Published online Nov 16, 2016. doi: 10.12998/wjcc.v4.i11.356
Peer-review started: March 10, 2016
First decision: May 19, 2016
Revised: August 24, 2016
Accepted: September 13, 2016
Article in press: September 18, 2016
Published online: November 16, 2016
Processing time: 249 Days and 19.1 Hours
To elucidate the profile of the salivary proteome.
Unstimulated whole mouth saliva was collected from 30 volunteers [15 proliferative verrucous leukoplakia (PVL) patients and 15 controls] and proteins were submitted for mass spectrometry-based proteomics using the discovery approach, followed by analyses of variance and logistic regression tests.
A total of two hundred and eighty-three proteins were confidently identified in saliva. By combining two low abundance proteins from the PVL group, angiotensinogen (AGT) and dipeptidyl peptidase 1 (DPP1), a model for group differentiation was built with a concordance index of 94.2%, identifying both proteins as potential etiologic biomarkers for PVL.
This study suggests that both AGT and DPP1 may be involved in developmental mechanisms of PVL.
Core tip: Saliva is a useful source for analysis of proteins that can be associated in many diseases, including proliferative verrucous leukoplakia (PVL). This study showed that angiotensinogen and dipeptidyl peptidase 1 may be important in PVL development.
- Citation: Flores IL, Santos-Silva AR, Coletta RD, Leme AFP, Lopes MA. Low expression of angiotensinogen and dipeptidyl peptidase 1 in saliva of patients with proliferative verrucous leukoplakia. World J Clin Cases 2016; 4(11): 356-363
- URL: https://www.wjgnet.com/2307-8960/full/v4/i11/356.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v4.i11.356
Proliferative verrucous leukoplakia (PVL) is a seldom variant of oral leukoplakia with an unique behavior characterized by persistent progression to malignancy[1-7]. The rate of malignant transformation of PVL ranges from 40%-100%[1,3,5,8-12]. This variability mostly depends on the time of patient follow-up and the criteria for diagnosis of PVL[13,14]. Due to a lack of specific baseline characteristics, the PVL diagnosis is made retrospectively, based on observation of progressive clinical and microscopical characteristics of the lesions[5]. Thus, evaluation of biopsy specimens from representative areas is important in establishing the existence of epithelial dysplasia or carcinoma along with clinical evolution[15].
Recent reviews on PVL have considered lesions with lichenoid aspects that progress to classical PVL phenotype in addition to the clinical aspect of multiple hyperkeratotic white plates with verrucous and expansive nature[6,7,16]. Four histological features are encountered over the course of the disease: Hyperplasia and hyperkeratosis with no epithelial dysplasia; multifocal expansion of lesions with or without varied grade of dysplasia; verrucous hyperplasia; and verrucous carcinoma or frankly invasive oral squamous cell carcinoma (OSCC)[5-7]. Nevertheless, not all lesions present with histopathological progression involving verrucous hyperplasia and verrucous carcinoma before OSCC. Currently, four main stages may be encountered during PVL development: Initial focal involvement, geographic expansion (multifocal) over time, development of a warty appearance and finally, development of a cancer[6,7].
Despite well-accepted clinical and histopathological features, the underlying molecular aspects involved in the pathogenesis and progression of PVL are still unknown and need further investigation. An intriguing characteristic of this entity is the prevalence in older women with no exposition to known etiological factors for leukoplakia, including tobacco and alcohol consumption, suggesting a possible existence of active molecular events[17]. Although proteomic and genomic analysis of OSCC/oral leukoplakia have been described, no proteomic approaches specifically for PVL have been identified[18]. Even so, some events and candidates have been proposed, such as aberrations in cell cycle regulation, homozygous p14ARF and p16INK4a gene deletion, heterozygosity loss, variable expression of the tumor suppressor gene p53, and a rare homozygous deletion of exon 1β of the gene p14[19,20]. DNA ploidy has also been suggested to predict the malignant transformation of PVL in OSCC[5,19].
Additionally, the p53, Ki-67, Mcm-2 and Mcm-5 proteins have been identified through immunohistochemistry to have increased expression in OSCC lesions that progressed from PVL[3,5]. PVL lesions with mild or moderate dysplasia, in addition to lesions that progressed to OSCC, had high expression of Mcm-2 and Mcm-5; such molecular indicators may be useful as markers of malignant transformation of the disease, especially Mcm-2[3,5]. Similar findings detected in other conventional dysplastic premalignant lesions and OSCC have reinforced the aggressive nature of this entity, but without clarifying the true meaning of these molecules with respect to the pathogenesis or progression of PVL[7,20].
Recently, the use of proteomic-based mass spectrometry (MS) has increased interest in salivary biomarkers for numerous diseases, including OSCC[21,22]. Our interest in the salivary proteome - studied here - stems from the biochemical aspects of proteins, which are considered the most important molecules in the salivary fluid[22,23]. Salivary protein activity provides important information about the pathogenesis of many oral diseases; in addition, they have presented growing potential to revolutionise the field of etiopathogenesis and diagnosis[23,24].
Therefore, the objective of this survey was to elucidate a salivary proteome profile of sample with PVL using MS technology, in order to identify proteins that may be involved in the etiology of PVL.
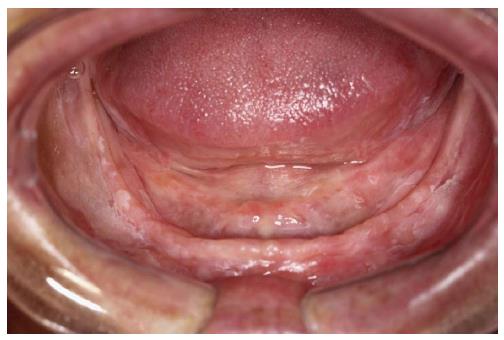
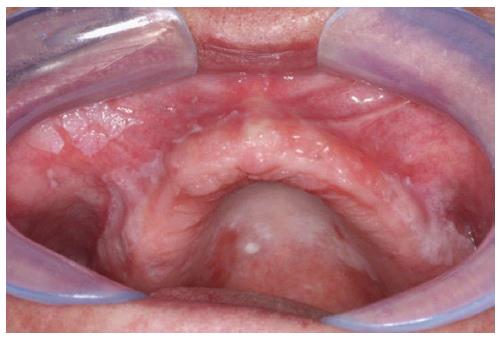
This study followed the guidelines of the Declaration of Helsinki and Tokyo for research in humans, and have been approved by the local ethics board of the University of Campinas (protocol number: 105/2013). All patients received verbal explanations concerning the study before signing a written informed consent. In this study, 15 patients with diagnosed PVL-according to clinical and microscopical findings (Figures 1 and 2) and after numerous appointments[3,5] - and 15 control patients with no history of cancer or any oral lesion, but possessing the same demographics, were chosen from patients assisted by the Orocentro (Oral Diagnosis Clinic) of Piracicaba Dental School. Clinicopathological data were collected retrospectively from patient charts. The inclusion criteria for the sample was a clinical and histopathological diagnosis of PVL according to the World Health Organization. We excluded patients that had an absence of saliva. Although all patients were female with no history of tobacco and alcohol consumption, these features were not part of our inclusion criteria but rather are peculiar features of patients with PVL.
After a mouthwash with 5 mL of drinking water for removal of oral residues, whole, unstimulated saliva produced over 5 min was collected in sterile plastic tubes of 50 mL. After collection, the saliva was promptly keep on dry ice and after stored at -80 °C in 2 mL, sterile, plastic tubes. Before preparation for MS analysis, 10 μL of PMSF and 2 μL of EDTA to each 1 mL of saliva, both at a final concentration of 1 mmol/L were added, followed by centrifugation at 12000 g for 10 min at 4 °C. The supernatant was collected and protein concentrations were settled with a Bradford test (Bio-Rad, Hercules, CA, United States) prior to protein digestion.
Proteins (20 μg) were treated with a solution with a final concentration of 5 mmol/L dithiothreitol for 25 min at 56 °C for reduction, followed by alkylation of cysteines with 14 mmol/L iodoacetamide for 30 min at room temperature in the darkness and with trypsin (1:50, w/w). The reaction was stopped with 1% formic acid and the specimens were dried in a vacuum concentrator and reconstituted in 0.1% formic acid.
An aliquot containing 1 μg of proteins was analysed on an ETD-enabled LTQ Velos Orbitrap mass spectrometer (Thermo Fisher Scientific) connected to nanoflow liquid chromatography (LC-MS/MS) by an EASY-nLC system (Proxeon Biosystem) through a Proxeon nanoelectrospray ion source. All steps for peptides preparation and all instrument methods for the LTQ Orbitrap Velos were set up following the protocol established by Flores et al[25].
Peak lists were generated from the raw data files using Proteome Discoverer version 1.4 (Thermo Fisher Scientific) with the Sequest search engine and searched against Human Uniprot (downloaded in June 2013; 88771 sequences; 35204890 residues) with carbamidomethylation as fixed modification, oxidation of methionine as variable modification, one trypsin missed cleavage and a tolerance of 10 ppm for precursor and 1 Da for fragment ions. All datasets were processed using the workflow feature in the Proteome Discoverer software and the resulting search data were further analysed in ScaffoldQ+v.3.3.1. The scoring parameters in Scaffold were set to obtain a false discovery rate of less than 1%. Using the number of total spectra output from ScaffoldQ+, differentially expressed proteins were identified using spectral counting.
A Comma-separated values file comprising the determined proteins and their normalised spectral counts have been created for 15 healthy patients (control) and 15 PVL patients (PVL). All statistical tests were carried out using SAS® software (version 9.3; SAS Institute Inc, Cary, NC, United States, 2010). Analysis of Variance test (ANOVA) was used to compare the abundance of each protein in relation to groups. Fold change (FC) calculation for proteins with differences in relation to abundance was performed according to the equation: MV in PVL group/MV in control group = Fold change (≥ + 1.00) (MV: Mean of abundance values).
Moreover, fold changes less than 1 were used the equation below: 1/FC (< + 1.00) = FC (-1.00).
Finally, to develop a method to predict the probability of pertinence to one specified group, an analysis based on the regression logistic model was conducted in conjunction with the stepwise method for variable selection as proteins were identified. Mean values are presented with the standard error. A P < 0.05 was considered significant in all statistical tests.
All thirty patients were females, with mean age of 68.13 ± 9.82 years for PVL patients and mean age of 65.20 ± 8.36 years for control patients. Demographic and clinicopathological data of the populations are shown in Tables 1 and 2.
| Control | Proliferative verrucous leukoplakia | |||
| n | % | n | % | |
| Age (yr) | ||||
| 50-59 | 5 | 33.33 | 4 | 26.66 |
| 60-69 | 3 | 20 | 3 | 20 |
| 70-79 | 7 | 46.66 | 7 | 46.66 |
| > 80 | 0 | 0 | 1 | 6.66 |
| Gender | ||||
| Female | 15 | 100 | 15 | 100 |
| Male | 0 | 0 | 0 | 0 |
| Risk factor | ||||
| Tobacco | 0 | 0 | 0 | 0 |
| Alcohol | 0 | 0 | 0 | 0 |
| Case | Anatomiclocation | Histopathologicfindings | Treatment | Follow-up time (yr) | Status |
| 1 | 1a, 3a, 3b, 4, 5, 6b | Epithelial dysplasia (3a, 6b) OSCC (3b) | Surgery | 11 | Alive |
| 2 | 2, 3b, 5a, 6a, 8, 9 | Epithelial dysplasia (2, 9) OSCC (2, 8) | Surgery | 5 | Alive |
| 3 | 2, 3b, 4, 10 | Acanthosis/hiperkeratosis (2, 10) Epithelial dysplasia (2) | - | 10 | Alive |
| 4 | 2, 5a, 6a, 7a | Acanthosis/hyperkera tosis (2) Epithelial dysplasia (2, 5a) | - | 20 | Alive |
| 5 | 2, 5a, 5b, 6b | Acanthosis/dysplasia (6b) | - | 2 | Alive |
| 6 | 2, 3a, 4, 5a, 9 | Epithelial dysplasia (3a) | - | 1 | Alive |
| 7 | 2, 3b | - | - | 2 | Alive |
| 8 | 2, 6a, 7b | Acanthosis/hyperkera tosis (7b) Epithelial dysplasia (7b) | - | 4 | Alive |
| 9 | 1b, 2, 3a, 3b, 4, 6a, 6b | Epithelial dysplasia (1b, 2) | - | 1 | Alive |
| 10 | 2, 3b, 6a | Epithelial dysplasia (3b) | - | 3 | Alive |
| 11 | 2, 3a, 3b, 4, 5a, 6b, 8 | Epithelial dysplasia (8) Verrucous carcinoma (5a) OSCC (4) | Surgery + RT | 6 | Alive |
| 12 | 2, 4, 5b, 6b, 8 | Acanthosis/hiperkeratosis (5b) Epithelial dysplasia (2) | - | 3 | Alive |
| 13 | 2, 3b | Epithelial dysplasia (3b) OSCC (3b) | - | 3 | Alive |
| 14 | 1a, 1b, 2, 3a, 3b, 4, 6a, 6b | Epithelial atrophy/ hyperkeratosis (3b) | - | 1 | Alive |
| 15 | 1a, 1b, 2, 3a, 3b, 5a, 5b, 6a, 6b, 8 | Epithelial dysplasia (5a, 5b) | - | 10 | Alive |
Using ScaffoldQ+v.3.3.1, a file of 283 proteins with less than 1% false discovery rate was created. A complete list of proteins and their respective normalized spectral counts in each patient is shown in Supplemental Table 1.
Thirty-one proteins had statistically significant differences in relation to abundance between the control and PVL groups according to ANOVA. Among these 31 proteins, 25 had higher abundance in the control group, and six proteins were higher in PVL group (Table 3).
| Accession number | Protein (31) | Control | PVL | P value | FC | ||
| SC | SD | SC | SD | ||||
| sp|P58876|H2B1D_HUMAN | Histone H2B type 1-D | 0.12 | 0.32 | 1.09 | 1.73 | 0.0413 | 9.08 |
| sp|P04264|K2C1_HUMAN | Keratin, type II cytoskeletal 1 | 2.33 | 2.45 | 5.24 | 4.67 | 0.0418 | 2.24 |
| sp|P80748|LV302_HUMAN | Ig lambda chain V-III region LOI | 0.45 | 0.50 | 1.01 | 0.66 | 0.0154 | 2.24 |
| sp|P31025|LCN1_HUMAN | Lipocalin-1 | 7.03 | 4.46 | 13.44 | 6.84 | 0.005a | 1.91 |
| sp|P04080|CYTB_HUMAN | Cystatin-B | 1.67 | 1.38 | 3.19 | 2.25 | 0.0338 | 1.91 |
| sp|Q01469|FABP5_HUMAN | Fatty acid-binding protein, epidermal | 2.22 | 1.36 | 3.99 | 2.04 | 0.009a | 1.79 |
| sp|P68032|ACTC_HUMAN | Actin, alpha cardiac muscle 1 | 9.66 | 3.79 | 6.78 | 3.71 | 0.0446 | -1.42 |
| sp|P02814|SMR3B_HUMAN | Submaxillary gland androgen-regulated protein 3B | 18.26 | 9.40 | 11.05 | 5.19 | 0.0148 | -1.66 |
| sp|P06744|G6PI_HUMAN | Glucose-6-phosphate isomerase | 4.21 | 2.89 | 2.12 | 2.05 | 0.0309 | -2.00 |
| sp|P30740|ILEU_HUMAN | Leukocyte elastase inhibitor | 3.93 | 1.57 | 1.87 | 1.75 | 0.002a | -2.12 |
| sp|Q06830|PRDX1_HUMAN | Peroxiredoxin-1 | 2.11 | 1.41 | 0.87 | 1.04 | 0.0106 | -2.43 |
| sp|P62328|TYB4_HUMAN | Thymosin beta-4 | 1.09 | 1.01 | 0.40 | 0.64 | 0.0323 | -2.77 |
| sp|Q96HE7|ERO1A_HUMAN | ERO1-like protein alpha | 0.93 | 0.67 | 0.32 | 0.59 | 0.0126 | -2.94 |
| sp|P23528|COF1_HUMAN (+1) | Cofilin-1 | 1.66 | 1.79 | 0.54 | 0.99 | 0.0423 | -3.12 |
| sp|Q02818|NUCB1_HUMAN | Nucleobindin-1 | 0.82 | 0.56 | 0.27 | 0.47 | 0.007a | -3.12 |
| sp|P52566|GDIR2_HUMAN | Rho GDP-dissociation inhibitor 2 | 1.61 | 1.76 | 0.49 | 0.79 | 0.0317 | -3.33 |
| sp|Q01518|CAP1_HUMAN | Adenylyl cyclase-associated protein 1 | 3.18 | 2.86 | 0.83 | 0.97 | 0.005a | -3.84 |
| sp|P26038|MOES_HUMAN | Moesin | 2.44 | 2.52 | 0.60 | 0.63 | 0.0107 | -4.16 |
| sp|P61586|RHOA_HUMAN | Transforming protein RhoA | 0.53 | 0.63 | 0.13 | 0.36 | 0.0453 | -4.16 |
| sp|P27797|CALR_HUMAN | Calreticulin | 0.61 | 0.65 | 0.13 | 0.35 | 0.0172 | -4.76 |
| sp|P06737-2|PYGL_HUMAN (+1) | Isoform 2 of Glycogen phosphorylase, liver form | 1.70 | 2.11 | 0.34 | 0.63 | 0.0239 | -5.00 |
| sp|P61158|ARP3_HUMAN | Actin-related protein 3 | 0.97 | 1.33 | 0.13 | 0.35 | 0.0268 | -7.69 |
| sp|P43490|NAMPT_HUMAN | Nicotinamide phosphoribosyl transferase | 0.53 | 0.64 | 0.07 | 0.26 | 0.0139 | -7.69 |
| tr|A6NNY3|A6NNY3_HUMAN | Chitinase-3-like protein 2 | 0.61 | 0.75 | 0.07 | 0.28 | 0.0143 | -9.09 |
| sp|P30041|PRDX6_HUMAN | Peroxiredoxin-6 | 0.60 | 0.87 | 0.05 | 0.21 | 0.0255 | -12.5 |
| sp|P01019|ANGT_HUMAN | Angiotensinogen | 0.83 | 0.73 | 0.07 | 0.26 | 0.0006a | -12.5 |
| sp|P0C0L5|CO4B_HUMAN | Complement C4-B | 0.93 | 1.02 | 0.07 | 0.28 | 0.004a | -14.28 |
| sp|P53634|CATC_HUMAN | Dipeptidyl peptidase 1 | 0.66 | 0.98 | 0.00 | 0.00 | 0.015 | NC |
| sp|P22392-2|NDKB_HUMAN (+1) | Isoform 3 of Nucleoside diphosphate kinase B | 0.58 | 0.60 | 0.00 | 0.00 | 0.000a | NC |
| sp|P22314|UBA1_HUMAN | Ubiquitin-like modifier-activating enzyme 1 | 0.38 | 0.59 | 0.00 | 0.00 | 0.0189 | NC |
| sp|Q9UHA7|IL36A_HUMAN | Interleukin-36 alpha | 0.43 | 0.68 | 0.00 | 0.00 | 0.0197 | NC |
Logistic regression screening tests were used as a form of group differentiation and two potential biomarkers met the final prediction: Angiotensinogen (AGT) and dipeptidyl peptidase 1 (DPP1). When likelihood analysis was applied to the two variables selected stepwise within the logistic regression, the following logistic function was obtained: Logit = 2.5647 - 12.4678 × P192 - 4.3839 × P206.
A negative logit indicated control group landmarks while a positive logit indicated PVL landmarks. The logit value can also be used to calculate the probability of PVL group using the function below, where “e” represents the Neperian constant equal to 2.71828:
PPVL= elogit(p)/(1 + elogit(p)).
Where PPVL represents the probability of relevance of an individual patient be of PVL group (PPVL), and the degree of confidence can be calculated by inserting the logit value. Combining the two selected variables in this study, a concordance index of 94.2% was found. The parameters selected by the stepwise method and their estimates are: Intersection (2.5647), P192 (DPP1) (-12.4678) and P206 (AGT) (-4.3839). The reliability values of the model built by the logistic regression test are: Percent concordant: 91.1; C value: 0.942 (area under Roc curve); P value: 0.0187; correct event: 14 control, 14 PVL; sensitivity: 93.3%; specificity: 93.3%.
PVL is a well-recognized form of oral leukoplakia which presents with particular features and certain molecular aspects. Previous studies have shown certain features of these intriguing lesion, where even no dysplastic lesions can present positivity for Mcm-2 positive, can be aneuploidy and can transform in cancer[3,5]. However, biomolecules and mechanisms involved in etiopathogenesis and progression remain obscure. A literature review of 84 English PVL articles revealed only 10 studies that described molecular aspects of PVL[3,5,10,20,26-31]. In addition, no previous studies investigated the presence of potential PVL biomarkers through proteome salivary analysis using LC-MS/MS. The approach presented here may combine this oral malignant condition as well as the secreted proteins associated with this lesion.
In comparison to serum, saliva presents with a lower amount of informative analytes, which presents a major challenge for research[32,33]. However, by using techniques well-suited for detecting small quantities such as LC-MS/MS, salivary components - including proteins that can be found in blood - can be also measured[34,35]. Thus, saliva can be considered as the blood stream of the oral cavity and proteins with different profile levels can be detected; analyses of these proteins can lead to their association with pathologies, leading to the discovery of potential biomarkers[34,35].
Among the 283 proteins identified in the saliva samples collected here, 31 proteins showed statistical significance difference in relation to abundance, with 25 proteins having higher abundance in the control group and six proteins having higher abundance in PVL group (Table 3). Amongst there, two proteins found in low abundance in PVL, which were AGT (ANOVA P = 0.0006, FC-12.5) and DPP1 (ANOVA P = 0.0150, FC: not calculated), that were identified as better biological parameters for model built associated to PVL patients according to regression logist test. The FC of DPP1 was not calculated due to its total loss of expression in PVL group confirming its exclusive presence in the control group.
Based on these findings, both proteins can be considered as the first salivary proteins with biomarker potential involved in the etiology of PVL. AGT (61 kDa) is a circulating protein, and mostly known for its role as a precursor of the renin-angiotensin-aldosterone system, where it is synthesized and secreted mostly by hepatocytes, adipocytes and astrocytes[35-37]. Physiologically, AGT is a unique precursor of the angiotensin peptides and the only natural renin substrate[36]. The hydrolysis of AGT into angiotensin I by renin results in further activation by angiotensin-converting enzyme, which transforms angiotensin I into angiotensin II and III[36,37]. Moreover, AGT is derived from serine protease inhibitor, which has a role in blood pressure control as a potent vasoconstrictor of arteries and veins, as well as in prothrombotic action[37,38].
Interestingly, recent studies have also related AGT with in vitro cell inhibition of human endothelial proliferation, migration and angiogenesis[37-39]. Anti-tumoral effects of AGT - which include blocking of primary tumor growth, suppression of intratumoral vascularisation, and decreased number of metastases - were observed in in vivo models[37,39]. Moreover, AGT was found down-regulated in tissue samples of esophageal squamous cell carcinoma and in the serum of subjects with recurrent head and neck cancer, demonstrating that it may be normally involved in impeding tumorigenesis[38,40]. A previous study also demonstrated that women with low amount of AGT presented an elevated risk of breast cancer[38,41].
In the present study, low AGT abundance was observed in PVL patients with a fold change of -12.5, suggesting that lower expression of this molecule can be associated with increased risk for PVL and indicating a possible role of AGT as an etiologic marker. No difference was observed in the expression or abundance within the PVL group in relation to patients that developed oral cancer, but these results were expected since patients with PVL probably will develop cancer during follow-up.
DPP1 (51kDa) also called as cathepsin c, cathepsin J, dipeptidyl transferase and dipeptidyl aminopeptidase (UNIPROT P53634) is a lysosomal cysteine enzyme that participates in intracellular protein degradation[42]. The activity of DPP1 is crucial in the differentiation of promyelocytes into mature neutrophils, and in the production of neutrophil elastase, proteinase-3, and cathepsin G[42,43]. The most common pathology involved with DPP1 mutations is Papillon-Lefèvre syndrome, an autosomal recessive condition related to the loss of enzymatic activities of these three serine proteases in neutrophils, causing palmoplantar keratosis and severe, early periodontal disease[44].
In addition to its involvement in physiological processes, an intrinsic relation among squamous carcinogenesis and DPP1 expression was demonstrated in relation to tumor growth and angiogenesis[45]. Among the tumors involved, squamous cell carcinoma of the oral cavity, nasopharynx and thyroid exhibited increased DPP1 expression when compared to control samples[45]. Nevertheless, DPP1 did not show an effect in the formation or progression in pancreatic endocrine tumors, presenting expression in innate immune cells[46]. Interestingly, dipeptidyl peptidase IV (DPPIV) is another cathepsin expressed in normal epithelial cells, but has decreased expression in various cancers, such as melanoma, lung and prostatic cancer suggesting that a decrease DPPIV expression can be a critical event during cancer progression[47-50].
In the present study, lower expression of DPP1 was found in PVL patients; although some articles demonstrated increased expression of this molecule and its association with tumor progression, one study showed a loss of DPP1 expression that was related with cancer[46]. No significant abundance differences were observed between PVL patients at various disease stages, including patients that developed oral cancer. This finding reinforces the relentless malignant evolution of PVL and the diagnostic potential of DPP1. Cathepsins appear to play an important role in preventing tumor formation and/or progression, with AGT showing similar capabilities in PVL patients. Nevertheless, a deeper investigation of these proteins to analyze their role in PVL disease is necessary.
This study revealed evidence about the promising etiologic potential of the proteins AGT and DPP1, and how these biomolecules may be associated with the development of PVL. However, our study focused on only a limited number of patients, all of whom are undergoing a very dynamic disease process; a larger sample size and standardization of sample collection with successive investigations could improve future studies. Moreover, more saliva samples would improve logit formula validation, an important step in following patients that go on to develop oral cancer.
In conclusion, there were no known etiologic factors of PVL previously; however, close contact of saliva with the PVL lesions presented a potential source for the detection of possible biomolecules through salivary proteome examination. Using this method, our results identified that AGT and DPP1 appear to play a role in PVL etiology and progression. Additional studies about the profile of the salivary proteome are necessary and may contribute to improving our understanding of this pattern of expression for AGT and DPP1.
We would like to thank our technician, Romenia Ramos Domingues, of the Mass Spectrometry Lab of the Brazilian Biosciences National Laboratory - LNBio, CNPEM, Campinas, Brazil.
Proliferative verrucous leukoplakia (PVL) is a seldom variant of oral leukoplakia with an unique behavior characterized by persistent progression to malignancy. This variability mostly depends on the time of patient follow-up and the criteria for diagnosis of PVL. Due to a lack of specific baseline characteristics, the PVL diagnosis is made retrospectively, based on observation of progressive clinical and microscopical characteristics of the lesions. Four histological features are encountered over the course of the disease: (1) hyperplasia and hyperkeratosis with no epithelial dysplasia; (2) multifocal expansion of lesions with or without varied grade of dysplasia; (3) verrucous hyperplasia; and (4) verrucous carcinoma or frankly invasive oral squamous cell carcinoma (OSCC).
Currently, four main stages may be encountered during PVL development: Initial focal involvement, geographic expansion (multifocal) over time, development of a warty appearance and finally, development of a cancer. Although proteomic and genomic analysis of OSCC/oral leukoplakia have been described, no proteomic approaches specifically for PVL have been identified. Recently, the use of proteomic-based mass spectrometry (MS) has increased interest in salivary biomarkers for numerous diseases, including OSCC.
In the present study, lower expression of dipeptidyl peptidase 1 (DPP1) was found in PVL patients; although some articles demonstrated increased expression of this molecule and its association with tumor progression. No significant abundance differences were observed between PVL patients at various disease stages, including patients that developed oral cancer. This finding reinforces the relentless malignant evolution of PVL and the diagnostic potential of DPP1. Cathepsins appear to play an important role in preventing tumor formation and/or progression, with angiotensinogen (AGT) showing similar capabilities in PVL patients.
This study revealed evidence about the promising etiologic potential of the proteins AGT and DPP1, and how these biomolecules may be associated with the development of PVL.
The manuscript is interesting and presents a novel diagnostic tool. The purpose, background, and results of this study are interesting and this manuscript is well written.
Manuscript source: Invited manuscript
Specialty type: Medicine, research and experimental
Country of origin: Brazil
Peer-review report classification
Grade A (Excellent): A, A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Boffano P, Gouvea AF, Inoue T, Rattan V S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Hansen LS, Olson JA, Silverman S. Proliferative verrucous leukoplakia. A long-term study of thirty patients. Oral Surg Oral Med Oral Pathol. 1985;60:285-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 244] [Cited by in RCA: 246] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 2. | van der Waal I, Reichart PA. Oral proliferative verrucous leukoplakia revisited. Oral Oncol. 2008;44:719-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 46] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Gouvêa AF, Vargas PA, Coletta RD, Jorge J, Lopes MA. Clinicopathological features and immunohistochemical expression of p53, Ki-67, Mcm-2 and Mcm-5 in proliferative verrucous leukoplakia. J Oral Pathol Med. 2010;39:447-452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Carrard VC, Brouns ER, van der Waal I. Proliferative verrucous leukoplakia; a critical appraisal of the diagnostic criteria. Med Oral Patol Oral Cir Bucal. 2013;18:e411-e413. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Gouvêa AF, Santos Silva AR, Speight PM, Hunter K, Carlos R, Vargas PA, de Almeida OP, Lopes MA. High incidence of DNA ploidy abnormalities and increased Mcm2 expression may predict malignant change in oral proliferative verrucous leukoplakia. Histopathology. 2013;62:551-562. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 42] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Gillenwater AM, Vigneswaran N, Fatani H, Saintigny P, El-Naggar AK. Proliferative verrucous leukoplakia: recognition and differentiation from conventional leukoplakia and mimics. Head Neck. 2014;36:1662-1668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 7. | Gillenwater AM, Vigneswaran N, Fatani H, Saintigny P, El-Naggar AK. Proliferative verrucous leukoplakia (PVL): a review of an elusive pathologic entity! Adv Anat Pathol. 2013;20:416-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 8. | Zakrzewska JM, Lopes V, Speight P, Hopper C. Proliferative verrucous leukoplakia: a report of ten cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:396-401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 85] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Silverman S, Gorsky M. Proliferative verrucous leukoplakia: a follow-up study of 54 cases. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;84:154-157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 146] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 10. | Fettig A, Pogrel MA, Silverman S, Bramanti TE, Da Costa M, Regezi JA. Proliferative verrucous leukoplakia of the gingiva. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2000;90:723-730. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 76] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 11. | Bagan JV, Jimenez Y, Sanchis JM, Poveda R, Milian MA, Murillo J, Scully C. Proliferative verrucous leukoplakia: high incidence of gingival squamous cell carcinoma. J Oral Pathol Med. 2003;32:379-382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 87] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 12. | Morton TH, Cabay RJ, Epstein JB. Proliferative verrucous leukoplakia and its progression to oral carcinoma: report of three cases. J Oral Pathol Med. 2007;36:315-318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 13. | Cabay RJ, Morton TH, Epstein JB. Proliferative verrucous leukoplakia and its progression to oral carcinoma: a review of the literature. J Oral Pathol Med. 2007;36:255-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 80] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Bagan J, Scully C, Jimenez Y, Martorell M. Proliferative verrucous leukoplakia: a concise update. Oral Dis. 2010;16:328-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.9] [Reference Citation Analysis (1)] |
| 15. | Arduino PG, Bagan J, El-Naggar AK, Carrozzo M. Urban legends series: oral leukoplakia. Oral Dis. 2013;19:642-659. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 16. | Müller S. Oral manifestations of dermatologic disease: a focus on lichenoid lesions. Head Neck Pathol. 2011;5:36-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 17. | Batsakis JG, Suarez P, el-Naggar AK. Proliferative verrucous leukoplakia and its related lesions. Oral Oncol. 1999;35:354-359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 114] [Cited by in RCA: 101] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 18. | de Jong EP, Xie H, Onsongo G, Stone MD, Chen XB, Kooren JA, Refsland EW, Griffin RJ, Ondrey FG, Wu B. Quantitative proteomics reveals myosin and actin as promising saliva biomarkers for distinguishing pre-malignant and malignant oral lesions. PLoS One. 2010;5:e11148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 77] [Cited by in RCA: 67] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 19. | Klanrit P, Sperandio M, Brown AL, Shirlaw PJ, Challacombe SJ, Morgan PR, Odell EW. DNA ploidy in proliferative verrucous leukoplakia. Oral Oncol. 2007;43:310-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 64] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 20. | Kresty LA, Mallery SR, Knobloch TJ, Li J, Lloyd M, Casto BC, Weghorst CM. Frequent alterations of p16INK4a and p14ARF in oral proliferative verrucous leukoplakia. Cancer Epidemiol Biomarkers Prev. 2008;17:3179-3187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 47] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 21. | Jou YJ, Lin CD, Lai CH, Chen CH, Kao JY, Chen SY, Tsai MH, Huang SH, Lin CW. Proteomic identification of salivary transferrin as a biomarker for early detection of oral cancer. Anal Chim Acta. 2010;681:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 106] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 22. | Wang Q, Gao P, Cheng F, Wang X, Duan Y. Measurement of salivary metabolite biomarkers for early monitoring of oral cancer with ultra performance liquid chromatography-mass spectrometry. Talanta. 2014;119:299-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Pfaffe T, Cooper-White J, Beyerlein P, Kostner K, Punyadeera C. Diagnostic potential of saliva: current state and future applications. Clin Chem. 2011;57:675-687. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 450] [Cited by in RCA: 488] [Article Influence: 34.9] [Reference Citation Analysis (0)] |
| 24. | Zhang A, Sun H, Wang P, Wang X. Salivary proteomics in biomedical research. Clin Chim Acta. 2013;415:261-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 25. | Flores IL, Kawahara R, Miguel MC, Granato DC, Domingues RR, Macedo CC, Carnielli CM, Yokoo S, Rodrigues PC, Monteiro BV. EEF1D modulates proliferation and epithelial-mesenchymal transition in oral squamous cell carcinoma. Clin Sci (Lond). 2016;130:785-799. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 34] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 26. | Migliorati CA, Ranken R, Kaplan MJ, Silverman S. Reactivity of monoclonal antibodies 17.13 and 63.12 with 141 oral mucosal lesions. J Oral Pathol Med. 1992;21:412-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Kannan R, Bijur GN, Mallery SR, Beck FM, Sabourin CL, Jewell SD, Schuller DE, Stoner GD. Transforming growth factor-alpha overexpression in proliferative verrucous leukoplakia and oral squamous cell carcinoma: an immunohistochemical study. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:69-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 28. | Gopalakrishnan R, Weghorst CM, Lehman TA, Calvert RJ, Bijur G, Sabourin CL, Mallery SR, Schuller DE, Stoner GD. Mutated and wild-type p53 expression and HPV integration in proliferative verrucous leukoplakia and oral squamous cell carcinoma. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1997;83:471-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 56] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 29. | Campisi G, Giovannelli L, Ammatuna P, Capra G, Colella G, Di Liberto C, Gandolfo S, Pentenero M, Carrozzo M, Serpico R. Proliferative verrucous vs conventional leukoplakia: no significantly increased risk of HPV infection. Oral Oncol. 2004;40:835-840. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 77] [Cited by in RCA: 66] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 30. | Bagan JV, Jimenez Y, Murillo J, Gavaldá C, Poveda R, Scully C, Alberola TM, Torres-Puente M, Pérez-Alonso M. Lack of association between proliferative verrucous leukoplakia and human papillomavirus infection. J Oral Maxillofac Surg. 2007;65:46-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 62] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 31. | Bagan JV, Jiménez Y, Murillo J, Poveda R, Díaz JM, Gavaldá C, Margaix M, Scully C, Alberola TM, Torres Puente M. Epstein-Barr virus in oral proliferative verrucous leukoplakia and squamous cell carcinoma: A preliminary study. Med Oral Patol Oral Cir Bucal. 2008;13:E110-E113. [PubMed] |
| 32. | Miller SM. Saliva testing--a nontraditional diagnostic tool. Clin Lab Sci. 1994;7:39-44. [PubMed] |
| 33. | Nagler R, Bahar G, Shpitzer T, Feinmesser R. Concomitant analysis of salivary tumor markers--a new diagnostic tool for oral cancer. Clin Cancer Res. 2006;12:3979-3984. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 121] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 34. | Bigler LR, Streckfus CF, Dubinsky WP. Salivary biomarkers for the detection of malignant tumors that are remote from the oral cavity. Clin Lab Med. 2009;29:71-85. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 62] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 35. | Krishna Prasad RB, Sharma A, Babu HM. An insight into salivary markers in oral cancer. Dent Res J (Isfahan). 2013;10:287-295. [PubMed] |
| 36. | Gaillard-Sanchez I, Mattei MG, Clauser E, Corvol P. Assignment by in situ hybridization of the angiotensinogen gene to chromosome band 1q4, the same region as the human renin gene. Hum Genet. 1990;84:341-343. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 37. | Vairaktaris E, Yapijakis C, Vylliotis A, Derka S, Vassiliou S, Nkenke E, Serefoglou Z, Ragos V, Critselis E, Avgoustidis D. Angiotensinogen polymorphism is associated with risk for malignancy but not for oral cancer. Anticancer Res. 2008;28:1675-1679. [PubMed] |
| 38. | Gourin CG, Zhi W, Adam BL. Proteomic identification of serum biomarkers for head and neck cancer surveillance. Laryngoscope. 2009;119:1291-1302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 39] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 39. | Bouquet C, Lamandé N, Brand M, Gasc JM, Jullienne B, Faure G, Griscelli F, Opolon P, Connault E, Perricaudet M. Suppression of angiogenesis, tumor growth, and metastasis by adenovirus-mediated gene transfer of human angiotensinogen. Mol Ther. 2006;14:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Zhou G, Li H, Gong Y, Zhao Y, Cheng J, Lee P, Zhao Y. Proteomic analysis of global alteration of protein expression in squamous cell carcinoma of the esophagus. Proteomics. 2005;5:3814-3821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 41. | González-Zuloeta Ladd AM, Arias Vásquez A, Siemes C, Yazdanpanah M, Coebergh JW, Hofman A, Stricker BH, van Duijn CM. Differential roles of Angiotensinogen and Angiotensin Receptor type 1 polymorphisms in breast cancer risk. Breast Cancer Res Treat. 2007;101:299-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Thong B, Pilling J, Ainscow E, Beri R, Unitt J. Development and validation of a simple cell-based fluorescence assay for dipeptidyl peptidase 1 (DPP1) activity. J Biomol Screen. 2011;16:36-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 43. | Owen CA. Leukocyte cell surface proteinases: regulation of expression, functions, and mechanisms of surface localization. Int J Biochem Cell Biol. 2008;40:1246-1272. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 62] [Cited by in RCA: 59] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 44. | Toomes C, James J, Wood AJ, Wu CL, McCormick D, Lench N, Hewitt C, Moynihan L, Roberts E, Woods CG. Loss-of-function mutations in the cathepsin C gene result in periodontal disease and palmoplantar keratosis. Nat Genet. 1999;23:421-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 336] [Cited by in RCA: 345] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 45. | Ruffell B, Affara NI, Cottone L, Junankar S, Johansson M, DeNardo DG, Korets L, Reinheckel T, Sloane BF, Bogyo M. Cathepsin C is a tissue-specific regulator of squamous carcinogenesis. Genes Dev. 2013;27:2086-2098. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 64] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 46. | Gocheva V, Zeng W, Ke D, Klimstra D, Reinheckel T, Peters C, Hanahan D, Joyce JA. Distinct roles for cysteine cathepsin genes in multistage tumorigenesis. Genes Dev. 2006;20:543-556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 398] [Cited by in RCA: 430] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 47. | Bogenrieder T, Finstad CL, Freeman RH, Papandreou CN, Scher HI, Albino AP, Reuter VE, Nanus DM. Expression and localization of aminopeptidase A, aminopeptidase N, and dipeptidyl peptidase IV in benign and malignant human prostate tissue. Prostate. 1997;33:225-232. [PubMed] |
| 48. | Wesley UV, Albino AP, Tiwari S, Houghton AN. A role for dipeptidyl peptidase IV in suppressing the malignant phenotype of melanocytic cells. J Exp Med. 1999;190:311-322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 135] [Cited by in RCA: 134] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 49. | Wesley UV, Tiwari S, Houghton AN. Role for dipeptidyl peptidase IV in tumor suppression of human non small cell lung carcinoma cells. Int J Cancer. 2004;109:855-866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 50. | Wesley UV, McGroarty M, Homoyouni A. Dipeptidyl peptidase inhibits malignant phenotype of prostate cancer cells by blocking basic fibroblast growth factor signaling pathway. Cancer Res. 2005;65:1325-1334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 116] [Article Influence: 5.8] [Reference Citation Analysis (0)] |