Human papillomavirus in oesophageal squamous cell carcinoma. J Clin Pathol. 1990;43:909-912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 26. | Lagergren J, Bergström R, Nyrén O. Association between body mass and adenocarcinoma of the esophagus and gastric cardia. Ann Intern Med. 1999;130:883-890. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 480] [Cited by in RCA: 441] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 27. | Franco EL, Duarte-Franco E, Ferenczy A. Cervical cancer: epidemiology, prevention and the role of human papillomavirus infection. CMAJ. 2001;164:1017-1025. [PubMed] |
| 28. | Mehanna H, Beech T, Nicholson T, El-Hariry I, McConkey C, Paleri V, Roberts S. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head Neck. 2013;35:747-755. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 683] [Cited by in RCA: 619] [Article Influence: 51.6] [Reference Citation Analysis (0)] |
| 29. | Li X, Gao C, Yang Y, Zhou F, Li M, Jin Q, Gao L. Systematic review with meta-analysis: the association between human papillomavirus infection and oesophageal cancer. Aliment Pharmacol Ther. 2014;39:270-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 66] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 30. | Petrick JL, Wyss AB, Butler AM, Cummings C, Sun X, Poole C, Smith JS, Olshan AF. Prevalence of human papillomavirus among oesophageal squamous cell carcinoma cases: systematic review and meta-analysis. Br J Cancer. 2014;110:2369-2377. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |