Published online May 16, 2015. doi: 10.12998/wjcc.v3.i5.470
Peer-review started: December 24, 2014
First decision: January 20, 2015
Revised: January 24, 2015
Accepted: February 9, 2015
Article in press: February 11, 2015
Published online: May 16, 2015
Processing time: 137 Days and 3.7 Hours
Congenital pulmonary airway malformation (CPAM), previously known as congenital cystic adenomatoid malformation is a congenital disorder of the lung similar to bronchopulmonary sequestration. In CPAM, usually an entire lobe of lung is replaced by a non-working cystic piece of abnormal lung tissue. This abnormal tissue will never function as normal lung tissue. The underlying cause for CPAM is not known. It occurs in approximately 1 in every 30000 pregnancies. The association between CPAM and malignancy has been well documented. There is a small risk (0.7%) of malignant transformation within the cyst. So early diagnosis and surgical resection is important to prevent the grave complications. Herein, we are reporting two interesting cases of CPAM and one belonged to Type II and other belonged to Type III of Stocker’s classification.
Core tip: Congenital pulmonary airway malformation (CPAM) is a rare disease with various clinical presentations and having the risk of future malignant transformation. Early pulmonary resection for asymptomatic CPAM is required and recommended to make a definitive diagnosis and determine the prognosis of the disease.
- Citation: Bolde S, Pudale S, Pandit G, Ruikar K, Ingle SB. Congenital pulmonary airway malformation: A report of two cases. World J Clin Cases 2015; 3(5): 470-473
- URL: https://www.wjgnet.com/2307-8960/full/v3/i5/470.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i5.470
Congenital pulmonary airway malformation (CPAM) is rare condition with a reported incidence of 1:25000 to 1:35000. About 15%-50% of cases of congenital cystic lung disease are reported to be CPAM[1-3].
This condition was first discovered by Stoerk[1] in 1897. The abnormality is mainly attributed to a maturation defect. It is classified into 5 major types based on clinical and pathological features. It is important to make early pathological diagnosis by surgical excision in congenital cystic lung diseases to determine the prognosis. Most CPAM lesions are manageable with the proper assessment, diagnosis and surgical interventions[4].
A male baby was born to primigravida mother with a history of 8 mo amenorrhoea. The mother’s prenatal parameters are within normal limits. The baby was having high grade fever and respiratory symptoms. High resolution computed tomography of thorax revealed approximately 4 cm × 3.7 cm × 4.8 cm sized round to oval lesion involving right lower lobe. It showed multiple tiny communicating cystic areas in the central portion and solid peripheral portion. Patchy consolidation was noted surrounding this lesion. Possibilities of CPAM (Type II) or pulmonary blastoma were suggested.
Clinical diagnosis of low birth weight with early onset sepsis and CPAM Type III was kept. Lobectomy was planned and performed. The resected specimen of right lower lobe of lung was sent for histopathological evaluation.
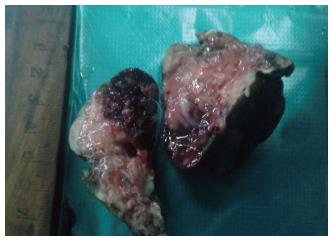
Right lower lung lobectomy specimen was solid, firm in consistency measuring 6 cm × 5 cm × 3 cm in size. The cut surface showed solid gray white area with slit like opening and few small cysts measuring smaller than 0.3 cm in diameter. Foci of large areas of haemorrhages were also noted (Figure 1).
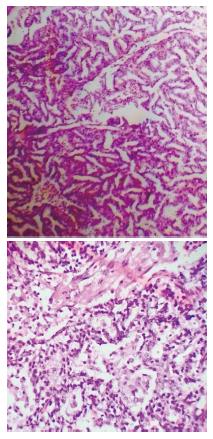
Microscopy revealed small cystic and predominantly solid areas. Cystic areas showed bronchiole like structures lined by simple cuboidal epithelium which are surrounded by alveolar spaces (Consistent with immature lungs). Some alveoli and cystic spaces filled with acute inflammatory exudates with microabscess formation. Intraalveolar haemorrhages were also noted (Figure 2).
Finally, the case was diagnosed as CPAM [congenital cystic adenomatoid malformation (CCAM)] Type III with acute pneumonia and focal intraalveolar hemorrhages.The patient is doing well on follow up till date since 4 mo.
A 31 years old female G3P2L2 with history of nine months of amenorrhoea came to the outpatient department with USG abdomen and pelvis report suggestive of CCAM with Polyhydramnios, displacing the heart towards left. Labour was induced. After birth baby did not cry, for which resuscitation was done but in vain, the baby was cyanosed. Clinically it was diagnosed as; female child with Very Low Birth Weight with CPAM. Baby was shifted to NICU (Neonatal intensive care unit). Baby was initially responding but after few hours, succumbed to death and autopsy was performed.
Autopsy findings revealed Heart and Lung together weighing 40 grams. Left lung measured 5 cm × 2 cm × 1 cm. It was firm in consistency. Right lung measured 7 cm × 3 cm × 3 cm. Pleural surface of right lung and cut surface showed tiny cysts ranging in size from 0.5-2 cm in diameter involving the entire parenchyma of right lung. No other anomalies were noted.
Microscopically sections studied from right middle and lower lobe showed multiple, cystically dilated abnormal bronchiole like structures lined by cuboidal to columnar epithelium resting on thin fibromuscular wall. Sections from left lung and right upper lobe show evidence of hyaline membrane disease characterised by alveoli and airways lined by eosinophilic membrane.
On autopsy findings, the case was finally diagnosed as CPAM Type II (Figure 3).
CPAM is an unusual condition characterised by immature, malformed lung tissue with cystic appearance. CPAM is seen mainly in newborns, still born infants and is an unusual condition in children beyond infancy. It is a hamartomatous lesion that is usually symptomatic in first few days of life. The patients with CPAM can present as neonates with severe, progressive respiratory distress due to cyst expansions. Hydrops may be present[3].
Exact etiology of CPAM is not known, it is to be considered as hamartomatous malformation and abnormal proliferation of the pulmonary tissue at different sites.
It has been proposed that designation of this lesion “CCAM” be changed to “CPAM” to reflect the fact that the lesions described as cystic are present in only 3 of the 5 types and “Adenomatoid” only in one type (Type III). CPAM more accurately encompasses all five types of this classification[1].
CPAM Type 0-Acinar dysplasia/agenesis is rare malformation largely incompatible with life. Lungs are small, firm with diffusely granular surface. Microscopically, it shows bronchus like structures with muscle, glands and numerous cartilage plates and loose, vascular mesenchymal tissue[1].
CPAM Type I-It accounts for nearly 65% of cases. It is operable with good prognosis. Grossly, lesion is predominantly cystic type (measuring 3-10 cm in diameter) surrounded by smaller cysts. Microscopically, the large, thin walled cysts are lined by ciliated pseudostratified columnar epithelium with some mucin producing cells. The wall composed of fibromuscular and elastic tissue and occasional cartilage plate[1].
CPAM Type II-it accounts for 10%-15% of cases and mainly seen in first year of life. It has poor prognosis because it is frequently associated with other congenital anomalies. Grossly lesion is composed of medium sized cysts measuring 0.5 to 2.0 cm in diameter that are evenly distributed and blend with the adjacent normal parenchyma. Our second case belonged to this type of CPAM. Cysts were arranged in back to back fashion and bronchiole like structures and lined by cuboidal to columnar epithelium with thin underlying fibromuscular layer. Mucous cells and cartilage plates were absent. CPAM Type 2 has been noted in nearly 50% cases of extralobar sequestration[1].
CPAM Type III- Chin and Tang (1949) have described this lesion. It is infrequent and accounts only about 5% of cases. It is small cystic or solid type, exclusively seen in first few days to months of life with characteristic male preponderance. It is commonly associated with maternal polyhydramnios, foetal anasarca. So it has high mortality rate. Increased Alfa-fetoprotein level can be noted in second trimester. Grossly cysts are small measuring less than 0.2 cm in diameter, affecting large bulky mass involving an entire lobe or even an entire lung. Microscopically the lesion resembles an immature lung devoid of bronchi. It consists of irregular, stellate shaped bronchiole like structures lined by cuboidal epithelial cells, surrounded by alveolar ductules and saccules that are lined by cuboidal epithelium giving the adenomatoid appearance that is why originally named as CCAM. Mucous cells, cartilage, rhabdomyomatous cells are not seen[1,4].
CPAM Type IV-It is hamartomatous malformation of the distal acinus and accounts for 10%-15% of cases with an age range of newborn to 4 years. This lesion involves a single lobe. Grossly large, thin walled cysts are lined by flattened epithelium-alveolar lining cells with underlying loose, fibrovascular mesenchymal tissue[1].
Clinical examination and chest X-ray invariably identify CPAM. On antenatal ultrasonograpy CPAM lesions can be identified in a population of infants who are asymptomatic at birth[5,6]. Most of these CPAM (10%-15%) are associated with other congenital anomalies, e.g., CPAM Type II is associated with bilateral renal agenesis, extralobar pulmonary sequestration, cardiovascular malformation, etc.
The lack of bronchial cartilage distinguishes CPAM from bronchogenic cyst and the distinctive rows of mucous cells from simple foregut cyst[2].
Congenital lobar emphysema can be distinguished from CPAM by the presence of bronchovascular markings extending to the periphery of the involved lobe and by atelectasis of adjacent tissue[7].The distinction from a sequestration of the intralobar variety may be difficult but a systemic blood supply would favour sequestration[8].
The association between CPAM and malignancy has been well documented. Malformation and proliferation cause hamartomas over the tracheobronchial tree. Type I CPAM may involve malignant transformation of mucinous bronchioloalveolar carcinoma[9-11]. Type II CPAM may involve malignant transformation- Rhabdomyosarcoma. Type III CPAM requires examination of the entire lesion to exclude pulmonary blastoma by confirming whether or not sarcomatous differentiation is present in the solid parts[8,12,13].
Surgical resection in asymptomatic infants is more beneficial with fewer complications compared with intervention following development of symptoms. It is the gold standard for management of CPAM for both pathological diagnosis and treatment[6].
Case 1: The baby was having high grade fever and respiratory symptoms;Case 2: It was female child with very low birth weight (LBW) with Congenital pulmonary airway malformation (CPAM).
Case 1: Clinical diagnosis of LBW with early onset sepsis and CPAM Type III was kept; Case 2: Clinically it was diagnosed as; female child with very LBW with CPAM.
Case 1: Pulmonary blastoma; Case 2: Diagnosed on autopsy.
Case 1: On CT diagnosis of pulmonary blastoma or CPAM were suggested; Case 2: Diagnosed on autopsy.
Case 1: Finally, the case was diagnosed as CPAM (congenital cystic adenomatoid malformation) Type III with acute pneumonia; Case 2: On autopsy findings, the case was finally diagnosed as CPAM Type II.
Case 1: Lobectomy was planned and performed; Case 2: Died immediately.
Early pulmonary resection for asymptomatic CPAM is required and recommended to make a definitive diagnosis and determine the prognosis of the disease.
This is an interesting case report.
P- Reviewer: Hori T, Noussios G S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Stocker JT. The Respiratory Tract. Pediatric Pathology. 2nd ed. Vol. 1, Chapter 13. Philadelphia: Lippiacott Company 1992; 466-473. |
| 2. | Sittig SE, Asay GF. Congenital cystic adenomatoid malformation in the newborn: two case studies and review of the literature. Respir Care. 2000;45:1188-1195. [PubMed] |
| 3. | Chan C, Lee YS, Taso PC, Jeng MJ, Soong WJ. Congenital Pulmonary Airway Malformation Type IV: A case report. Journal compilation. 2013; Available from: http://www.pedipulm.org.tw/ezcatfiles/rb02/img/img/231/9-2.6.48.pdf. |
| 4. | Sirithangkul S, Chuengchitraks S, Staworn D, Laohapand C, Silarat T. Late manifestation of congenital cystic adenomatoid malformation with lung abscess: a case report. J Med Assoc Thai. 2010;93 Suppl 6:S223-S227. [PubMed] |
| 5. | Malik R, Pandya VK, Agarwal G, Jain M. Congenital Cystic AdenomatoidMalformation: A case report. Ind J RadiolImg. 2006;16:859-860. |
| 6. | Marshall KW, Blane CE, Teitelbaum DH, van Leeuwen K. Congenital cystic adenomatoid malformation: impact of prenatal diagnosis and changing strategies in the treatment of the asymptomatic patient. AJR Am J Roentgenol. 2000;175:1551-1554. [PubMed] |
| 7. | Hansen T, Cooper Weisman L. Congenital Diseases affecting the Lung Parenchyma. In contemporary Diagnosis & Management of Neonatal Respiratory Diseases. Newton, PA: Handbooks in Health Care Co 1995; 164-179. |
| 8. | Stocker JT. Cystic lung disease in infants and children. Fetal Pediatr Pathol. 2009;28:155-184. [PubMed] |
| 9. | Granata C, Gambini C, Balducci T, Toma P, Michelazzi A, Conte M, Jasonni V. Bronchioloalveolar carcinoma arising in congenital cystic adenomatoid malformation in a child: a case report and review on malignancies originating in congenital cystic adenomatoid malformation. Pediatr Pulmonol. 1998;25:62-66. [PubMed] |
| 10. | Ramos SG, Barbosa GH, Tavora FR, Jeudy J, Torres LA, Tone LG, Trad CS. Bronchioloalveolar carcinoma arising in a congenital pulmonary airway malformation in a child: case report with an update of this association. J Pediatr Surg. 2007;42:E1-E4. [PubMed] |
| 11. | West D, Nicholson AG, Colquhoun I, Pollock J. Bronchioloalveolar carcinoma in congenital cystic adenomatoid malformation of lung. Ann Thorac Surg. 2007;83:687-689. [PubMed] |
| 12. | 12Cass DL, Quinn TM, Yang EY, Liechty KW, Crombleholme TM, Flake AW, Adzick NS. Increased cell proliferation and decreased apoptosis characterize congenital cystic adenomatoid malformation of the lung. J Pediatr Surg. 1998;33:1043-1046; discussion 1047. [PubMed] |
| 13. | Oliveira C, Himidan S, Pastor AC, Nasr A, Manson D, Taylor G, Yanchar NL, Brisseau G, Kim PC. Discriminating preoperative features of pleuropulmonary blastomas (PPB) from congenital cystic adenomatoid malformations (CCAM): a retrospective, age-matched study. Eur J Pediatr Surg. 2011;21:2-7. [PubMed] |