Published online Jul 16, 2015. doi: 10.12998/wjcc.v3.i7.614
Peer-review started: January 9, 2014
First decision: March 12, 2014
Revised: May 28, 2015
Accepted: June 9, 2015
Article in press: June 11, 2015
Published online: July 16, 2015
Processing time: 566 Days and 20.9 Hours
With the term “oncological screening”, we define the overall performances made to detect early onset of tumors. These tests are conducted on a population that does not have any signs or symptoms related to a neoplasm. The whole population above a certain age, only one sex, only subjects with a high risk of developing cancer due to genetic, professional, discretionary reasons may be involved. Screening campaigns should be associated, when risk factors that can be avoided are known, with campaigns for the prevention of cancer by means of suitable behavior. The goal of cancer screening cannot however be limited to the diagnosis of a greater number of neoplasms. Screening will be useful only if it leads to a reduction in overall mortality or at least in mortality related to the tumor. Screening should then allow the diagnosis of the disease at a stage when there is a possibility of healing, possibility that is instead difficult when the disease is diagnosed at the appearance of signs or symptoms. This is the reason why not all campaigns of cancer screening have the same effectiveness. In Italy, every year there are about 150000 deaths due to cancer. Some of these tumors can be cured with a very high percentage of success if diagnosed in time. Cervical cancer can be diagnosed with non-invasive tests. The screening test used all over the world is Papanicolaou (Pap) test. This test may be carried out over the entire healthy population potentially exposed to the risk of contracting cancer. Public health has begun the screening campaigns in the hope of saving many of the approximately 270000 new cases of cancer reported each year. Screening is done following protocols that guarantee quality at the national level: these protocols are subject to change over time to reflect new realities or to correct any errors in the system. A simplified sketch of a possible route of cancer screening is as follows: (1) after selecting the target population, for example all women between 25 and 64 years (in the case of monitoring of cervical cancer), an invitation letter with the date and time of the appointment, planned according to the acceptance capacity of the hospital, is sent to all individuals; (2) an examination, which depending on the individual and the type of cancer to be monitored, for example, can be a Pap smear, is performed and the patient can go home; (3) once available the results of examinations, if negative, they shall be communicated to the person concerned that will be notified by mail and will be recalled for a second test at a few years of distance, in the case of non-negativity, instead, the patient is contacted by telephone and informed of the need to carry out further examinations: it is said that the patient is in the “phase two” of the screening pathway; (4) in phase two, reached by only a small portion of the interested parties (usually less than 3%-5%), more in-depth tests are carried out, which, depending on the individual and the type of cancer, can be: cytological and colposcopic examinations, the removal of a fragment of tissue (biopsy) and subsequent histological examination, additional tests such as ultrasound, radiography, or others such as computerized tomography, magnetic resonance imaging, positron emission tomography, etc., in case of negativity, the concerned person will be called for new control tests at a a few years of distance, in case of non-negativity, it will be proposed instead an oncologic therapeutic plan and/or surgery to treat the diagnosed tumor; and (5) once the treatment plan is completed, the individual enters the follow-up protocol, which is monitored over time to see if the tumor has been completely removed or if instead it is still developing. Cervical cancer is undoubtedly the most successful example of a cancer screening campaign. Paradoxically, its effectiveness is one of the strongest reasons to criticize the usefulness of vaccination against human papillomavirus (HPV) in countries where the screening service with Pap test is organized in an efficient manner. Cervical cancer screening protocols are directed to sexually active women aged 25-64 years: they provide the Pap test performed by examining under a microscope or by staining with a specific “thin prep” the material taken from the cervix with a small spatula and a brush. It is recommended to repeat the test every two or three years. It is important to emphasize that women vaccinated against HPV must continue the screening with Pap test. Although some screening programs (e.g., Pap smears) have had remarkable success in reducing mortality from a specific cancer, any kind of screening is free from inherent limitations. The screening methods are in fact applied to large parts of the apparently healthy population. In particular, the limits for certain cancers may be as obvious as to prohibit the introduction of an organized screening program. Potential limitations of organized screenings are basically of two types: organizational and medical. The limits of organizational type relate to the ability of a program to recruit the whole target population. Although well organized, a screening program will hardly be able to exceed a coverage of 70%-80% of the target population, and in fact the results of the current programs are often much smaller. The limits of medical type are represented by the possibility of reducing the overall mortality, or specific mortality, using a specific screening campaign.
Core tip: Most cases of cervical cancer are preventable and, if caught early, highly curable. Despite this, cervical cancer is the second most common cause of cancer death and a leading cause of morbidity in women worldwide. Unfortunately, cure is less likely when the disease is diagnosed at an advanced stage. Although the human papillomavirus is considered the major causative agent of cervical cancer, yet the viral infection alone is not sufficient for cancer progression.
- Citation: Comparetto C, Borruto F. Cervical cancer screening: A never-ending developing program. World J Clin Cases 2015; 3(7): 614-624
- URL: https://www.wjgnet.com/2307-8960/full/v3/i7/614.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i7.614
Human papillomavirus (HPV) belongs to the diverse group of sexually transmitted viruses that manifest affinity to the squamous epithelia of the skin and mucous membranes. It has been proved that types 16 and 18 in particular could lead to cervical cancer. High-risk strains of HPV (HR-HPV) types have been found in cervical cancer worldwide[1]. The Papanicolaou (Pap) smear was the mainstay of screening in women for over 60 years[2]. All current guidelines recommend colposcopy for women with high-grade squamous intraepithelial lesions (H-SIL), with a view to performing a biopsy or conization. Randomized controlled trials and retrospective comparisons much more strongly suggest that regular well-organized smear testing prevents a number of deaths due to cervical cancer. It should be remembered that many cellular atypia found on cervical smears never progress to cancer. The frequency of overdiagnosis has not been studied. Smear-based screening appears to have very few serious adverse effects. In practice, despite the lack of solid evidence, it seems unreasonable not to recommend screening for cervical cancer. Organized screening is preferable to opportunistic screening performed without quality controls and without research to optimize screening strategy[3,4]. The available technology for prevention and its developments allows real opportunities for cervical cancer elimination in defined populations to be foreseen[5-8].
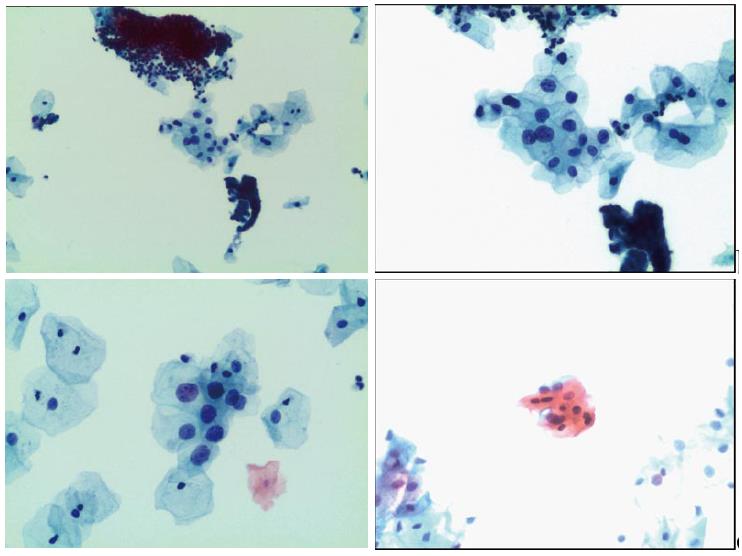
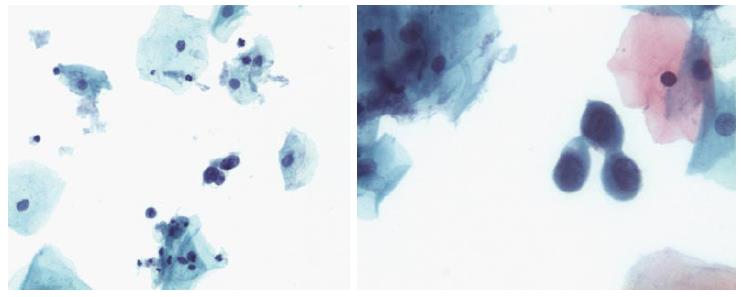
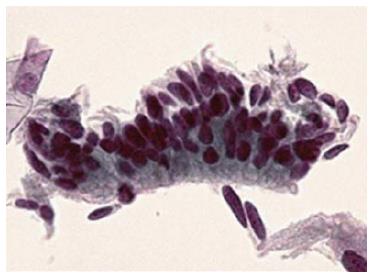
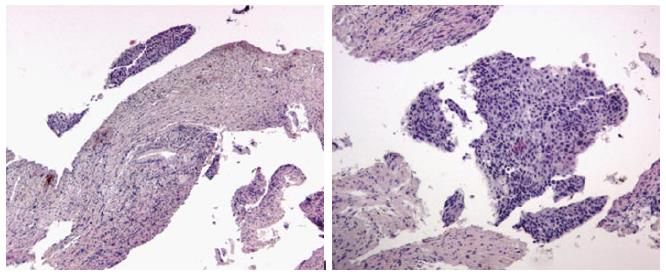
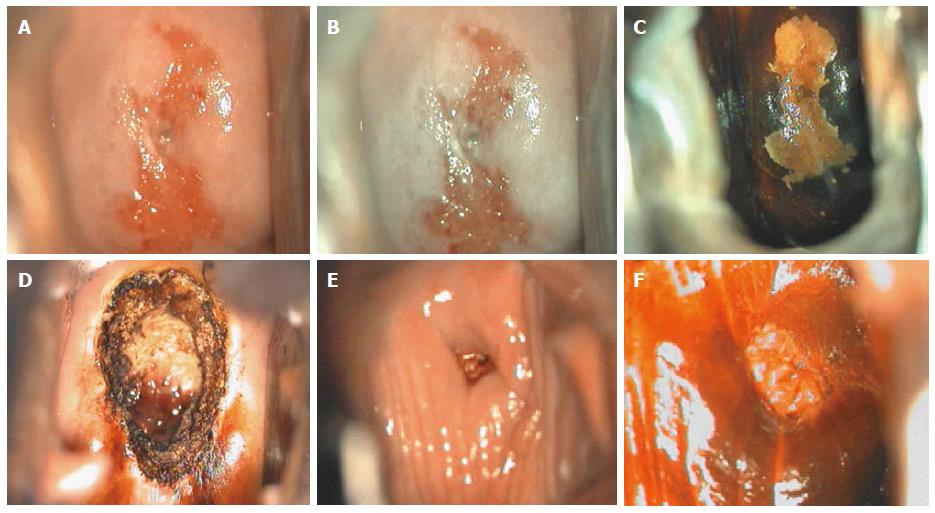
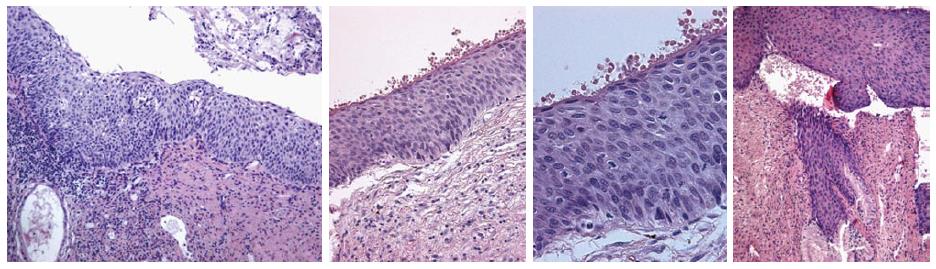
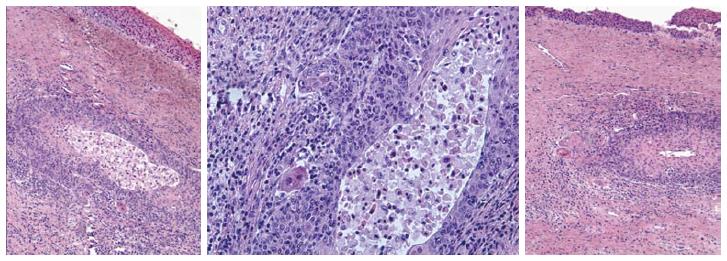
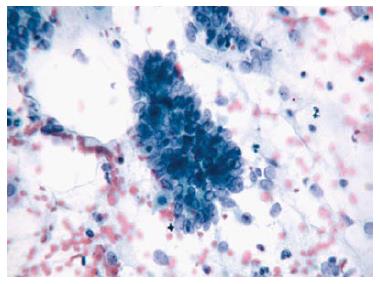
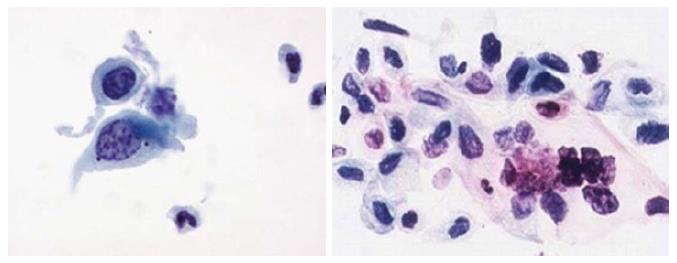
Two quality metrics for gynecologic cytology are available: “prospective rescreening” and “retrospective rescreening”. Most laboratories (> 85%) prospectively rescreen more than 10% of Pap tests interpreted as negative for intraepithelial lesion or malignancy. Most (72%) report inclusion of less than 20% high-risk cases. Most laboratories use multiple measures to define “high risk”. Most laboratories (96.2%) retrospectively rescreen Pap tests from the preceding 5 years only. In most laboratories (71.4%), only Pap test results with H-SIL or worse prompt retrospective review. Upgraded diagnoses from negative for intraepithelial lesion or malignancy to atypical squamous cells (ASC), cannot exclude H-SIL (ASC-H), should be monitored (Figures 1-5)[9-18].
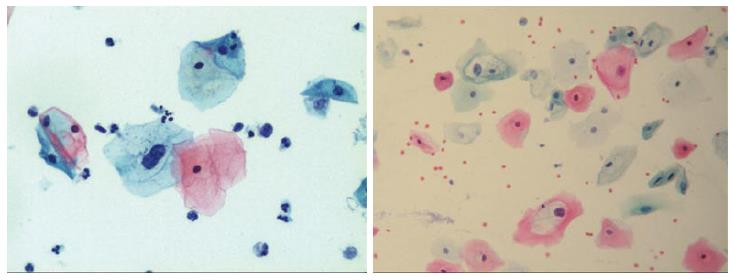
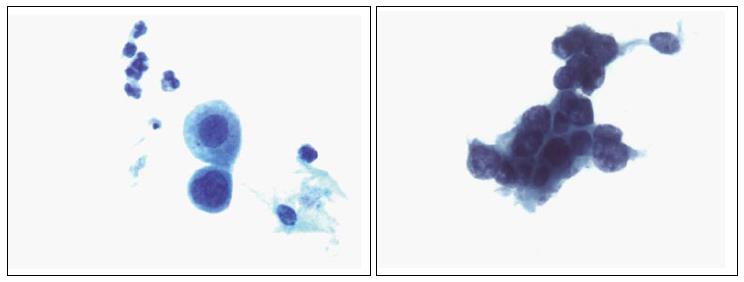
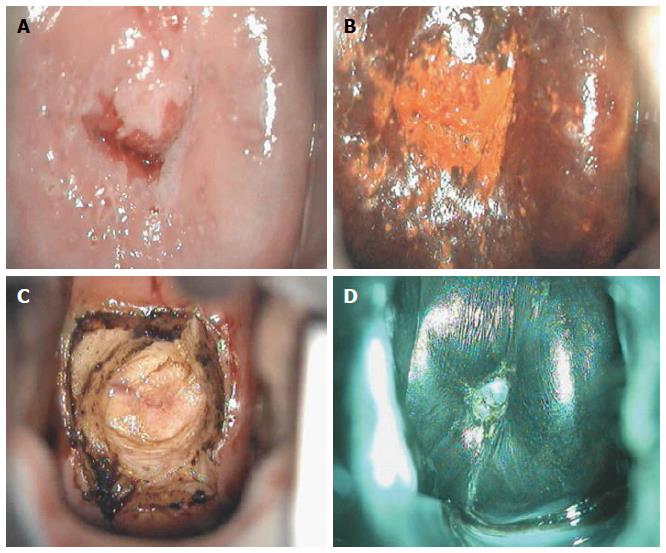
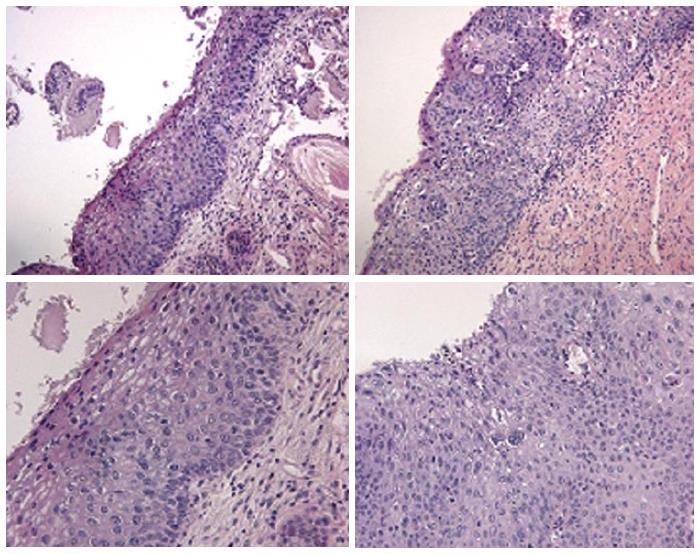
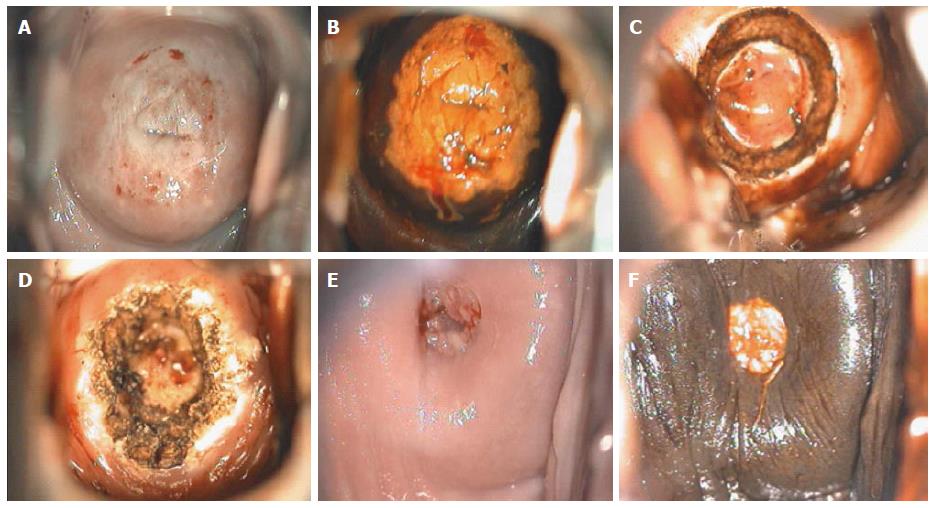
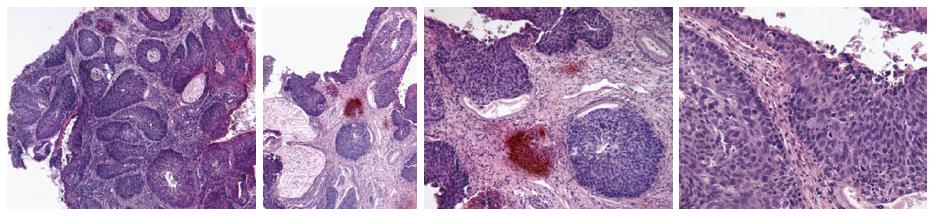
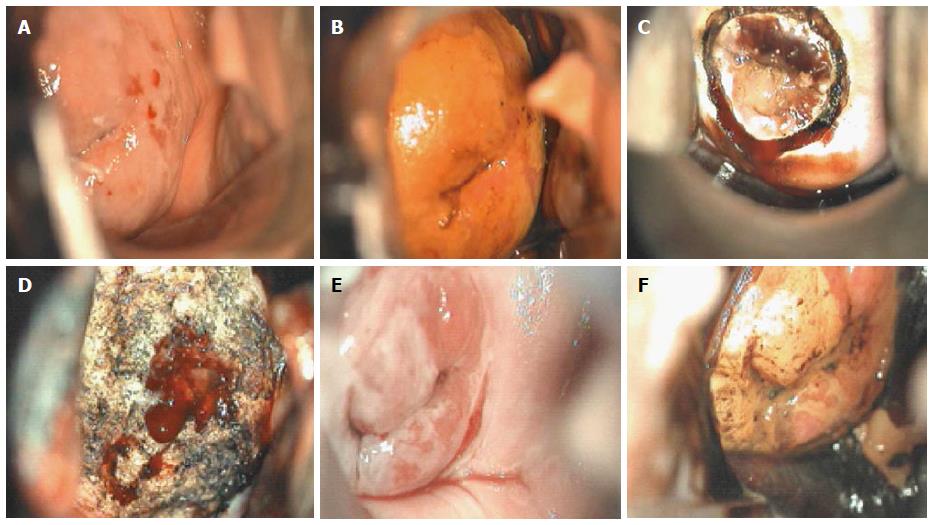
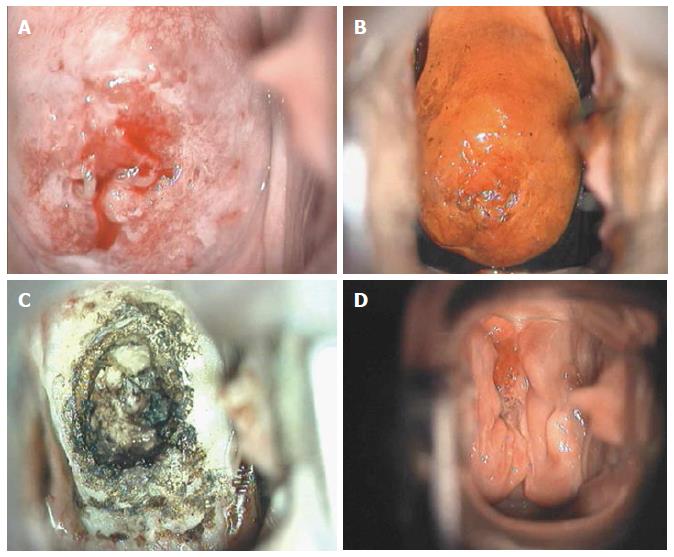
Though in the 1980s colposcopically-directed biopsy excluded over 90% of cervical intraepithelial neoplasia (CIN) 3 or worse (CIN3+), recent reviews found sensitivity of colposcopically-directed biopsy for CIN3+ of 50%-65%. Studies from China showed that the sensitivity of colposcopically-directed biopsy for CIN3+ is higher for large CIN3+ than for small CIN3+ and higher for associated high-grade cervical cytology than for low-grade cervical cytology. Colposcopically-directed biopsy excluded over 90% of CIN3+ in the 1980s because colposcopy clinics in the 1980s evaluated women with high-grade cytology that had large CIN3+. It no longer excludes CIN3+ well because current colposcopy clinics evaluate women with low-grade cytology that have small CIN3+. When colposcopically-directed biopsy is used to exclude CIN3+, our understanding of the natural history of CIN is skewed, errors occur in defining appropriate screening practice, and inaccurate diagnosis results in incorrect treatment. The impression that CIN is more common on the anterior lip of the cervix is an artifact introduced by the inaccuracy of colposcopy. An unjustified enthusiasm for screening with visual inspection with acetic acid (VIA) occurred when the sensitivity of VIA for CIN3+ was inflated by screening studies using colposcopically-directed biopsy as the gold-standard for CIN3+. As the diagnosis of CIN3+ solely by endocervical curettage (ECC) is uncommon in women under age 25, the ECC may be omitted in women under age 25 years. If multiple cervical biopsies are performed, to limit discomfort, a bronchoscopy biopsy instrument which obtains 2-mm biopsies should be used (Figures 6-17)[19,20]. A novel technique uses a high-resolution microendoscope (HRME) to diagnose cervical dysplasia. HRME imaging reduces the limitations of existing cervical cancer screening methods currently in use in low-resource settings and so has the potential to contribute to cervical cancer prevention in the developing world[21,22].
Pre-cancerous lesions of cervix (CIN) are usually treated with excisional or ablative procedures. In the United Kingdom, the National Health Service cervical screening guidelines suggest that over 80% of treatments should be performed in an outpatient setting (colposcopy clinics). Treatment methods commonly used for precancerous lesions are conization, loop electrosurgical excision procedure (LEEP), laser ablation, and cryotherapy. Recently, outpatient LEEP has replaced cryotherapy in many countries. However, a greater awareness of the importance of cervical cancer in the developing world and a greater awareness of the long-term consequences of LEEP like cervical insufficiency, have renewed interest in cryotherapy. Among the trials, cure rates ranged from 56.8% to 96.6% in prospective controlled studies and from 70% to 95.5% in observational trials. Cryotherapy has very low rates of complication and serious complications that require medical therapy or affect the reproductive future results are extremely rare. Side effects include vaginal discharge and cramping which are temporary, usually self-limited, and well tolerated after preventive patient counseling. When surveyed, women highly accept cryotherapy. Compared to other methods of treatment, cryotherapy is very affordable and feasible to integrate screening programs and treatment for cervical cancer[23]. Often, due to improper judgment of interventional indications for cervical lesions, overtreatment to various degrees takes place, influencing patients’ health and lives[24,25].
Cytopathology experts, interested stakeholders, and representatives from the College of American Pathologists (CAP), the Centers for Disease Control and Prevention, the American Society of Cytopathology (ASC), the Papanicolaou Society of Cytopathology, the American Society for Clinical Pathology, and the American Society of Cytotechnology convened the Gynecologic Cytopathology Quality Consensus Conference to present preliminary consensus statements developed by working groups, including the Cytologic-Histologic Correlations Working Group 4, using results from surveys and literature review. Conference participants voted on statements, suggested changes where consensus was not achieved, and voted on proposed changes. To document existing practices in gynecologic cytologic-histologic correlation (see the Bethesda System cytologic reports in Table 1), to develop consensus statements on appropriate practices, to explore standardization, and to suggest improvement in these practices, the material is based on survey results from US laboratories, review of the literature, and the CAP Web site for consensus comments and additional survey questions[26-30].
| Specimen type: |
| Conventional smear (Pap smear) |
| Liquid-based preparation |
| Other |
| Specimen adequacy: |
| Satisfactory for evaluation (describe presence or absence of endocervical/transformation zone component and any other quality indicators, e.g. partially obscuring blood, inflammation, etc.) |
| Unsatisfactory for evaluation… (specify reason) |
| Specimen rejected/not processed (specify reason) |
| Specimen processed and examined, but unsatisfactory for evaluation of epithelial abnormality because of (specify reason) |
| General categorization (optional): |
| Negative for intraepithelial lesion or malignancy conventional smear (Pap smear) |
| Other: see interpretation/result (e.g., endometrial cells in a woman ≥ 40 yr of age) |
| Epithelial cell abnormality: see interpretation/result (specify “squamous” or “glandular” as appropriate) |
| Interpretation/result: |
| Negative for intraepithelial lesion or malignancy: when there is no cellular evidence of neoplasia, state this in the general categorization above and/or in the interpretation/result section of the report, whether or not there are organisms or other non-neoplastic findings |
| Organisms: |
| Trichomonas vaginalis |
| Fungal organisms morphologically consistent with Candida spp. |
| Shift in flora suggestive of bacterial vaginosis |
| Bacteria morphologically consistent with Actinomyces spp. |
| Cellular changes consistent with HSV |
| Other non neoplastic findings (optional to report; list not inclusive): |
| Reactive cellular changes associated with: |
| Inflammation (includes typical repair) |
| Radiation |
| IUD |
| Glandular cells status post hysterectomy |
| Atrophy |
| Other: |
| Endometrial cells (in a woman ≥ 40 yr of age): specify if “negative for SIL” |
| Epithelial cell abnormalities: |
| Squamous cell: |
| ASC: |
| Of undetermined significance (ASC-US) |
| Cannot exclude H-SIL (ASC-H) |
| Low-grade SIL (L-SIL) (encompassing: HPV/mild dysplasia/CIN1) |
| High-grade SIL (H-SIL) (encompassing: moderate and severe dysplasia, CIS/CIN2 and CIN3): |
| With features suspicious for invasion (if invasion is suspected) |
| SCC |
| Glandular cell: |
| Atypical: |
| Endocervical cells (NOS or specify in comments) |
| Endometrial cells (NOS or specify in comments) |
| Glandular cells (NOS or specify in comments) |
| Atypical |
| Endocervical cells, favor neoplastic |
| Glandular cells, favor neoplastic |
| Endocervical adenocarcinoma in situ |
| Adenocarcinoma: |
| Endocervical |
| Endometrial |
| Extrauterine |
| NOS |
| Other malignant neoplasms (specify) |
| Ancillary testing: provide a brief description of the test methods and report the result so that it is easily understood by the clinician |
| Automated review: if case examined by automated device, specify device and result |
| Educational notes and suggestions (optional): suggestions should be concise and consistent with clinical follow-up guidelines published by professional organizations (references to relevant publications may be included) |
Vaccination against HPV is expected to decrease the incidence of cervical cancer in most countries. However, it is also expected to influence the effectiveness of screening. In the future, maintaining Pap test as the primary test for cervical screening may become too expensive. As the prevalence of cervical dysplasia decreases, the positive predictive value of citology also decrease, and consequently, more women will undergo unnecessary diagnostic procedures and follow-up. The HPV deoxy ribonucleic acid (DNA) test has recently emerged as the best tool to replace cytology as primary screening. It is less subjected to human errors and much more sensitive than the Pap test in detecting high-grade cervical lesions. By incorporating this test the overall quality of screening programs will improve and will allow spacing out the screening tests, while maintaining safety and reducing costs. Although HPV testing is less specific than Pap test, this problem could be solved by reserving the latter for triaging cases of HPV positivity. Since most HPV-positive smears contain significant anomalies, Pap cytology is expected to perform with sufficient accuracy in these cases. Pap triage of HPV-positive patients would also provide a low-cost strategy to monitor the effectiveness of the vaccine in the long term. Although HPV typing could be implemented as a screening tool for the population, further research is needed to determine the optimal age to begin screening, the role of the HPV test and other markers of disease progression, and adequate follow-up procedures for the HPV-positive and smear-negative women[31-33].
P- Reviewer: Giraldi G, Yokoyama Y S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Ciesielska U, Nowińska K, Podhorska-Okołów M, Dziegiel P. The role of human papillomavirus in the malignant transformation of cervix epithelial cells and the importance of vaccination against this virus. Adv Clin Exp Med. 2012;21:235-244. [PubMed] |
| 2. | Tambouret RH. The evolution of the Papanicolaou smear. Clin Obstet Gynecol. 2013;56:3-9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 3. | Cervical cancer screening. Organised screening to avoid unnecessary conisation. Prescrire Int. 2010;19:172-177, 179. [PubMed] |
| 4. | Nygård M. Screening for cervical cancer: when theory meets reality. BMC Cancer. 2011;11:240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Bosch FX. Human papillomavirus: science and technologies for the elimination of cervical cancer. Expert Opin Pharmacother. 2011;12:2189-2204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 6. | Crosbie EJ, Kitchener HC. Human papillomavirus as a target for management, prevention and therapy. Int J Hyperthermia. 2012;28:478-488. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 7. | Adriaensen WJ, Matheï C, Buntinx FJ, Arbyn M. A framework provided an outline toward the proper evaluation of potential screening strategies. J Clin Epidemiol. 2013;66:639-647. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 21] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Jimbo M, Rana GK, Hawley S, Holmes-Rovner M, Kelly-Blake K, Nease DE, Ruffin MT. What is lacking in current decision aids on cancer screening? CA Cancer J Clin. 2013;63:193-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 49] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 9. | Brainard JA, Birdsong GG, Elsheikh TM, Hartley DA, Naik K, Neal MH, Souers RJ, Henry MR. Prospective and retrospective review of gynecologic cytopathology: findings from the College of American Pathologists Gynecologic Cytopathology Quality Consensus Conference working group 2. Arch Pathol Lab Med. 2013;137:175-182. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Hoda RS, Loukeris K, Abdul-Karim FW. Gynecologic cytology on conventional and liquid-based preparations: a comprehensive review of similarities and differences. Diagn Cytopathol. 2013;41:257-278. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 11. | Reuschenbach M, von Knebel Doeberitz M. Diagnostic tests for the detection of human papillomavirus-associated cervical lesions. Curr Pharm Des. 2013;19:1358-1370. [PubMed] |
| 12. | Booth CN, Bashleben C, Filomena CA, Means MM, Wasserman PG, Souers RJ, Henry MR. Monitoring and ordering practices for human papillomavirus in cervical cytology: findings from the College of American Pathologists Gynecologic Cytopathology Quality Consensus Conference working group 5. Arch Pathol Lab Med. 2013;137:214-219. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 13. | Rebolj M, Pribac I, Frederiksen ME, Lynge E. The problem of false-positive human papillomavirus DNA tests in cervical screening. Curr Pharm Des. 2013;19:1439-1449. [PubMed] |
| 14. | Dillner J. Primary human papillomavirus testing in organized cervical screening. Curr Opin Obstet Gynecol. 2013;25:11-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 35] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 15. | Lui R. Clinical utility of HPV testing. Clin Obstet Gynecol. 2013;56:17-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Arbyn M, Roelens J, Cuschieri K, Cuzick J, Szarewski A, Ratnam S, Reuschenbach M, Belinson S, Belinson JL, Monsonego J. The APTIMA HPV assay versus the Hybrid Capture 2 test in triage of women with ASC-US or LSIL cervical cytology: a meta-analysis of the diagnostic accuracy. Int J Cancer. 2013;132:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 96] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 17. | Snijders PJ, Verhoef VM, Arbyn M, Ogilvie G, Minozzi S, Banzi R, van Kemenade FJ, Heideman DA, Meijer CJ. High-risk HPV testing on self-sampled versus clinician-collected specimens: a review on the clinical accuracy and impact on population attendance in cervical cancer screening. Int J Cancer. 2013;132:2223-2236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 163] [Cited by in RCA: 206] [Article Influence: 15.8] [Reference Citation Analysis (0)] |
| 18. | Patanwala IY, Bauer HM, Miyamoto J, Park IU, Huchko MJ, Smith-McCune KK. A systematic review of randomized trials assessing human papillomavirus testing in cervical cancer screening. Am J Obstet Gynecol. 2013;208:343-353. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 19. | Pretorius RG, Belinson JL. Colposcopy. Minerva Ginecol. 2012;64:173-180. [PubMed] |
| 20. | Herfs M, Crum CP. Laboratory management of cervical intraepithelial neoplasia: proposing a new paradigm. Adv Anat Pathol. 2013;20:86-94. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 21. | Schmeler KM. Preventing cervical cancer globally. Cancer Prev Res (Phila). 2012;5:1257-1259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 22. | McCluggage WG. New developments in endocervical glandular lesions. Histopathology. 2013;62:138-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 76] [Cited by in RCA: 62] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | McClung EC, Blumenthal PD. Efficacy, safety, acceptability and affordability of cryotherapy: a review of current literature. Minerva Ginecol. 2012;64:149-171. [PubMed] |
| 24. | Tianmin X, Weiqin C, Manhua C, Yang L, Lihui S, Tianshu W, Xiaocui L. Status quo and prevention of overtreatment in cervical diseases. Eur J Gynaecol Oncol. 2012;33:300-303. [PubMed] |
| 25. | Sauvaget C, Muwonge R, Sankaranarayanan R. Meta-analysis of the effectiveness of cryotherapy in the treatment of cervical intraepithelial neoplasia. Int J Gynaecol Obstet. 2013;120:218-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 64] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 26. | Shafi MI, Petry U, Bosch XF, Gissman L, Kocken M, Helmerhorst TJ, Stanley M, Nazeer S. European consensus statement on “HPV Vaccination and Colposcopy”. J Low Genit Tract Dis. 2011;15:309-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 27. | Crothers BA, Jones BA, Cahill LA, Moriarty AT, Mody DR, Tench WD, Souers RJ. Quality improvement opportunities in gynecologic cytologic-histologic correlations: findings from the College of American Pathologists Gynecologic Cytopathology Quality Consensus Conference working group 4. Arch Pathol Lab Med. 2013;137:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Priebe AM. 2012 cervical cancer screening guidelines and the future role of HPV testing. Clin Obstet Gynecol. 2013;56:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 28] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 29. | Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, Solomon D, Wentzensen N, Lawson HW. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. J Low Genit Tract Dis. 2013;17:S1-S27. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 478] [Cited by in RCA: 508] [Article Influence: 42.3] [Reference Citation Analysis (0)] |
| 30. | Massad LS, Einstein MH, Huh WK, Katki HA, Kinney WK, Schiffman M, Solomon D, Wentzensen N, Lawson HW. 2012 updated consensus guidelines for the management of abnormal cervical cancer screening tests and cancer precursors. Obstet Gynecol. 2013;121:829-846. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 496] [Cited by in RCA: 548] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 31. | Syrjänen K, Di Bonito L, Gonçalves L, Murjal L, Santamaria M, Mahovlic V, Karakitsos P, Onal B, Schmitt FC. Cervical cancer screening in Mediterranean countries: implications for the future. Cytopathology. 2010;21:359-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 32. | Tornesello ML, Buonaguro L, Buonaguro FM. Mutations of the TP53 gene in adenocarcinoma and squamous cell carcinoma of the cervix: a systematic review. Gynecol Oncol. 2013;128:442-448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 33. | Hou F, Ma D, Cui B. Treg cells in different forms of uterine cancer. Clin Chim Acta. 2013;415:337-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |