Published online Dec 16, 2015. doi: 10.12998/wjcc.v3.i12.988
Peer-review started: May 19, 2015
First decision: June 18, 2015
Revised: September 21, 2015
Accepted: October 1, 2015
Article in press: October 8, 2015
Published online: December 16, 2015
Processing time: 204 Days and 5.7 Hours
Sarcoidosis is a systemic inflammatory condition in which noncaseating epithelioid cell granulomas appear within one or several body sites. Sarcoid reaction (also referred to as sarcoidal or sarcoid-like reaction) occurs in patients who do not fulfill the diagnostic criteria for systemic sarcoidosis but present with similar clinical and histological features. As sarcoma-associated sarcoid reactions are rare, we describe the features of sarcoid reaction that developed in a man with liposarcoma and summarize reports of other oncology patients with sarcoma-associated sarcoid reactions. A 68-year-old man with retroperitoneal liposarcoma presented for evaluation of erythematous dermal plaques on his left leg. Microscopic examination of a tissue specimen revealed multiple epithelioid granulomas in the superficial and mid-reticular dermis. Correlation of the clinical presentation and histopathologic findings established a diagnosis of liposarcoma-associated cutaneous sarcoid reaction. Sarcoid reactions have been described in only seven individuals with sarcoma, including two patients with leiomyosarcoma and one patient with either carcinosarcoma, Kaposi sarcoma, liposarcoma, malignant peripheral nerve sheath tumor, rhabdosarcoma, or synovial sarcoma. Sarcoidal granulomas most commonly develop within the locoregional draining lymph nodes. Sarcoid reactions may also affect other organs, such as the lungs, skin, and spleen.
Core tip: Sarcoid reaction is an inflammatory condition in which noncaseating epithelioid cell granulomas develop within one or several body sites. Several malignancies, including lymphomas and carcinomas, have been linked to sarcoid reaction. We describe the first case of a patient presenting with liposarcoma-associated sarcoid reaction and summarize the literature on rare patients with sarcoma-associated sarcoid reactions. It is imperative that clinicians consider sarcoid reaction in the evaluation of oncology patients prior to initiating treatment.
- Citation: Beutler BD, Cohen PR. Sarcoma-associated sarcoid reaction: Report of cutaneous sarcoid reaction in a patient with liposarcoma. World J Clin Cases 2015; 3(12): 988-992
- URL: https://www.wjgnet.com/2307-8960/full/v3/i12/988.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v3.i12.988
Sarcoidosis is a multisystem inflammatory condition characterized by the appearance of noncaseating epithelioid cell granulomas within one or several body sites. Sarcoid reaction-also called sarcoidal or sarcoid-like reaction-refers to the presence of noncaseating epithelioid cell granulomas in patients who do not fulfill the diagnostic criteria for systemic sarcoidosis. Many malignancies-including cancers of the thyroid, breast, and kidney-have been associated with sarcoid reaction. However, sarcoma-associated sarcoid reactions are rare[1].
Sarcoid reactions most commonly affect the lungs, intrathoracic lymph nodes, and skin. In addition, oncology patients often develop sarcoidal granulomas within the locoregional lymph nodes that drain the cancer. Diagnosis is typically established through imaging and/or biopsy. Similar to sarcoidosis, sarcoid reaction is typically asymptomatic and self-limiting; therefore, treatment is seldom required[2]. Immunohistochemical analyses have revealed that granulomas found in sarcoid reactions are B cell-positive while those found in sarcoidosis are B cell-negative.
We describe a man with liposarcoma who presented with cutaneous sarcoid reaction and summarize the characteristics of other sarcoma patients with sarcoid reaction.
In July 2014, a 68-year-old man with liposarcoma, which was diagnosed in 2008, presented for evaluation of a red rash on his leg that had been present for 30 mo. The tumor was 20 cm × 15 cm and located in the retroperitoneal space. The liposarcoma was inoperable and therefore treatment with oral pazopanib hydrochloride (200 mg taken four times per day) had been initiated. However, metastasis to the lymph nodes was subsequently detected.
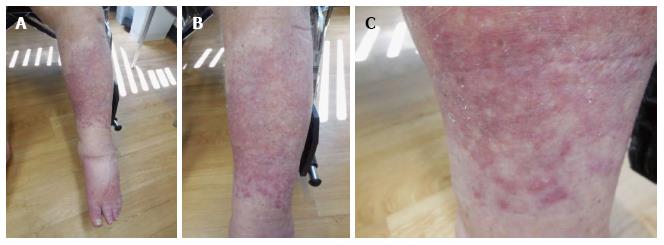
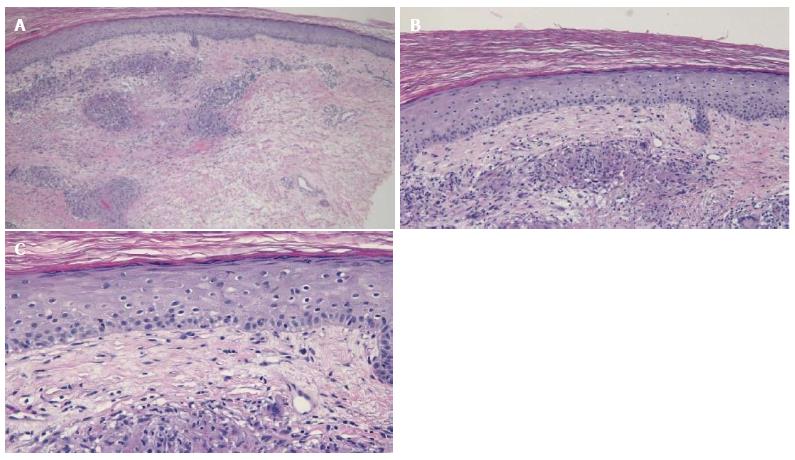
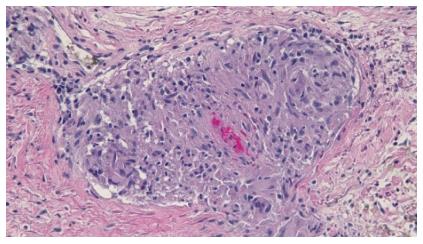
Cutaneous examination revealed multiple smooth-surfaced erythematous dermal plaques affecting his left pretibial area (Figure 1). Pathologic examination of a punch biopsy showed multiple epithelioid granulomas in the superficial and mid-reticular dermis. Histiocytes could also be seen within the interstitium. There was mild lymphocytic and neutrophilic inflammation surrounding the sarcoidal granulomas (Figures 2 and 3). Bacterial, fungal, and mycobacterial cultures of biopsy-obtained skin specimens were negative for organisms.
Laboratory studies revealed an elevated erythrocyte sedimentation rate of 68 mm/h (reference range: 0-20 mm/h). With the exception of his serum albumin level being low at 2.92 g/dL (reference range: 3.5-5.5 g/dL), his serum chemistry levels were normal. Notably, his alpha-2-macroglobulin was elevated at 1.17 g/dL (reference range: 0.6-1.1 g/dL); this finding was consistent with subacute tumor-associated inflammation. Laboratory results for the following studies were negative or normal: anti-nuclear antibody, angiotensin converting enzyme, anti-dsDNA, anti-La (Sjogren’s syndrome B), anti-Ro (Sjogren’s syndrome A), anti-streptolysin O titer, glycohemoglobin, hepatitis antibodies, lipid profile, rheumatoid factor, Smith antibody, syphilis enzyme immunoassay, thyroxine 4, thyroid stimulating hormone, and vitamin D1,25-dihydroxy.
Based on correlation of the clinical presentation, histopathologic findings, and laboratory studies, a diagnosis of liposarcoma-associated cutaneous sarcoid reaction was established. The patient did not fulfill the criteria for systemic sarcoidosis. His skin condition was asymptomatic; therefore, no treatment was initiated. He died of malignancy-associated kidney failure shortly after the diagnosis of sarcoid reaction was established.
The first account of sarcoid reaction can be traced back to 1869, when the British physician Jonathan Hutchinson described a patient with “peculiar patches of dark purplish color on his extremities”[3]. The condition was further characterized throughout the late-19th and early-20th centuries. In 1899, Boeck[4] described 24 patients with “benign miliary lupoids”. Twelve years later, Wolbach[5] detailed the histologic features of sarcoidal granulomas in a report of five patients with “widely distributed miliary sized lesions of granulomatous character”. However, the association between sarcoid reaction and malignancy was not identified until 1917, when Herxheimer[6] observed sarcoidal granulomas affecting patients with breast, rectal, and cystic duct carcinomas.
Investigators continued to study sarcoid reaction throughout the first decades of the 1900s. By 1937, it was clear that sarcoid reaction could be definitively distinguished from systemic sarcoidosis. In a report of six patients with sarcoidal granulomas, Nickerson explained that sarcoid reaction refers to the localized development of noncaseating epithelioid cell granulomas; sarcoidosis, in contrast, is a multisystem inflammatory disease characterized not only by the development of noncaseating epithelioid cell granulomas, but also by various systemic symptoms and serum abnormalities[7]. Interestingly, although sarcoidal granulomas are histologically identical in both conditions, immunohistochemical analyses have revealed that granulomas found in sarcoid reactions are B cell-positive while those found in sarcoidosis are B cell-negative[8].
Sarcoid reactions have been described in association with numerous hematologic malignancies and solid tumors[9]. However, sarcoma-associated sarcoid reactions are rare. Indeed, to the best of our knowledge, only eight patients (including our patient) with sarcoma-associated sarcoid reactions have been described in the English literature (Table 1)[10-16].
| C | Dx: SM | Dx: SR | G | Sarcoma | Diagnostic tests | Sarcoid reaction | Ref. |
| 1 | 22 | 24 | F | Synovial sarcoma of the left thigh | CT; biopsy | Left lower lobe subpleural nodules | [10] |
| 2 | 30 | 30 | M | Malignant peripheral nerve sheath tumor1 | FDG PET/CT; biopsy | Hilar and mediastinal lymphadenopathy | [11] |
| 3 | 48 | 50 | F | Leiomyosarcoma of the stomach | Chest radiograph; biopsy | Bilateral hilar and paratracheal lymphadenopathy | [12] |
| 4 | 57 | 57 | M | Leiomyosarcoma of the rectum | Biopsy | Granulomas within the tumor tissue | [13] |
| 5 | 58 | 59 | F | Uterine carcinosarcoma | FDG PET; CT; biopsy | Hilar, pretracheal, and mediastinal lymphadenopathy | [14] |
| 6 | 60 | 60 | M | Rhabdomyosarcoma of the esophagus | Biopsy | Granulomas within the lymph nodes draining the neoplasm and in the spleen | [15] |
| 7 | 62 | 68 | M | Retroperitoneal liposarcoma | Biopsy | Red dermal plaques of granulomas | Current report |
| 8 | 74 | 74 | M | Kaposi sarcoma of the foot | Biopsy | Red-purple cutaneous patches; granulomas within the tumor tissue and in the stroma surrounding the tumor | [16] |
Leiomyosarcoma-either of the rectum or stomach-was associated with sarcoid reaction in 25% of individuals (two of eight). The other patients had either carcinosarcoma, Kaposi sarcoma, liposarcoma, malignant peripheral nerve sheath tumor, rhabdosarcoma, or synovial sarcoma. However, the number of patients is too small to observe any definitive relationship between specific sarcoma type and the development of sarcoid reaction.
Sarcoma-associated sarcoid reaction was observed in five men and three women. The age at sarcoma diagnosis ranged from 22 to 74 years; the median age was 57.5 years. The women were significantly younger than the men; they ranged in age from 22 to 58 years, with a median age of 48 years. In contrast, men ranged in age from 30 to 74 years; their median age was 60 years.
The primary site of origin was most commonly the gastrointestinal tract; three of eight cases presented with neoplasms affecting this region. These included tumors from the esophagus, stomach, and rectum. Other sites of origin, each present in one individual, included the genitourinary tract (uterus), pelvic cavity, retroperitoneum, skin (Kaposi sarcoma), and synovium.
Five of eight cases were diagnosed with sarcoma and subsequently developed sarcoid reaction. The interval between the diagnosis of sarcoma and sarcoid reaction ranged from three months to six years, with a mean interval of 2.3 years. The two conditions were diagnosed concurrently in the remaining three patients (cases 4, 6, and 8).
The sites of sarcoma-associated sarcoid reaction in 4 of 8 cases were the lungs and mediastinal lymph nodes. Cutaneous involvement was observed in two patients, including our own. In addition, two individuals-either with Kaposi sarcoma or leiomyosarcoma of the rectum-developed nodules within the tumor tissue. Other sites of sarcoma-associated sarcoid reaction included the locoregional draining lymph nodes, the spleen, and the stroma surrounding the neoplasm.
The diagnosis of sarcoma-associated sarcoid reaction was established primarily by imaging studies and histologic examination of tissue samples. Imaging studies were performed on five patients-either chest radiography (one patient), computed tomography scan (two patients), or combination positron emission tomography and computed tomography scan (two patients). In addition, all eight patients underwent biopsy in order to identify noncaseating epithelioid cell granulomas and exclude disease metastasis. In most patients, sarcoid reactions resolve spontaneously; therefore, treatment is rarely required.
The pathogenesis of sarcoma-associated sarcoid reaction is unknown. It has been postulated that the development of sarcoidal granulomas in oncology patients represents a host immune defense mechanism. Indeed, the occurrence of sarcoid reaction within tumor tissue is associated with a better prognosis, a reduced risk of metastasis or recurrence, or both; T lymphocytes and dendritic cells, which are typically found within granulomas, are thought to play a central role in this response[17,18]. Sarcoid reaction is characterized by the development of noncaseating epithelioid cell granulomas in patients who do not fulfill the diagnostic criteria for sarcoidosis or sarcomas.
A 68-year-old man with a six-year history of retroperitoneal liposarcoma presented for evaluation of a red rash on his leg that had been present for 30 mo.
Multiple smooth-surfaced erythematous dermal plaques affecting the left pretibial area.
Discoid lupus erythematous, granuloma annulare, lichen planus, lymphocytoma cutis, plaque psoriasis.
Elevated erythrocyte sedimentation rate of 68 mm/h (reference range: 0-20 mm/h) and elevated alpha-2-macroglobulin at 1.17 g/dL (reference range: 0.6-1.1 g/dL).
Multiple epithelioid granulomas in the superficial and mid-reticular dermis.
The cutaneous condition was asymptomatic and therefore no treatment was administered.
Sarcoid reactions have been described in only eight individuals, including the authors’ patient, with various sarcomas; these include two patients with leiomyosarcoma and one patient with either carcinosarcoma, Kaposi sarcoma, liposarcoma, malignant peripheral nerve sheath tumor, rhabdosarcoma, or synovial sarcoma.
Sarcoidosis is a systemic inflammatory disease in which noncaseating epithelioid cell granulomas develop in multiple organ systems. In contrast, sarcoid reaction - also called sarcoidal or sarcoid-like reaction - refers to the presence of noncaseating epithelioid cell granulomas in patients who do not fulfill the diagnostic criteria for systemic sarcoidosis.
Sarcoid reaction may occasionally mimic metastases in patients with solid tumors, including sarcomas, and should therefore be considered in the evaluation of oncology patients in order to prevent misdiagnosis and unnecessary treatment.
This is a straightforward clinical case study reporting on a liposarcoma patient that presents with a cutaneous sarcoid reaction. At the same time the authors review the literature listing all cases of soft tissues sarcoma that are linked to a sarcoid reaction.
P- Reviewer: Brcic I, Wiemer EAC S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Brincker H. Sarcoid reactions in malignant tumours. Cancer Treat Rev. 1986;13:147-156. [PubMed] |
| 2. | Young RC, Rachal RE, Cowan CL. When should sarcoidosis be treated? J Natl Med Assoc. 1986;78:811-821. [PubMed] |
| 3. | James DG, Sharma OP. From Hutchinson to now: a historical glimpse. Curr Opin Pulm Med. 2002;8:416-423. [PubMed] |
| 4. | Boeck C. Multiple benign sarcoid of the skin. J Cutan Genitourin Dis. 1899;17:543-550. |
| 5. | Wolbach SB. A new type of cell inclusion, not parasitic, associated with disseminated granulomatous lesions. J Med Res. 1911;24:243-258. |
| 7. | Nickerson DA. Boeck’s sarcoid. Report of six cases in which autopsies were made. Arch Pathol Lab Med. 1937;24:19-29. |
| 8. | Brincker H. Interpretation of granulomatous lesions in malignancy. Acta Oncol. 1992;31:85-89. [PubMed] |
| 9. | Cohen PR, Kurzrock R. Sarcoidosis and malignancy. Clin Dermatol. 2007;25:326-333. [PubMed] |
| 10. | Hashem SA, Sultan I, Al-Hussaini M, Hawari F, Yaser S. Pulmonary sarcoid-like reaction in metastatic synovial sarcoma. Respir Med CME. 2011;4:20-23. |
| 11. | Chowdhury FU, Sheerin F, Bradley KM, Gleeson FV. Sarcoid-like reaction to malignancy on whole-body integrated (18)F-FDG PET/CT: prevalence and disease pattern. Clin Radiol. 2009;64:675-681. [PubMed] |
| 12. | Parra ER, Canzian M, Saber AM, Coêlho RS, Rodrigues FG, Kairalla RA, de Carvalho CR, Capelozzi VL. Pulmonary and mediastinal “sarcoidosis” following surgical resection of cancer. Pathol Res Pract. 2004;200:701-705. [PubMed] |
| 13. | Nakamura M, Iemura A, Kojiro M, Umetani H, Isobe M. Leiomyosarcoma of the rectum with sarcoid-like reaction--a case report. Kurume Med J. 1990;37:171-175. [PubMed] |
| 14. | Froio E, D’Adda T, Fellegara G, Martella E, Caruana P, Pruneri G, Pesci A, Rindi G. Uterine carcinosarcoma metastatic to the lung as large-cell neuroendocrine carcinoma with synchronous sarcoid granulomatosis. Lung Cancer. 2009;64:371-377. [PubMed] |
| 15. | Sumiyoshi A, Sannoe Y, Tanaka K. Rhabdomyosarcoma of the esophagus--a case report with sarcoid-like lesions in its draining lymph nodes and the spleen. Acta Pathol Jpn. 1972;22:581-589. [PubMed] |
| 16. | Kandemir NO, Yurdakan G, Bektas S, Tekin NS. Classic Kaposi sarcoma with sarcoid-like granulomas: a case report and literature review. Exp Mol Pathol. 2009;87:89-93. [PubMed] |
| 17. | Kawasaki Y, Maemura K, Kurahara H, Mataki Y, Iino S, Sakoda M, Ueno S, Shinchi H, Takao S, Natsugoe S. Gallbladder adenocarcinoma with sarcoid-like reaction in regional lymph nodes: report of a case. BMC Cancer. 2014;14:946. [PubMed] |