Published online Jun 16, 2015. doi: 10.12998/wjcc.v3.i6.519
Peer-review started: October 20, 2014
First decision: November 14, 2014
Revised: February 6, 2015
Accepted: March 18, 2015
Article in press: March 20, 2015
Published online: June 16, 2015
Processing time: 242 Days and 9.5 Hours
A 54-year-old female with Anderson-Fabry disease (AFD)-R342Q missense mutation on exon 7 in alpha-galactosidase A (GLA) gene - presented with sustained ventricular tachycardia. Imaging confirmed the presence of a new left ventricular apical aneurysm (LVAA) and a significantly reduced intra-cavitary gradient compared to two years prior. AFDcv is an X-linked lysosomal storage disorder caused by GLA enzyme deficiency. The phenotypic expression of AFD in the heart is not well described. Cardiac involvement can include left ventricular hypertrophy (LVH), which is typically symmetric, but can also mimic hypertrophic cardiomyopathy (HCM). Left ventricular apical aneurysm is a rare finding in HCM. We suggest a shared mechanism of LVAA formation in AFD and HCM, independent of the underlying cardiomyopathy. Mechanisms of LVAA formation in HCM include genetic predisposition and long-standing left ventricular wall stress from elevated intra-cavitary systolic pressures due to mid-cavitary obstruction. Both mechanisms are supported in this patient (a brother with AFD also developed a small LVAA). Screening for AFD should be considered in cases of unexplained LVH, particularly in patients with the aneurysmal variant of HCM.
Core tip: Left ventricular apical aneurysm (LVAA) is a very rare phenomenon in patients with Anderson-Fabry disease (AFD); however, this patient with genetically confirmed AFD presented with a new LVAA. The authors believe that formation of the LVAA is the result of long-standing elevated intra-cavitary systolic pressures and possibly genetic predisposition, similar to what can be seen in hypertrophic cardiomyopathy.
- Citation: Poulin MF, Shah A, Trohman RG, Madias C. Advanced Anderson-Fabry disease presenting with left ventricular apical aneurysm and ventricular tachycardia. World J Clin Cases 2015; 3(6): 519-524
- URL: https://www.wjgnet.com/2307-8960/full/v3/i6/519.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i6.519
Anderson-Fabry disease (AFD) is an X-linked lysosomal storage disorder caused by alpha-galactosidase (GLA) enzyme deficiency. The phenotypic expression of AFD in the heart is not well described. Cardiac involvement can include left ventricular hypertrophy (LVH), which is typically symmetric, but can also mimic hypertrophic cardiomyopathy (HCM). We report the case of a middle-aged female previously diagnosed with AFD who developed a left ventricular apical aneurysm (LVAA), which has been rarely reported in AFD. We suggest a shared mechanism of LVAA formation in AFD and HCM, independent of the underlying cardiomyopathy.
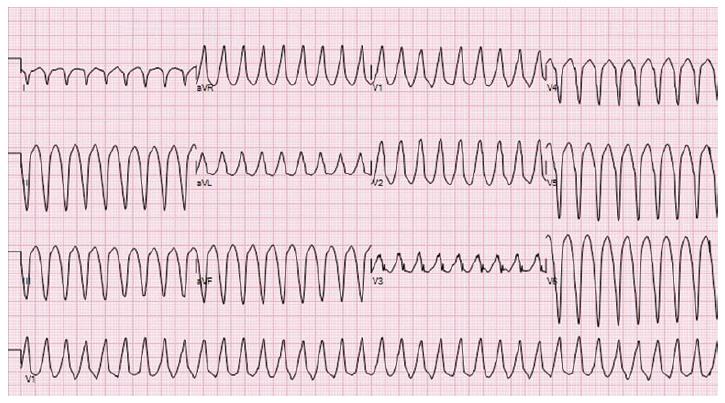
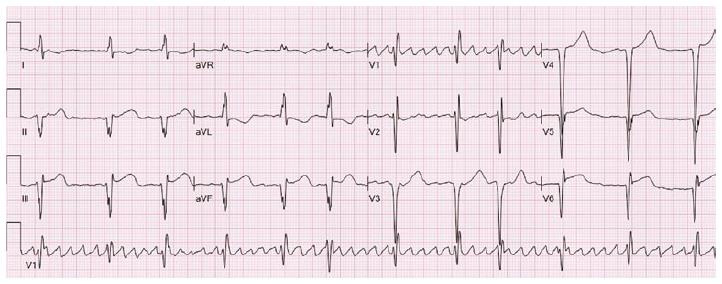
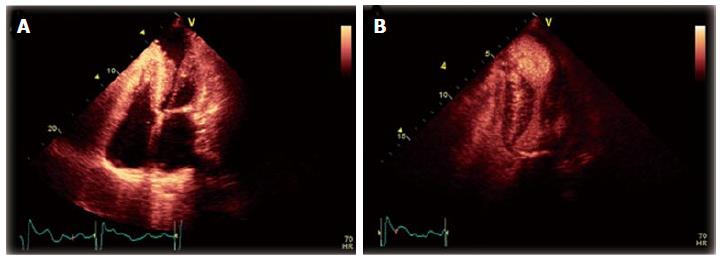
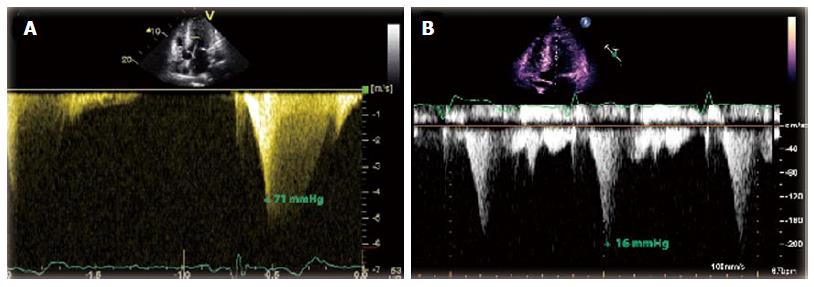
A 54-year-old female with AFD and well controlled hypertension presented with chest pain, palpitations and dizziness after missing two doses of metoprolol. Her initial electrocardiogram (ECG) displayed sustained monomorphic ventricular tachycardia (VT) with a cycle length of 214 ms. The VT had a right bundle, superior axis morphology suggesting a left ventricular (LV) apical origin (Figure 1)[1]. She initially received a bolus of amiodarone that failed to terminate the VT. Post direct current cardioversion, serial ECGs showed atrial fibrillation with persistent ST segment elevation in the inferior leads (Figure 2). A coronary angiogram demonstrated non-occlusive coronary artery disease. Transthoracic echocardiogram (TTE) revealed that her ejection fraction was newly reduced from 75% to 40% and identified a thin-walled LVAA that was not seen on TTE two years prior (Figure 3A and B). The mid and basal segments of the LV still had marked concentric hypertrophy with systolic mid-cavitary obliteration; however, the intra-cavitary gradient at rest was significantly lower than on the prior study; it had decreased from 71 to 16 mmHg (Figure 4). Contrast-enhanced cardiovascular magnetic resonance (CMR) confirmed these findings and demonstrated late gadolinium enhancement (LGE) of the aneurysmal LV apex extending to the lateral and inferolateral walls, consistent with myocardial scarring (Figure 5). The patient was restarted on metoprolol and had no further episodes of VT. A defibrillator (ICD) was implanted prior to discharge.
AFD is a rare, X-linked lysosomal storage disorder caused by GLA enzyme deficiency, which results in an abnormal accumulation of sphingolipid catabolites in multiple organs, including the heart. Although the prevalence of AFD is likely underestimated, mutations are present in 1:6000 to 1:40000 females. Clinical manifestations in heterozygous females vary widely from no apparent clinical disease to full expression of the disease[2]. Cardiac involvement can lead to myocardial hypertrophy, restrictive cardiomyopathy, coronary artery disease, arrhythmias (atrial and ventricular) as well as aortic and mitral valvulopathy[3]. Our patient’s disease manifested in childhood with acroneuropathy and hypohydrosis, and subsequently progressed to include cutaneous lesions, lacunar infarcts and LVH. A few years prior to presentation she was diagnosed with hypertension, but there was no manifest renal involvement from AFD. Her blood pressure was well controlled with a combination of an angiotensin converting enzyme inhibitor and a beta blocker. The diagnosis of AFD was established at age fifteen by genetic testing with a R342Q missense mutation on exon 7 in the GLA gene. A brother had the same mutation. The R342Q mutation has been shown to lead to a complete loss of the GLA activity, and to be associated with a classical phenotypic presentation of AFD[4,5].
Despite a relatively early diagnosis, our patient had been reluctant to initiate AFD therapy until her symptoms worsened. Enzyme replacement therapy with Agalsidase beta (Fabrazyme®, Genzyme, Cambridge, MA, United States) had been initiated eighteen months prior to presentation. She received monthly infusion of 65 mg for one year, followed by an infusion of 60 mg every 2 wk. Despite being compliant with the treatment, her disease slowly progressed, with worsening of her neuropathy, cutaneous lesions, development of cerebral vasculopathy and formation of the LVAA.
LVH is a relatively common finding in AFD and has been shown to correlate with severity of the disease. LVH in AFD can mimic HCM and has been identified among the causes of late onset HCM in women. In addition, women tend to present at a later age than men, and often have milder symptoms, making the diagnosis of AFD more challenging in female patients[6,7]. The pattern of hypertrophy in AFD is typically symmetric, as opposed to asymmetric with preferential septal involvement in HCM. Contrast-enhanced CMR typically demonstrates evidence of LGE, particularly in the LV subepicardial and mesocardial inferior and inferolateral segments. The LGE is believed to be the result of both intra-myocyte accumulation of sphingolipids and fibrosis[8]. Development of a LVAA is uncommon in HCM (reported in only 2% of patients) but portends a poor prognosis with a high adverse event rate, including sudden death, appropriate ICD discharges, thromboembolic stroke, and progressive heart failure[9].
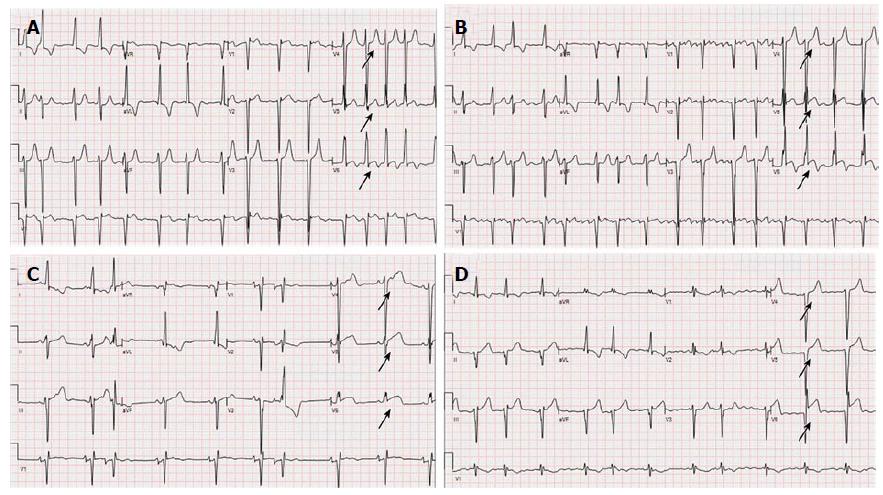
In contrast to AFD, HCM is a relatively common genetic disease and is the most common cause of sudden cardiac death in young people. Its estimated prevalence is 1 in 500. In HCM, the inheritance pattern is autosomal dominant. The mutant proteins that cause HCM are incorporated into intact filaments of the sarcomere and have been described as “poisonous peptides”. Local environmental factors such as pressure, protein defect and modifier genes interact to induce the subsequent phenotype[10]. In HCM, development of ST elevation in leads V4 through V6 has been associated with LVAA formation[11]. Similar findings were seen upon reviewing our patient’s serial ECGs (ranging from 4 years to 3 mo) prior to her presentation (Figure 6). To the best of the authors’ knowledge, the development of LVAA has only been previously described once in AFD. In a recent report, the case of a middle-aged female diagnosed with HCM and LVAA is described. That patient was later found to have a missence mutation in the GLA gene (p.R118C) confirming the diagnosis of AFD[12].
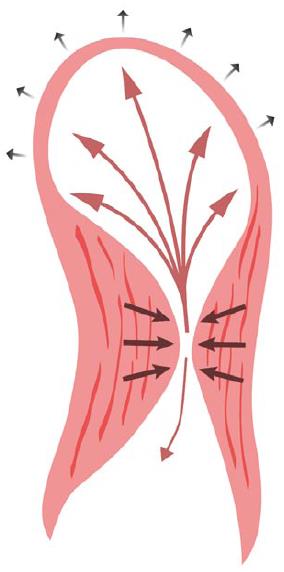
The proposed mechanisms for LVAA formation in HCM are genetic predisposition and long-standing LV wall stress from elevated intra-cavitary systolic pressures as a result of mid-cavitary obstruction[8]. Both mechanisms are potential contributors in our patient, as a brother with AFD also developed a small LVAA prior to his death. We hypothesized that the formation of an apical aneurysm may be considered a hemodynamic adaptation to sustained elevation of systolic intra-cavitary LV pressure. The apex can dilate in response to the chronically elevated wall pressures during systole, with some blood flow directed towards the aneurysm, which offers less resistance than the mid-cavitary obliteration (Figure 7). Over time, this results in decreased flow across the mid-cavitary obstruction and a concordant smaller peak pressure gradient[13]. This phenomenon is exhibited in our patient by the considerable fall in her peak intra-cavitary gradient after the LVAA developed. Of note, our patient had chronic atrial fibrillation and it could be speculated that the LVAA formation was the result of a thromboembolic event. However, this scenario seems unlikely given that she had been appropriately anticoagulated with coumadin for years and had no other evidence of (non-cerebral) systemic embolization.
The phenotypic expression of AFD in the heart is not well described and can mimic even rare forms of HCM. The findings in this case suggest a shared mechanism(s) of aneurysm formation in AFD and HCM, independent of the underlying cardiomyopathy. Screening for AFD should be strongly considered in cases of unexplained LVH, particularly in patients with the aneurysmal variant of HCM. As previously demonstrated in HCM, it is likely that LVAA formation in AFD portends a poor prognosis.
A middle-aged female with Anderson-Fabry disease (AFD) presented with chest pain, palpitations and dizziness for a few hours after missing two doses of her beta-blocker.
She was found to have sustained ventricular tachycardia originating from the left ventricular (LV) apex, and further imaging confirmed the presence of a new LV apical aneurysm (LVAA).
The differential diagnosis included aneurysm from hypertrophic cardiomyopathy (HCM), aneurysm from prior infarct, apical diverticulum and Takotsubo cardiomyopathy.
The diagnosis of AFD was established by genetic testing with the presence of R342Q missense mutation on exon 7 in the GLA gene.
A transthoracic echocardiogram revealed that her intra-cavitary gradient had significantly decreased from two years prior and identified a new LVAA, which was confirmed by a cardiovascular magnetic resonance imaging.
The findings in this case suggest a shared mechanism(s) of aneurysm formation in AFD and HCM: genetic predisposition and long standing LV wall stress from elevated intra-cavitary systolic pressures as a result of mid-cavitary obstruction.
She was placed back on her beta blocker and a defibrillator was implanted prior to discharge.
Left ventricular hypertrophy in AFD can mimic HCM, however the pattern of hypertrophy is typically symmetric in AFD as opposed to asymmetric with preferential septal involvement in HCM. Development of a LVAA is uncommon in HCM (reported in only 2% of patients) and has only been (recently) reported once in AFD.
Development of a LVAA is a very rare phenomenon in patients with AFD.
The findings in this case suggest a shared mechanism(s) of aneurysm formation in AFD and HCM, independent of the underlying cardiomyopathy. Screening for AFD should be strongly considered in cases of unexplained LVH, particularly in patients with the aneurysmal variant of HCM.
Authors showed a case of AFD complicated by apical aneurysm and ventricular tachycardia. This report is written and presents well.
P- Reviewer: Kurisu S, Pesquero JB, Satoh H, Ucar SK S- Editor: Song XX L- Editor: A E- Editor: Wu HL
| 1. | Callans DJ, Menz V, Schwartzman D, Gottlieb CD, Marchlinski FE. Repetitive monomorphic tachycardia from the left ventricular outflow tract: electrocardiographic patterns consistent with a left ventricular site of origin. J Am Coll Cardiol. 1997;29:1023-1027. [PubMed] |
| 2. | Mauer M, Kopp JB. Clinical features and diagnosis of Fabry disease [accessed 2014 Nov 27]. Available from: http://www.uptodate.com/contents/clinicalfeatures and diagnosis of fabry disease&selectedTitle=1~3. |
| 3. | Desnick R, Ioannou Y, Eng C. Alpha-Galactosidase A deficiency: Fabry disease. New-York: McGraw-Hil 1995; . |
| 4. | Wu X, Katz E, Della Valle MC, Mascioli K, Flanagan JJ, Castelli JP, Schiffmann R, Boudes P, Lockhart DJ, Valenzano KJ. A pharmacogenetic approach to identify mutant forms of α-galactosidase A that respond to a pharmacological chaperone for Fabry disease. Hum Mutat. 2011;32:965-977. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 66] [Cited by in RCA: 72] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 5. | Germain DP, Shabbeer J, Cotigny S, Desnick RJ. Fabry disease: twenty novel alpha-galactosidase A mutations and genotype-phenotype correlations in classical and variant phenotypes. Mol Med. 2002;8:306-312. [PubMed] |
| 6. | Chimenti C, Pieroni M, Morgante E, Antuzzi D, Russo A, Russo MA, Maseri A, Frustaci A. Prevalence of Fabry disease in female patients with late-onset hypertrophic cardiomyopathy. Circulation. 2004;110:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 174] [Cited by in RCA: 178] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 7. | Linhart A, Elliott PM. The heart in Anderson-Fabry disease and other lysosomal storage disorders. Heart. 2007;93:528-535. [PubMed] |
| 8. | Moon JC, Sachdev B, Elkington AG, McKenna WJ, Mehta A, Pennell DJ, Leed PJ, Elliott PM. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium. Eur Heart J. 2003;24:2151-2155. [PubMed] |
| 9. | Maron MS, Finley JJ, Bos JM, Hauser TH, Manning WJ, Haas TS, Lesser JR, Udelson JE, Ackerman MJ, Maron BJ. Prevalence, clinical significance, and natural history of left ventricular apical aneurysms in hypertrophic cardiomyopathy. Circulation. 2008;118:1541-1549. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 385] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 10. | Roberts R, Ulrich U. Current Concepts of the Pathogenesis and Treatment of Hypertrophic Cardiomyopathy. Circulation. 2005;112:293-296. [RCA] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 45] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 11. | Ichida M, Nishimura Y, Kario K. Clinical significance of left ventricular apical aneurysms in hypertrophic cardiomyopathy patients: the role of diagnostic electrocardiography. J Cardiol. 2014;64:265-272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 35] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Caetano F, Botelho A, Mota P, Silva J, Leitão Marques A. Fabry disease presenting as apical left ventricular hypertrophy in a patient carrying the missense mutation R118C. Rev Port Cardiol. 2014;33:183.e1-183.e5. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Hsieh BPC, Tauras J, Taub C. Continuous Apex to Left Ventricle Blood Flow Pattern in Hypertrophic Cardiomyopathy with Apical Aneurysm and Midventricular Obstruction. Echocardiogr-J Card. 2012;29:E131-E133. [RCA] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |