Published online Nov 16, 2017. doi: 10.12998/wjcc.v5.i11.390
Peer-review started: December 19, 2016
First decision: March 28, 2017
Revised: May 24, 2017
Accepted: June 12, 2017
Article in press: June 13, 2017
Published online: November 16, 2017
Processing time: 336 Days and 3.3 Hours
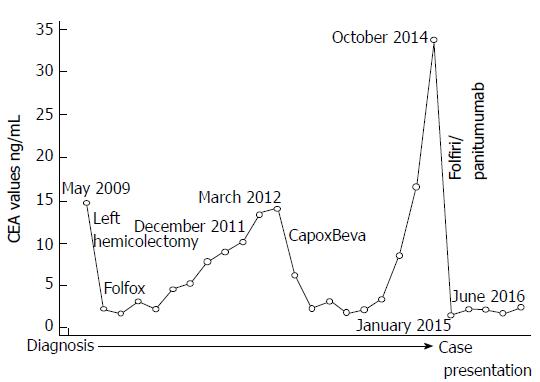
Here we report a case of a 70-year-old man who received adjuvant chemotherapy with fluorouracile, folinic acid and oxaliplatin after a left hemicolectomy for a stage IIIb adenocarcinoma in May 2009. During follow-up he de-veloped abdominal lymphnodes metastases evidenced by positron emission tomography- computed tomography (PET-CT) scan and increase of carcinoembryonic antigen (CEA) level. Chemotherapy with capecitabine, oxaliplatin and bevacizumab was started in April 2012. Restaging showed a complete response and normalization of CEA. The patient received maintenance therapy with bevacizumab which was stopped in December 2013 for patient choice. In October 2014, a new increase in CEA was documented and PET-CT scan showed lung metastases. Analysis of RAS status revealed the absence of mutations, then the patient started a second-line chemotherapy with fluorouracile, folinic acid, irinotecan (folfiri) and panitumumab achieving, in January 2015, a complete response and normalization of CEA. Thereafter, folfiri was discontinued for toxicity; furthermore, upon the third occurrence of a grade 3 dermatologic toxicity, panitumumab was continued from June 2015 at 60% of the original dose and it was administered every three weeks. Until presentation of this case, the patient maintains a complete response, has no symptoms of disease and CEA is normal. Interestingly, this patient presented a high proportion of circulating natural killer (NK) cells (35.1%) with high cytotoxic activity against tumor cells. Study on the role of NK in patients with advanced colorectal cancer are ongoing.
Core tip: The case presented here shows a patients with metastatic colorectal cancer (mCRC) and long-lasting responses to different treatments including chemotherapies and targeted therapies; in particular, the patient had a long-lasting complete response to panitumumab. Additionally, he presented a high proportion of circulating natural killer (NK) cells displaying high cytotoxic activity against tumor cells in vitro. Interestingly, we previously reported that patients affected by mCRC with high NK-cell cytotoxicity showed a significantly higher response rate and a longer progression-free survival compared with patients with low NK-cell cytotoxicity. Study on the role of NK in patients with mCRC should be improved.
- Citation: Ottaiano A, Napolitano M, Capozzi M, Tafuto S, Avallone A, Scala S. Natural killer cells activity in a metastatic colorectal cancer patient with complete and long lasting response to therapy. World Journal of Clinical Cases 2017; 5(11): 390-396
- URL: https://www.wjgnet.com/2307-8960/full/v5/i11/390.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v5.i11.390
Colorectal cancer (CRC) is one of most common cancers and leading causes of mortality worldwide. Unfortunately, about twenty percent of patients with CRC have clinical evidence of metastatic disease at diagnosis and about 50% of patients will develop metastases later[1].
To date, first and second-line treat-ment of metastatic CRC (mCRC) are based on combinations of chemotherapy (fluorouracil, oxaliplatin, irinotecan) and biologic drugs (bevacizumab, cetuximab, panitumumab). Anti-EGFR agents (cetuximab and panitumumab) are reserved for RAS wild-type (RAS wt) tumors. In fact, when RAS is mutated, PI3K results in constitutive activation of its downstream signaling pathway so that tumor cells become independent of EGFR signaling inactivation by anti-EGFR drugs. Large randomized multicenter phase III clinical trials confirmed the predictive value of KRAS for anti-EGFR therapy[2,3] and a meta-analysis of 11 studies showed that KRAS status was closely associated with the response rate (P < 0.001) and PFS (P < 0.005)[4]. KRAS mutation is a predictive marker for the efficacy of anti-EGFR agents in the treatment of mCRC as stated in guidelines from the National Comprehensive Cancer Network, European Society for Medical Oncology, and Japanese Society for Cancer of the Colon and Rectum, which recommend the use of antibodies to EGFR only for mCRC patients with WT K-RAS. In addition, N-RAS mutations have been recently included, defining the “RAS status” as the new validated marker of response to antibodies to EGFR[5].
Panitumumab is a fully humanized monoclonal antibody against EGFR approved in RAS wt mCRC as first-line therapy in association with folfox (fluorouracile, folinic acid, oxaliplatin), second-line in association with folfiri (fluorouracile, folinic acid, irinotecan) and as monotherapy following disease progression after prior treatment with fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy. The use of chemotherapy and biologic drugs in a flexible and personalized context of multidisciplinary approach (continuum of care) has improved survival of patients with mCRC which in some cases exceed two years[6].
In last years, many evidences are accumulating on the role of immune system cells in controlling tumors at different stages of disease (from the initiation to the metastatic spread and growth)[7,8]. The issue is very complex since the interactions between components of the immune system and tumor cells are largely unknown. We recently reported that polymorphisms of receptors involved in ADCC (antibody-mediated cellular cytotoxicity) as well as the NK cells activity of patients affected by mCRC were predictive and prognostic[9]. Additionally, MDSCs (myeloid-derived suppressor cells) and Tregs (regulatory T-cell) are a component of the immune system that may promote tumor progression[10] by inhibiting both innate and adaptive immune responses. In particular they are able to suppress conventional effector immune cells (T cells, NK cells, macrophages) which play an important role in anti-tumor responses.
Here we report a case of a mCRC patient responding to first and second-line chemotherapies and with a durable complete response to panitumumab single agent. NK cell activity of peripheral blood lymphocytes (PBL) was evaluated. As additional information, also MDSCs and Tregs were characterized.
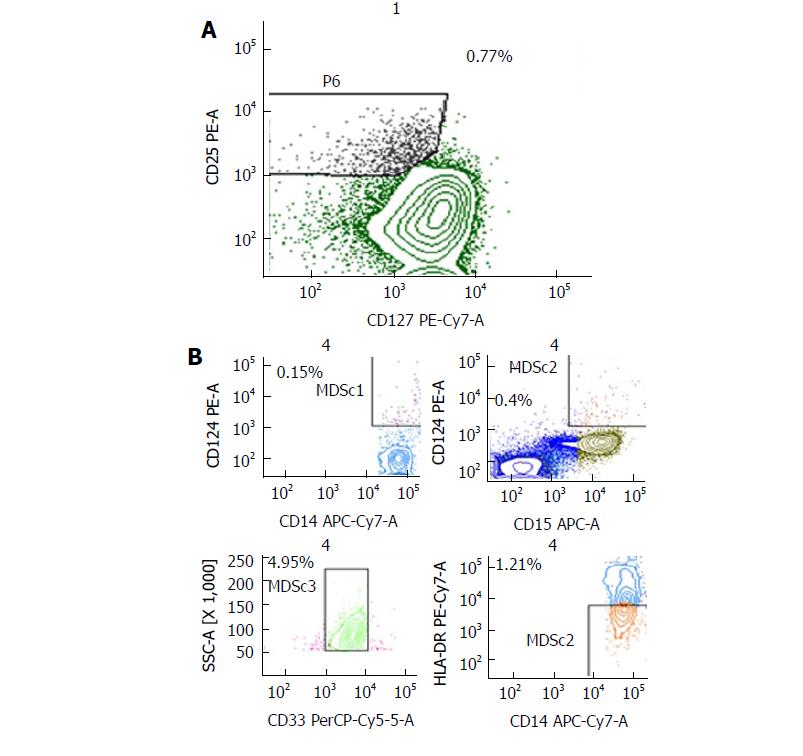
Flow cytometry was performed on fresh venous blood (BD Biosciences), using a FACSCanto II 6-colour flow cytometer and analyzed using BD FACSDiva™ Software version 6.2 (BD Bioscience, San Jose, CA, United States), daily calibrated with Calibrite beads (Fitc, Pe, PerCP and APC) and Compbeads (Pe-Cy7 and APC-Cy7; Becton Dickinson, San Jose, CA, United States). For identification of circulating Tregs the following fluorochrome labeled anti-human monoclonal antibodies were used: CD4, CD25, CD127, FOXP3, CD45RA, CD45R0. The classical populations were CD4+/CD25+/CD127low/FOXP3int/CD45RA+ (naïve Treg cells) versus CD4+/CD25+/CD127low/FOXP3high/CD45RA- (activated Treg cells). CD39 (ENTPD1), CD152 (CTLA-4), CD184 (CXCR4), and CD279 (PD-1) were also evaluated (data not shown). For identification of circulating MDSCs: Anti-Lineage 1 antibodies (CD3, CD14, CD16, CD19, CD20, CD56), CD11b, CD33, HLA-DR, CD15 and CD14 (BD Bioscience, San Diego, CA, United States). The classical populations are shown (four types indicated as MDSC1, 2, 3, 4): 1, CD14+/CD124+; 2, CD15+/CD124+; 3, CD33+/SSC+, 4, CD14+/HLADR-/low. For circulating NK cells: CD3, CD16, CD56, CD158a, CD158b, CD161 and CD279 (PD-1). Monoclonal antibodies were used together with the appropriate corresponding isotype controls.
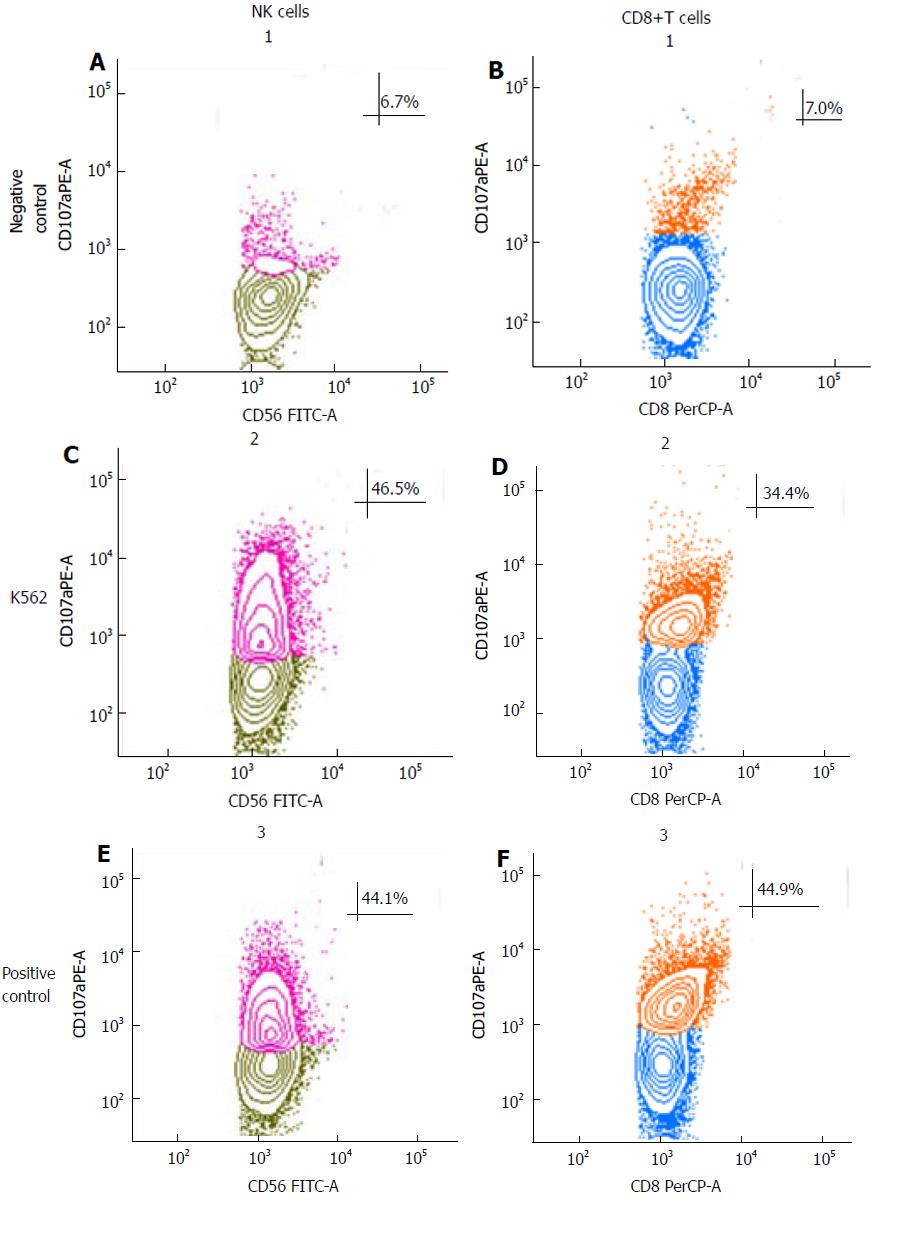
NK-cell mediated cytotoxicity was evaluated using the degranulation lysosomal marker LAMP-1 or CD107a as described[11]. Blood was transferred to cell culture flasks and diluted with one volume RPMI-1640 containing 10% heat-inactivated fetal bovine serum and supplemented with 100 U/mL IL-2. Samples were incubated overnight at 37 °C in a humidified 5% CO2 for 18 h. The cytotoxic activity of NK cells was tested against NK sensitive cell line K562, as previously described[12]. Briefly, 200 μL of IL-2 preactivated blood were co-cultured with 2 × 105 K562 at 5:1, 10:1 and 20:1 effector:target (e:r) ratios (only 10:1 e:r ratio experiments are shown), medium alone served as the negative control and the positive control were stimulated with phorbol-12-myristate-13-acetate (PMA) (2.5 μg/mL) and ionomycin (0.5 μg/mL) (Sigma), in presence of PE-conjugate anti-CD107a antibody (BD Bioscience, San Jose, CA, United States) at 37 °C in 5% CO2. Control samples were incubated without target cells to detect spontaneous degranulation. Following a 3-h culture, cells were stained with FITC-conjugated anti-CD56, PerCP anti-CD8 and Pe-Cy7 anti CD3 (BD Bioscience, San Jose, CA, United States). NK cells were defined as CD3-CD56+ in the lymphocyte gate. CD107a expression on CD3-CD56+ NK cells and CD8 cytotoxic T cells were analyzed using a FACSCanto II 6-colour flow cytometer whit BD FACSDiva™ Software version 6.2 (BD Bioscience, San Jose, CA, United States).
No ethics approval was needed for the care of this patient as all treatments were in accordance with institutional best practice. A 70-year-old man with no relevant medical history was diagnosed in May 2009 with left-sided adenocarcinoma of the colon. He had abdominal pain and hematochezia for two weeks, then underwent colonscopy which revealed the colon cancer. CEA (CarcinoEmbryonic Antigen) was 12.3 ng/mL (Figure 1). A left hemicolectomy was performed in July 2009; pathology revealed a stage IIIb well-differentiated adenocarcinoma. He received adjuvant chemotherapy with folfox6 (oxaliplatin 85 mg/m2 on day 1, leucovorin 200 mg/m2 as a 2-h infusion on day 1 and 5-FU 400 mg/m2 IV bolus on day 1 followed by a 5-FU 2.400 mg/m2 46-h continuous infusion, repeated every 14 d) from August 2009 to November 2009 (6 cycles), stopped for his choice. During follow-up a progressive increases of CEA was registered (Figure 1). In December 2011 a CT (Computed Tomography) was completely negative, the CEA was 10.1 ng/mL. In March 2012 the CEA was 14.0 ng/mL and a PET/CT scan showed foci of abnormal uptake in abdominal nodes (SUV 3.06) and pericolic tissue (SUV 2.18). After discussion, the patient refused any surgical procedure; chemotherapy with capecitabine, oxalipltain and bevacizumab (CapOxBeva) was started in April 2012 (oxaliplatin, 130 mg/m2 on day 1, capecitabine, 1000 mg/m2 twice daily on days 1-14 every 3 wk, bevacizumab 7.5 mg/kg on day 1 of the 3-weekly cycle) for seven cycles; oxaliplatin was reduced at the sixth cycle and then stopped for persistent peripheral neuropathy. PET/CT scan at October 2012 showed a complete response and normalization of CEA (2.4 ng/mL). Thereafter, the patient received maintenance therapy consisting of bevacizumab (7.5 mg/kg) once every 3 wk and capecitabine (the drug was reduced for hand/foot syndrome to 750 mg/mq bis/die from day 1 to day 14 every 21 d). No adverse events were documented and the maintenance therapy was stopped in December 2013 due to patient preference.
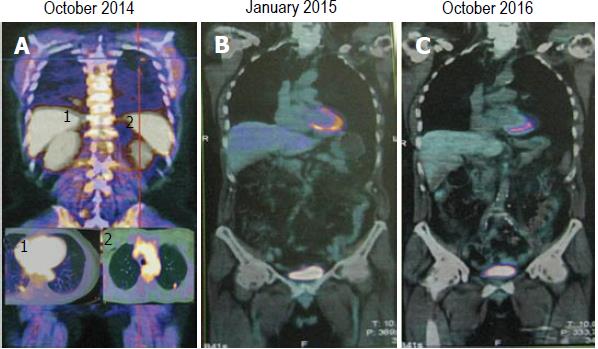
During follow-up a new increase in tumor markers was documented (October 2014, CEA: 33.8 ng/mL) (Figure 1). PET/CT scan showed glucose uptake in lungs (lower lobe SUV: 2.8, middle lobe SUV: 2.06) (Figure 2A). Analysis of RAS status revealed the absence of KRAS mutations, thus the patient started at October 2014 a second-line chemotherapy with folfiri/panitumumab (irinotecan 180 mg/m2 on day 1, leucovorin 200 mg/m2 as a 2-h infusion on day 1 and 5-FU 400 mg/m2 IV bolus on day 1 followed by a 5-FU 2.400 mg/m2 46-h continuous infusion, panitumumab 6 mg/kg on day 1, repeated every 14 d). At January 2015 a PET/CT scan showed a complete response (Figure 2B) with normalization of CEA (1.6 ng/mL) (Figure 1) but the patient experienced neutropenia, asthenia and diarrhea grade 3 according to NCI-CTC v4.0 so that folfiri was discontinued. The patient refused any rechallenge with chemotherapy. Upon the third occurrence of a grade 3 dermatologic toxicity (treated as per protocol), panitumumab was continued from June 2015 at 60% of the original dose; additionally, it was administered every three weeks as strong and explicit patient request.
Until presentation of this case (October 2016, Figure 2C) the patient maintains a complete response, has no symptoms of disease (the performance status according to ECOG is 0) and CEA is normal (Figure 1). He continues panitumumab without side effects with a very good quality of life.
At June 2016, when the complete response was confirmed with single agent panitumumab, the patient was characterized two times with respect to NK cells activity, Tregs and MSDCc cells in peripheral blood before panitumumab administration and after 10 d. The results were quite overlapping. We excluded NK cells cytotoxicity evaluation right after therapy to avoid the interference of premedication drugs (i.e., corticosteroids and antihistamine). The results showed refer to a blood sample obtained just before panitumumab administration. Interestingly, the patient had 35.1% of circulating CD3+/CD56+ lymphocytes which is a high value considering the normal range (11.0%-28.0%)[13]. Furthermore, a large part of these cells where highly cytotoxic NK lymphocytes (30.4% of CD3-/CD56dim) showing high cytotoxicity activity against K562 cells (Figure 3). Furthermore, a characterization of Tregs and MDSC cells was performed and is described in Figure 4. A prospective study on the predictive and prognostic role of NK cell cytotoxicity in patients with mCRC is ongoing at the National Cancer Institute of Naples.
We report on a case of a patient with oligometastatic disease who received diagnosis of mCRC about four years ago. We studied some immunological characteristics of this patient with a descriptive and exploratory aim; high natural killer cells activity was found. Interestingly, surgery or stereotactic radiotherapy was never used for his choice; thus, the patient was exclusively treated with systemic therapy. We decided to monitor the therapeutic response with PET/CT in order to have comparable exams and to reveal early changes of tumor activity; in this case, there was high concordance between CEA values and PET/CT results.
According to RAS status, he was treated with chemotherapy and panitumumab as second-line treatment. Interestingly, a long-lasting metabolic complete response was achieved both in first and second-line therapies and it was maintained although the use of a reduced panitumumab dose density and intensity. Only recently we have extended the analysis of RAS status of this patient and no mutations of BRAF and NRAS were found.
Recently, researchers are focusing their attention to immune system and immune checkpoints in order to restore and/or potentiate cellular-mediated antitumor immunity. One of the most studied inhibitory check point is the PD-1/PD-1L pathway which suppress immune responses[14]. PD-1L is mainly expressed by B and T cells, macrophages and dendritic cells. Tumor-expressing PD-1 are able to induce an immunosuppressive status and to evade host immune surveillance by inhibiting T-cell-mediated anti-tumor activity. In advanced colorectal cancer inhibition of this pathway shows efficacy only in deficient MMR (mismatch repair) tumors (3%-6% of advanced CRC patients)[15,16]. The hypothesis is that the immune system could recognize many more somatic mutations (neoantingen load) than proficient MMR tumors. Additionally, dMMR neoplasms present prominent lymphocyte infiltrates. The PD-1/PD-L1 is not relevant to the main concept of this clinical case study and this patient did not present a deficient MMR tumor (data not shown); however, some stimulating speculations can be raised: (1) Which are the relationship between NK and T cells in tumor microenvironment? and (2) can other effector cells (NK cells, macrophages, regulatory cells), play a role in mCRC patients? Is there any interaction between panitumumab and NK cells?
Interestingly, we previously reported that patients affected by mCRC with high NK-cell cytotoxicity, independently from the type of therapy, showed a significantly higher response rate and a longer progression-free survival (PFS) compared with patients with low NK-cell cytotoxicity[9]. Due to the complex and dynamic nature of the immune system we are evaluating the hypothesis that decrease over time of NK cell activity could associate with progression of the tumor. Thus, we are conducting a prospective study evaluating circulating NK cells [cytotoxicity level, circulating NK CD56dimvs CD56high, expression of KIRs (Killer-cell immunoglobulin-like receptors)] in order to evaluate their predictive and prognostic role in mCRC.
The patient had abdominal pain and hematochezia before undergoing surgery for colon cancer.
The diagnosis was done through colonscopy.
The differential diagnosis of ulcerative colitis was excluded by colonscopy and biopsy.
The diagnosis of colon cancer was definitively done through the pathological examination of biopsy and supported by carcinoembryonic antigen determination; phenotypic analysis of peripheral immune cell subsets and degranulation assay for natural killer (NK) cells were done through flow-cytometry.
The response to treatments as well as the evolution of the disease were studied by positron emission tomography/computed tomography.
Classical hematoxylin and eosin stain showed a well-differentiated adenocarcinoma of the left colon.
The patient received the following sequence of treatments: Adjuvant chemotherapy with fluorouracile, folinic acid and oxaliplatin, first-line therapy with capecitabine, oxaliplatin and bevacizumab, maintenance therapy with bevacizumab, second-line therapy with fluorouracile, folinic acid, irinotecan and panitumumab and, finally, panitumumab monotherapy.
The authors previously reported in Trotta et al (Cancer Immunol Res 2016; 4: 366-374) that patients affected by metastatic colorectal cancer (mCRC) with high NK-cell cytotoxicity, independently from the type of therapy, showed a significantly higher response rate and a longer progression-free survival compared with patients with low NK-cell cytotoxicity.
NK cells: Natural killer cells, a subset of lymphocytes with anti-tumor cytotoxic properties; CD: Cluster of differentiation, antigens used to identify molecules expressed by different leukocytes.
NK cells might be a predictive and/or prognostic factor in patients with mCRC; comprehension of their role deserves further studies.
This is a well written and interesting case.
Manuscript source: Unsolicited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Ilie M, Surlin VM S- Editor: Ji FF L- Editor: A E- Editor: Zhao LM
| 1. | DeSantis CE, Lin CC, Mariotto AB, Siegel RL, Stein KD, Kramer JL, Alteri R, Robbins AS, Jemal A. Cancer treatment and survivorship statistics, 2014. CA Cancer J Clin. 2014;64:252-271. [PubMed] [DOI] [Full Text] |
| 2. | Peeters M, Douillard JY, Van Cutsem E, Siena S, Zhang K, Williams R, Wiezorek J. Mutant KRAS codon 12 and 13 alleles in patients with metastatic colorectal cancer: assessment as prognostic and predictive biomarkers of response to panitumumab. J Clin Oncol. 2013;31:759-765. [PubMed] [DOI] [Full Text] |
| 3. | Heinemann V, von Weikersthal LF, Decker T, Kiani A, Vehling-Kaiser U, Al-Batran SE, Heintges T, Lerchenmüller C, Kahl C, Seipelt G. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab as first-line treatment for patients with metastatic colorectal cancer (FIRE-3): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:1065-1075. [PubMed] [DOI] [Full Text] |
| 4. | Adelstein BA, Dobbins TA, Harris CA, Marschner IC, Ward RL. A systematic review and meta-analysis of KRAS status as the determinant of response to anti-EGFR antibodies and the impact of partner chemotherapy in metastatic colorectal cancer. Eur J Cancer. 2011;47:1343-1354. [PubMed] [DOI] [Full Text] |
| 5. | Lech G, Słotwiński R, Słodkowski M, Krasnodębski IW. Colorectal cancer tumour markers and biomarkers: Recent therapeutic advances. World J Gastroenterol. 2016;22:1745-1755. [PubMed] [DOI] [Full Text] |
| 6. | Chibaudel B, Tournigand C, Bonnetain F, Richa H, Benetkiewicz M, André T, de Gramont A. Therapeutic strategy in unresectable metastatic colorectal cancer: an updated review. Ther Adv Med Oncol. 2015;7:153-169. [PubMed] [DOI] [Full Text] |
| 7. | Reeves E, James E. Antigen processing and immune regulation in the response to tumours. Immunology. 2017;150:16-24. [PubMed] [DOI] [Full Text] |
| 8. | Mohme M, Riethdorf S, Pantel K. Circulating and disseminated tumour cells - mechanisms of immune surveillance and escape. Nat Rev Clin Oncol. 2017;14:155-167. [PubMed] [DOI] [Full Text] |
| 9. | Trotta AM, Ottaiano A, Romano C, Nasti G, Nappi A, De Divitiis C, Napolitano M, Zanotta S, Casaretti R, D’Alterio C. Prospective Evaluation of Cetuximab-Mediated Antibody-Dependent Cell Cytotoxicity in Metastatic Colorectal Cancer Patients Predicts Treatment Efficacy. Cancer Immunol Res. 2016;4:366-374. [PubMed] [DOI] [Full Text] |
| 10. | Kalathil SG, Thanavala Y. High immunosuppressive burden in cancer patients: a major hurdle for cancer immunotherapy. Cancer Immunol Immunother. 2016;65:813-819. [PubMed] [DOI] [Full Text] |
| 11. | Alter G, Malenfant JM, Altfeld M. CD107a as a functional marker for the identification of natural killer cell activity. J Immunol Methods. 2004;294:15-22. [PubMed] |
| 12. | Claus M, Watzl C. Evaluation of human natural killer cell activities in whole blood. Curr Protoc Immunol. 2010;Chapter 7:Unit7.39. [PubMed] [DOI] [Full Text] |
| 13. | Valiathan R, Deeb K, Diamante M, Ashman M, Sachdeva N, Asthana D. Reference ranges of lymphocyte subsets in healthy adults and adolescents with special mention of T cell maturation subsets in adults of South Florida. Immunobiology. 2014;219:487-496. [PubMed] [DOI] [Full Text] |
| 14. | Luo M, Fu L. The effect of chemotherapy on programmed cell death 1/programmed cell death 1 ligand axis: some chemotherapeutical drugs may finally work through immune response. Oncotarget. 2016;7:29794-29803. [PubMed] [DOI] [Full Text] |
| 15. | Toh JW, de Souza P, Lim SH, Singh P, Chua W, Ng W, Spring KJ. The Potential Value of Immunotherapy in Colorectal Cancers: Review of the Evidence for Programmed Death-1 Inhibitor Therapy. Clin Colorectal Cancer. 2016;15:285-291. [PubMed] [DOI] [Full Text] |
| 16. | Sun X, Suo J, Yan J. Immunotherapy in human colorectal cancer: Challenges and prospective. World J Gastroenterol. 2016;22:6362-6372. [PubMed] [DOI] [Full Text] |