Published online Jun 16, 2015. doi: 10.12998/wjcc.v3.i6.504
Peer-review started: December 29, 2014
First decision: January 20, 2015
Revised: March 13, 2015
Accepted: April 10, 2015
Article in press: April 14, 2015
Published online: June 16, 2015
Processing time: 175 Days and 11.8 Hours
Obesity and diabetes is a co-pandemic and a major health concern that is expanding. It has many psychosocial and economic consequences due to morbidity and mortality of this disease combination. The pathophysiology of obesity and related diabetes is complex and multifactorial. One arm of this disease process is the genetic susceptibility. Other arm is dependent on the intricate neuro-humoral factors that converge in the central nerve system. Gut hormones and the adipose tissue derived factors plays an important role in this delicate network. Bariatric surgery provides the only durable option for treatment of obesity and furthermore it provides a remission in the concomitant diseases that accompany obesity. This review provides a brief insight to all these mechanisms and tries to deduce the possible reasons of remission of type 2 diabetes after bariatric surgery.
Core tip: Metabolic surgery in obese individuals results weight loss and beneficial effects on type 2 diabetes mellitus and metabolic syndrome.
- Citation: Cetinkunar S, Erdem H, Aktimur R, Sozen S. Effect of bariatric surgery on humoral control of metabolic derangements in obese patients with type 2 diabetes mellitus: How it works. World J Clin Cases 2015; 3(6): 504-509
- URL: https://www.wjgnet.com/2307-8960/full/v3/i6/504.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i6.504
Obesity is considered as abnormal accumulation of excess energy as fat tissue that results in major health problems and reduced life span of the individual[1]. Obesity is a major health problem and is now considered a worldwide pandemic. Its incidence increasing in an alarming rate and by 2015 around a million people is expected to be overweight worldwide[2]. Furthermore, it is considered as a government political problem for it results in economic, social losses due to morbidity and mortality during the course of the disease[3]. The risk stratification of obesity is made according to World Health Organization’s proposed classification of obesity (Table 1)[4]. Increasing body mass index (kg/m2) the morbidity and mortality rate of the patient increases. Obesity causes many concomitant systemic diseases. Among the diseases that occur with obesity are diabetes, hypertension, coronary artery diseases, dyslipidaemias, non-alcoholic fatty liver disease and metabolic syndrome are pronounced[5]. There are many problems when dealing with obese patient. For since it is a multifactorial disease the treatment is very hard furthermore there are many social security related problems when dealing with bariatric patient. There are also psychosocial implications against obese people such as job interview bias, unavailability of public bills and inability of social security coverage for obesity related diseases[6].
| Classification | WHO class | BMI (kg/m2) |
| Underweight | ≤ 18.5 | |
| Normal | 18.5-24.9 | |
| Overweight | 25.0-29.9 | |
| Obesity | I | 30.0-34.9 |
| II | 35.0-39.9 | |
| Extreme obesity | III | 40 |
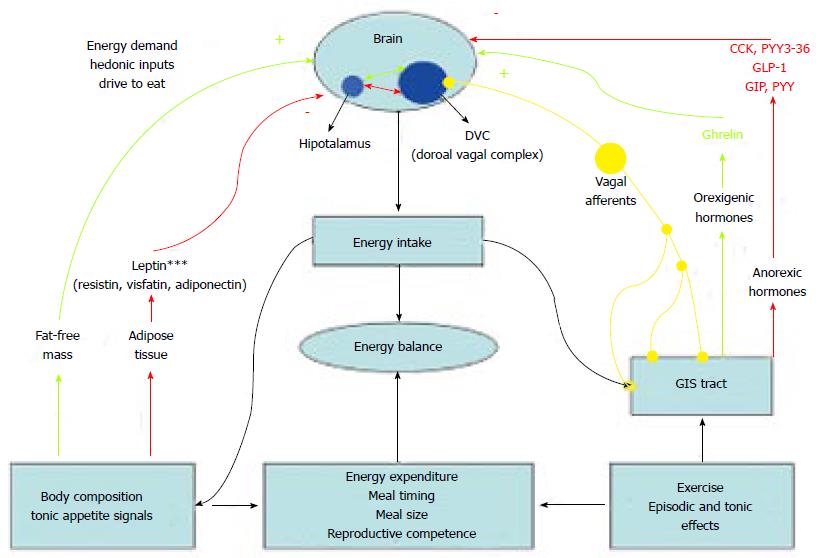
Obesity is multifactorial disease and many neuro-humoral factors are orchestrated in appetite control and energy haemostasis of the individual. Detailed review of these factors is out of the scope of this text. Here we will examine briefly the central nervous system related and gut humoral factors related control mechanisms of the appetite of the individual and furthermore we will give detailed information regarding type 2 diabetes mellitus (T2DM) and obesity pandemic. Later on, we will try to summarize the effects of bariatric surgery on humoral factors of obesity and diabetes.
Central nervous system as a pivotal point in orchestration of anorectic and orexigenic signals received from the periphery. Mainly the arcuate nucleus of the central nervous system integrates all the input[7]. This area of the brain contains neuropeptide YY rich neurons. The main orexigenic stimulus comes from Ghrelin and the anorectic stimuli come from the Glucagon like peptide-1 (GLP-1), plasma peptide tyrosine tyrosine 3-36 (PYY3-36), cholecystokinin and etc. All these stimuli integrate at the arcuate nucleus and this causes the individual to seek food in discrete time points between daily activities[8-10]. This is one of the main points that is deranged in the obese individual and a continuous eating behaviour occurs in the obese patient.
Peripheral axis consists of gut related hormones with vagus and the adipose tissue related humoral factor; namely the adipokines. These are all potent short and long terms stimuli for the control of energy haemostasis in the individual and as a result of this there is the food seeking behaviour characteristics of humans (and most vertebrates) ae determined.
Several factors secreted from the gastrointestinal tract regulate the caloric intake and the food seeking behaviour of the individual[7,8]. There are factors that increase the adipogenesis and there are counteracting factors that reduce the appetite and reduce adipose tissue formation. Main orexigenic factors secreted from the gastrointestinal system are ghrelin and insulin. The counteracting mechanisms on the other hand are GLP-1, NYY3-36, etc[9,10]. Furthermore vagus and the autonomic system are important afferent inputs of the gastrointestinal system to the arcuate nucleus forming the gut-brain feedback mechanisms[11,12].
Ghrelin is a 28-amino acid peptide. It has been shown to be produced from the fundic mucosa. In animal models, it was shown that ghrelin increased feeding and weight gain and had an orexigenic role in energy balance. The clock genes PER1 and PER2 ghrelin levels peak before meals and quickly decrease following meals. Arcuate nucleus in the central nervous system exhibits ghrelin receptors[13,14].
Insulin has many roles in energy balance. Serum glucose levels elevated after a meal stimulate insulin release by pancreatic beta cells. Additional secretagogues of insulin are amino acids such as alanine, glycine, and arginine, acetylcholine produced from vagal nerve endings, and incretins such as GLP-1 and glucose-dependent insulinotropic polypeptide[7,8]. Although central effects of insulin is reduced appetite and weight loss in preclinical studies in obese individuals show higher basal insulin levels[8].
GLP-1 has an important role in increasing secretion of insulin from the pancreas[7]. Both in vivo and in vitro researches have showed that GLP-1 increase insulin secretion in the beta cell. Moreover, glucagon secretion is inhibited by GLP-1 while insulin sensitivity is increased[7,15].
PYY3-36 is an anorectic factor on the arcuate nucleus through the Y2 receptors expressed at the neuronal level. It shows peak levels 1-2 h following the meals and the rise in serum levels are observed within 15 min following eating[8,16]. PYY3-36 anorectic effect is possibly facilitated by the vagus nerve[16].
Leptin and adiponectin are the two main humoral factors secreted from the adipose tissue and that play a role in the energy haemostasis of the individual. They all together form the adipose-brain feedback axis[8].
Leptin is produced in white and brown adipose tissue, placenta, ovaries, skeletal muscle, stomach, breast, bone marrow, pituitary, and liver[8]. Engineer and Garcia have showed that leptin affects hypothalamus, where it inhibits NPY/AgRP receptor neurons while stimulates α-MSH neurons[17]. In contrast to ghrelin, leptin acts on appetite and energy balance[7,18]. Harvey et al[8] has reported leptin resistance in obese patients.
Adiponectin is secreted from white adipose tissue[8]. Serum adiponectin levels are negatively correlated to serum insulin and glucose levels, body fat mass, and waist-to-hip ratio. It has been shown that fasting adiponectin levels have been reduced in the obese individuals[19]. Nevertheless, the response to a meal appear to be exaggerated in obese subjects.
Obesity is associated to many medical conditions, probably the most disturbing may be T2DM. Both obesity and T2DM are mainly related with insulin resistance[3]. The nonesterified fatty acids (NEFAs) secreted from adipose tissue in obese population may lead to the theory that insulin resistance and β-cell dysfunction are probably related[20,21]. Adipose tissue affects body metabolism by secreting hormones, glycerol, leptin, cytokines, adiponectin, proinflammatory substances, and NEFAs. Increased NEFA secretion is detected both in obesity and T2DM, and it is related with insulin resistance in both situations. In humans, insulin resistance starts to develop shortly after an acute increase of plasma NEFA levels.
Intra-abdominal fat is linked to the genes that secrete specific types of proteins responsible for the production of energy[22,23]. Omental adipocytes secrete larger amount of adiponectin than the subcutaneous-derived adipocytes[24,25]. Furthermore, adiponectin secreted from omental adipocytes is negatively associated with weight gain. The excretion of NEFAs to different tissue may be affected by their source. Abdominal fat is considered more lipolytic than subcutaneous fat, and it does not respond simply to the antilipolytic action of insulin, which makes intra-abdominal fat more significant in causing insulin resistance and diabetes[26]. All these factors produce a tendency towards insulin resistance and diabetes in the obese patients (Figure 1).
The cytotoxicity of fecal bile acids is associated with their concentration in fecal water, and particularly is related to the concentration of secondary bile acids[27,28]. Total bile acid levels in fecal water were decreased meaningfully in the course of orlistat treatment. The decrease in fecal water bile acids during orlistat treatment mainly was caused by a large reduction in deoxycholic acid. Small reductions were observed in fecal water with both the orlistat and placebo groups for all the other bile acids. This is relevant in that the secondary bile acids, which include deoxycholic acid, are the bile acids most frequently associated in colonic cell hyperproliferation[29,30].
Bariatric surgery consists of various interventions which can be divided as restrictive, malabsorptive, or combined restrictive and malabsorptive. The number of bariatric interventions (i.e., metabolic surgery) for the treatment of obesity is in exponential increase. This is partially due to effective and long-lasting weight loss; in addition, a good deal of improvement of co-morbidities after surgery compared with diet and physical activity[7]. In this subsection we will briefly evaluate the changes in the levels of above mentioned factors with respect to various bariatric procedures and at the end try to draw certain conclusion regarding the metabolic effects of bariatric (metabolic) surgery. We will briefly summarize the effect of bariatric surgery on each of the adipose and gut humoral factors.
In the case of ghrelin there are many report regarding Roux-en-Y gastric bypass (RY-GBP) and the serum ghrelin levels detected in various set points starting from ranging between 14 d postoperatively to 2 years[31,32]. Most of the researchers have found decreased ghrelin levels postoperatively and these results have been compared to non-resectional restrictive procedures such as adjustable gastric banding (AGB). Therefore we can say that the final effect of procedures involving gastric transection reduces the serum ghrelin levels[33,34].
The changes in ghrelin after sleeve gastrectomy (SG) were measured in different studies. Shak et al[35] stated that fasting ghrelin levels are decreased up to 5 years of follow-up. Moreover, some studies tried to evaluate and compare the effects of RY-GBP or AGB with the SG on fasting ghrelin levels, which showed to be decreased[36]. These studies showed that the ghrelin suppression after both SG and RY-GBP may be part of the mechanism that contributes to diabetes remission[35,36].
Serum insulin levels sow rapid drop with RY-GBP, biliopancreatic diversion and duodenal switch and SG however with AGB although insulin drops the incretin effect is not observed[7,35-38].
A strong GLP-1 response was reported 10 years after RY-GBP, suggesting a long-lasting effect. Furthermore, in T2DM patients, an improved GLP-1 response to meal intake is not enough to preserve normal glucose tolerance in the long term after RY-GBP. Similarly, some studies have shown unaffected fasting GLP-1 and a noteworthy increase in response to a glycemic challenge[39,40]. Studies have shown a significantly increased fasting level of PYY3-36 following RY-GBP. Following the AGB, fasting PYY3-36 or meal-stimulated response is variable and inconclusive[41,42]. Data regarding the effects on leptin are inconclusive and therefore research regarding area is urgently needed. Furthermore, adiponectin studied in RY-GBP in only one study and was found be increased following surgery[43]. This study is not enough to draw conclusions and therefore multi-centric high patient volume studies are needed to evaluate the role of metabolic surgery on adipose-brain axis in obesity.
Clinically all these changes in the humoral effects that the bariatric surgery produces is seen as remission of diabetes in obese individuals postoperatively. Even in patients who continue to have diabetes postoperatively have a better quality of life due to reduced medications and a better glycaemic control. This has been extensively studied and currently here even meta-analysis regarding this subject showing very good results in almost all procedure types[44-47].
Metabolic surgery in obese individuals results weight loss and beneficial effects on T2DM and metabolic syndrome.
P- Reviewer: Chiu CC, Vilallonga R S- Editor: Ji FF L- Editor: A E- Editor: Wu HL
| 1. | Naser KA, Gruber A, Thomson GA. The emerging pandemic of obesity and diabetes: are we doing enough to prevent a disaster? Int J Clin Pract. 2006;60:1093-1097. [PubMed] |
| 2. | Gallagher D, Heymsfield SB, Heo M, Jebb SA, Murgatroyd PR, Sakamoto Y. Healthy percentage body fat ranges: an approach for developing guidelines based on body mass index. Am J Clin Nutr. 2000;72:694-701. [PubMed] |
| 3. | Al-Goblan AS, Al-Alfi MA, Khan MZ. Mechanism linking diabetes mellitus and obesity. Diabetes Metab Syndr Obes. 2014;7:587-591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 459] [Cited by in RCA: 542] [Article Influence: 49.3] [Reference Citation Analysis (0)] |
| 4. | Aronne LJ. Classification of obesity and assessment of obesity-related health risks. Obes Res. 2002;10 Suppl 2:105S-115S. [PubMed] |
| 5. | Moon OR, Kim NS, Jang SM, Yoon TH, Kim SO. The relationship between body mass index and the prevalence of obesity-related diseases based on the 1995 National Health Interview Survey in Korea. Obes Rev. 2002;3:191-196. [PubMed] |
| 6. | Lyznicki JM, Young DC, Riggs JA, Davis RM. Obesity: assessment and management in primary care. Am Fam Physician. 2001;63:2185-2196. [PubMed] |
| 7. | Finelli C, Padula MC, Martelli G, Tarantino G. Could the improvement of obesity-related co-morbidities depend on modified gut hormones secretion? World J Gastroenterol. 2014;20:16649-16664. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 39] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 8. | Harvey EJ, Arroyo K, Korner J, Inabnet WB. Hormone changes affecting energy homeostasis after metabolic surgery. Mt Sinai J Med. 2010;77:446-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Yamada Y, Seino Y. Physiology of GIP--a lesson from GIP receptor knockout mice. Horm Metab Res. 2004;36:771-774. [PubMed] |
| 10. | Nguyen NQ, Debreceni TL, Bambrick JE, Chia B, Wishart J, Deane AM, Rayner CK, Horowitz M, Young RL. Accelerated intestinal glucose absorption in morbidly obese humans: relationship to glucose transporters, incretin hormones, and glycemia. J Clin Endocrinol Metab. 2015;100:968-976. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 91] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 11. | Nakazato M, Murakami N, Date Y, Kojima M, Matsuo H, Kangawa K, Matsukura S. A role for ghrelin in the central regulation of feeding. Nature. 2001;409:194-198. [PubMed] |
| 12. | Cowley MA, Smart JL, Rubinstein M, Cerdán MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480-484. [PubMed] |
| 13. | Briggs DI, Lockie SH, Wu Q, Lemus MB, Stark R, Andrews ZB. Calorie-restricted weight loss reverses high-fat diet-induced ghrelin resistance, which contributes to rebound weight gain in a ghrelin-dependent manner. Endocrinology. 2013;154:709-717. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (2)] |
| 14. | Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402:656-660. [PubMed] |
| 15. | Cobble M. Differentiating among incretin-based therapies in the management of patients with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4:8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 16. | Suzuki K, Jayasena CN, Bloom SR. The gut hormones in appetite regulation. J Obes. 2011;2011:528401. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 54] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Engineer DR, Garcia JM. Leptin in anorexia and cachexia syndrome. Int J Pept. 2012;2012:287457. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 46] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 18. | Swarbrick MM, Havel PJ. Physiological, pharmacological, and nutritional regulation of circulating adiponectin concentrations in humans. Metab Syndr Relat Disord. 2008;6:87-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 19. | Kubota N, Terauchi Y, Yamauchi T, Kubota T, Moroi M, Matsui J, Eto K, Yamashita T, Kamon J, Satoh H. Disruption of adiponectin causes insulin resistance and neointimal formation. J Biol Chem. 2002;277:25863-25866. [PubMed] |
| 20. | Arora S. Molecular basis of insulin resistance and its relation to metabolic syndrome. Rijeka, Croatia: InTech Europe 2012; . |
| 21. | Tsai AG, Williamson DF, Glick HA. Direct medical cost of overweight and obesity in the USA: a quantitative systematic review. Obes Rev. 2011;12:50-61. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 327] [Cited by in RCA: 290] [Article Influence: 20.7] [Reference Citation Analysis (0)] |
| 22. | Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444:840-846. [PubMed] |
| 23. | Karpe F, Dickmann JR, Frayn KN. Fatty acids, obesity, and insulin resistance: time for a reevaluation. Diabetes. 2011;60:2441-2449. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 594] [Cited by in RCA: 646] [Article Influence: 46.1] [Reference Citation Analysis (0)] |
| 24. | Jelic K, Luzio SD, Dunseath G, Colding-Jorgsensen M, Owens DR. A cross-sectional analysis of NEFA levels following standard mixed meal in a population of persons with newly diagnosed type 2 diabetes mellitus across a spectrum of glycemic control [Abstract]. Alexandria, VA: American Diabetes Association 2007; . |
| 25. | Roden M, Price TB, Perseghin G, Petersen KF, Rothman DL, Cline GW, Shulman GI. Mechanism of free fatty acid-induced insulin resistance in humans. J Clin Invest. 1996;97:2859-2865. [PubMed] |
| 26. | Fain JN, Madan AK, Hiler ML, Cheema P, Bahouth SW. Comparison of the release of adipokines by adipose tissue, adipose tissue matrix, and adipocytes from visceral and subcutaneous abdominal adipose tissues of obese humans. Endocrinology. 2004;145:2273-2282. [PubMed] |
| 27. | Stadler J, Stern HS, Yeung KS, McGuire V, Furrer R, Marcon N, Bruce WR. Effect of high fat consumption on cell proliferation activity of colorectal mucosa and on soluble faecal bile acids. Gut. 1988;29:1326-1331. [PubMed] |
| 28. | Wargovich MJ, Eng VW, Newmark HL, Bruce WR. Calcium ameliorates the toxic effect of deoxycholic acid on colonic epithelium. Carcinogenesis. 1983;4:1205-1207. [PubMed] |
| 29. | Bull AW, Marnett LJ, Dawe EJ, Nigro ND. Stimulation of deoxythymidine incorporation in the colon of rats treated intrarectally with bile acids and fats. Carcinogenesis. 1983;4:207-210. [PubMed] |
| 30. | DeRubertis FR, Craven PA, Saito R. Bile salt stimulation of colonic epithelial proliferation. Evidence for involvement of lipoxygenase products. J Clin Invest. 1984;74:1614-1624. [PubMed] |
| 31. | Carroll JF, Franks SF, Smith AB, Phelps DR. Visceral adipose tissue loss and insulin resistance 6 months after laparoscopic gastric banding surgery: a preliminary study. Obes Surg. 2009;19:47-55. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 37] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Hanusch-Enserer U, Brabant G, Roden M. Ghrelin concentrations in morbidly obese patients after adjustable gastric banding. N Engl J Med. 2003;348:2159-2160. [PubMed] |
| 33. | Hanusch-Enserer U, Cauza E, Brabant G, Dunky A, Rosen H, Pacini G, Tüchler H, Prager R, Roden M. Plasma ghrelin in obesity before and after weight loss after laparoscopical adjustable gastric banding. J Clin Endocrinol Metab. 2004;89:3352-3358. [PubMed] |
| 34. | Schindler K, Prager G, Ballaban T, Kretschmer S, Riener R, Buranyi B, Maier C, Luger A, Ludvik B. Impact of laparoscopic adjustable gastric banding on plasma ghrelin, eating behaviour and body weight. Eur J Clin Invest. 2004;34:549-554. [PubMed] |
| 35. | Shak JR, Roper J, Perez-Perez GI, Tseng CH, Francois F, Gamagaris Z, Patterson C, Weinshel E, Fielding GA, Ren C. The effect of laparoscopic gastric banding surgery on plasma levels of appetite-control, insulinotropic, and digestive hormones. Obes Surg. 2008;18:1089-1096. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 36. | Langer FB, Reza Hoda MA, Bohdjalian A, Felberbauer FX, Zacherl J, Wenzl E, Schindler K, Luger A, Ludvik B, Prager G. Sleeve gastrectomy and gastric banding: effects on plasma ghrelin levels. Obes Surg. 2005;15:1024-1029. [PubMed] |
| 37. | Catheline JM, Fysekidis M, Dbouk R, Boschetto A, Bihan H, Reach G, Cohen R. Weight loss after sleeve gastrectomy in super superobesity. J Obes. 2012;2012:959260. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 38. | Lee WJ, Ser KH, Chong K, Lee YC, Chen SC, Tsou JJ, Chen JC, Chen CM. Laparoscopic sleeve gastrectomy for diabetes treatment in nonmorbidly obese patients: efficacy and change of insulin secretion. Surgery. 2010;147:664-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 124] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 39. | Morínigo R, Lacy AM, Casamitjana R, Delgado S, Gomis R, Vidal J. GLP-1 and changes in glucose tolerance following gastric bypass surgery in morbidly obese subjects. Obes Surg. 2006;16:1594-1601. [PubMed] |
| 40. | Jiménez A, Casamitjana R, Flores L, Delgado S, Lacy A, Vidal J. GLP-1 and the long-term outcome of type 2 diabetes mellitus after Roux-en-Y gastric bypass surgery in morbidly obese subjects. Ann Surg. 2013;257:894-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 41. | Bose M, Machineni S, Oliván B, Teixeira J, McGinty JJ, Bawa B, Koshy N, Colarusso A, Laferrère B. Superior appetite hormone profile after equivalent weight loss by gastric bypass compared to gastric banding. Obesity (Silver Spring). 2010;18:1085-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 42. | Korner J, Inabnet W, Febres G, Conwell IM, McMahon DJ, Salas R, Taveras C, Schrope B, Bessler M. Prospective study of gut hormone and metabolic changes after adjustable gastric banding and Roux-en-Y gastric bypass. Int J Obes (Lond). 2009;33:786-795. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 279] [Cited by in RCA: 246] [Article Influence: 15.4] [Reference Citation Analysis (0)] |
| 43. | Dadson K, Liu Y, Sweeney G. Adiponectin action: a combination of endocrine and autocrine/paracrine effects. Front Endocrinol (Lausanne). 2011;2:62. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 50] [Cited by in RCA: 61] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 44. | Li JF, Lai DD, Lin ZH, Jiang TY, Zhang AM, Dai JF. Comparison of the long-term results of Roux-en-Y gastric bypass and sleeve gastrectomy for morbid obesity: a systematic review and meta-analysis of randomized and nonrandomized trials. Surg Laparosc Endosc Percutan Tech. 2014;24:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 110] [Cited by in RCA: 96] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 45. | Ribaric G, Buchwald JN, McGlennon TW. Diabetes and weight in comparative studies of bariatric surgery vs conventional medical therapy: a systematic review and meta-analysis. Obes Surg. 2014;24:437-455. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 212] [Cited by in RCA: 193] [Article Influence: 17.5] [Reference Citation Analysis (0)] |
| 46. | Li JF, Lai DD, Ni B, Sun KX. Comparison of laparoscopic Roux-en-Y gastric bypass with laparoscopic sleeve gastrectomy for morbid obesity or type 2 diabetes mellitus: a meta-analysis of randomized controlled trials. Can J Surg. 2013;56:E158-E164. [PubMed] |
| 47. | Gloy VL, Briel M, Bhatt DL, Kashyap SR, Schauer PR, Mingrone G, Bucher HC, Nordmann AJ. Bariatric surgery versus non-surgical treatment for obesity: a systematic review and meta-analysis of randomised controlled trials. BMJ. 2013;347:f5934. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 936] [Cited by in RCA: 940] [Article Influence: 78.3] [Reference Citation Analysis (0)] |