UNILATERAL NEUROPATHIC PAIN
In 2008, the special interest group for neuropathic pain of the International Association for the Study of Pain (IASP) proposed a new definition of neuropathic pain: “pain arising as a direct consequence of a lesion or disease affecting the somatosensory system”[1]. This new definition was accepted by the IASP and is now largely used all over the world. According to it, neuropathic pain conditions have several possible mechanisms which express different phenotypes and clinical presentations. On this basis, neuropathic pain has to be divided into at least central and peripheral neuropathic pain, depending on which part of the nervous system is involved. Peripheral neuropathic pain is sustained by peripheral nerve lesions that can have various types of presentation[2]. In particular, among the multiform pathophysiological expressions, one of the main differences is based on symmetrical or asymmetrical pathology sustaining the painful clinical syndromes. Nerve lesions associated with neuropathic pain can indeed be symmetrical, e.g., polyneuropathies, or asymmetrical, e.g., herpes zoster neuropathy. Although there are well-documented contralateral effects following a painful lesion[3], only asymmetrical nerve lesions sustain the so-called unilateral neuropathic pain.
Several forms of unilateral peripheral nerve lesions are possible[2,4]. They can be roughly divided into mononeuropathies, radiculopathies and plexopathies. The most common clinical presentation is mononeuropathy, i.e., a lesion involving only one peripheral nerve, such as the median nerve lesion in the carpal tunnel syndrome. Another common clinical presentation of unilateral peripheral nerve lesion is radiculopathy, a frequent consequence of an intervertebral disk herniation or other pathologies of the lumbar or cervical spine. Plexopathies are rarer than other unilateral nerve lesions, the most frequent being brachial plexopathy, commonly caused by trauma or disorders involving neighboring structures.
CLINICAL NEUROPHYSIOLOGICAL TESTS FOR THE DIAGNOSIS OF UNILATERAL PERIPHERAL NERVE LESIONS
In cases of suspected peripheral unilateral neuropathic pain, in order to demonstrate the presence of a peripheral nerve lesion sustaining the painful condition, the first diagnostic step following the clinical examination is usually the execution of neurophysiological tests, in particular electromyography (EMG) and electroneurography (ENG or nerve conduction studies)[5-8]. Unfortunately, those tests have two major limitations. The first is the impossibility of using them in every part of the body (e.g., the trunk) and the second is the fact that they investigate only large diameter fiber functions, part of the lemniscal system which is only one of the two tracts of the somatosensory system, the lesion (or disease) of which is mandatory for the diagnosis of neuropathic pain[1]. Another possible tool to test the function of peripheral large diameter/lemniscal fibers are somatosensory evoked potentials[7,9,10] that can be useful to study the proximal parts of the peripheral nervous system, but substantially share the same limitations of EMG and ENG. The small diameter fibers’ functions can be neurophysiologically investigated by Laser Evoked Potentials[10-12], although this test is still confined to specialized neurophysiological labs, is time consuming and currently is not used for routine diagnostic evaluation.
Finally, another test for evaluating small fiber afferent function is Quantitative Sensory Testing[13-15]. The controlled application of thermal stimuli indeed allows investigating the spinothalamic functions, both in its A-delta (cold stimuli) and C component (warm stimuli). The major limit of this method is the necessity for the patient’s cooperation. On the other hand, the most important advantage is the possibility of studying the entire body surface and above all to identify and measure hypersensitivity phenomena, such as thermal allodynia and hyperalgesia.
NEURODIAGNOSTIC SKIN BIOPSY
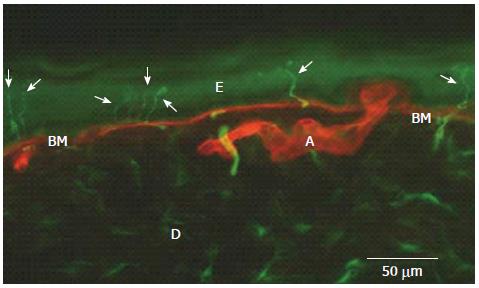
Neurodiagnostic skin biopsy (NSB) is a relatively new technique for the diagnosis of peripheral neuropathies[16-18]. It is an objective method based on a skin biopsy performed by a circular punch, usually 3 mm diameter, and on immunohistochemical staining techniques that allow identifying the epidermal nerve fibers. To this aim, both bright-field and immunofluorescence (Figure 1) can be used, allowing calculation of the Epidermal Nerve Fiber Density (ENFD)[19]. It is important to underline that epidermal nerve fibers are currently exclusively considered the free nerve endings of small diameter (A-delta and C) afferent fibers[20], a part of the spinothalamic tract physiologically conveying thermal and painful sensations from the periphery to the brain. Interestingly, among all the nerve fibers present in a peripheral nerve, the great majority are just small diameter fibers[21].
Figure 1 Epidermal nerve fibers (arrows) identified by immunofluorescence in the distal leg of a normal subject (in green, PGP 9.
5 staining of nerve fibers; in red, type IV collagen staining of basement membrane and blood vessels). E: Epidermis; D: Dermis; BM: Basement Membrane; A: Artery.
NSB FOR THE DIAGNOSIS OF PERIPHERAL NEUROPATHIES IN BODY PARTS IMPOSSIBLE TO INVESTIGATE BY CLINICAL NEUROPHYSIOLOGICAL TESTS
Due to the continuous advances in knowledge that have occurred in the last ten years, NSB is currently considered an important diagnostic tool for neurologists[22,23].
NSB has the important property of being used to investigate the skin, allowing obtaining a diagnosis of small fiber axonal neuropathy of peripheral nerves innervating every body part covered by skin[24,25].
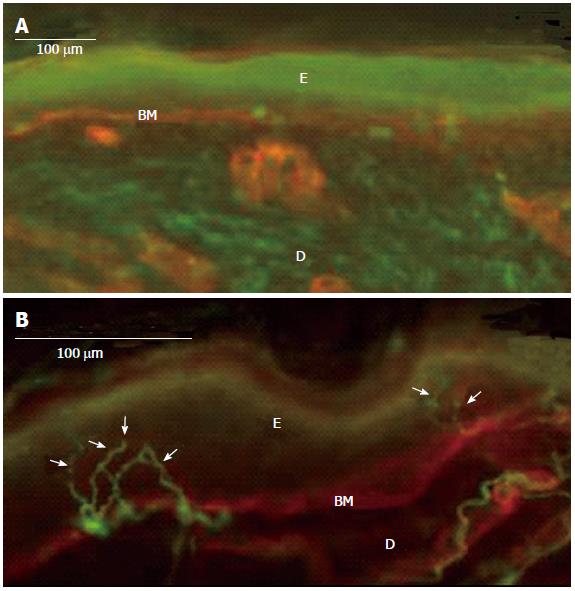
This feature appears to be very important because it allows going beyond the possibilities of neurophysiological tests which are available only for a limited number of peripheral nerves. It follows on that NSB allows reaching a diagnosis of neuropathy for “difficult” nerves, such as those of the trunk or occipital nerves frequently (and irregularly) involved in post-herpetic neuralgia[26]. An example of NSB clinical use is given in Figure 2 which shows a severe, unilateral decrease of ENFD in the neck skin of a patient with post-herpetic neuralgia.
Figure 2 Complete denervation of epidermis (and dermis) in an 82-year-old patient with a severe post-herpetic neuralgia (A), normal, contralateral, mirror skin innervation (13 fibers/mm) (B).
Immunofluorescence method: In green, PGP 9.5 staining of nerve fibers; in red, type IV collagen staining of basement membrane and blood vessels; Arrows: Epidermal nerve fibers; E: Epidermis; D: Dermis; BM: Basement Membrane.
Another important property of NSB is the ability to identify lesions involving small branches of peripheral nerves which cannot be investigated by neurophysiological tests. This feature of NSB appears to be particularly important in post-traumatic peripheral nerve lesions where one lesion is different from another.
In this context, it is important to highlight a recent paper where NSB showed a significant asymmetry in a spinal cord injury patient complaining of a bilateral burning and pricking pain at the level of injury[27]. Interestingly, NSB not only allowed demonstration of the presence of two different mechanisms leading to identical symptoms, but also to justify a different efficacy of the same treatment in the two sides. Continuing to talk of possible pain mechanisms, in a very recent paper, NSB findings suggested skin hyperinnervation as a possible cause for the development of dynamic mechanical allodynia following finger amputation[28].
Another recent study confirmed that NSB can allow getting information from the skin of several parts of the body. In that study, the epidermal innervation was studied in burn patients with unilateral injuries, allowing to suggest a possible correlation between the residual cutaneous innervation and the development of chronic pain[29].
NSB can also be useful in other clinical contexts. For example, it can also be used in differentiating neuropathic from referred pain, as demonstrated in a very recent paper in patients with endometriosis and unilateral thigh pain[30]. Moreover, it has been used for assessing the involvement of the peripheral nervous system in a dermatological manifestation of neurological disease, such as a dyshidrotic eczema in a patient with ulnar neuropathy or a unilateral pruritus on the paretic side of a stroke patient[31]. Finally, NSB can also be used to exclude a neuropathic pathophysiology of a clinical pain, as demonstrated in Parry-Romberg syndrome, a rare painful condition characterized by progressive hemifacial atrophy and unilateral facial pain[32].
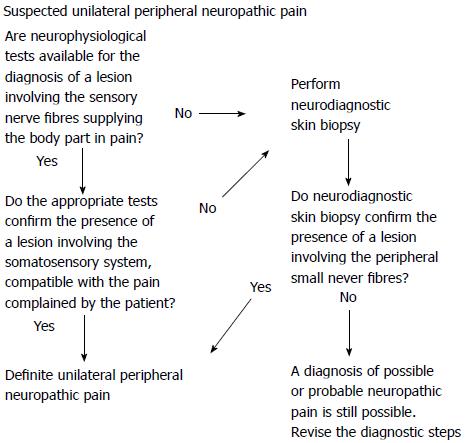
Considering the current evidence on NSB and the well-known specific diagnostic properties of neurophysiological tests, it is possible to suggest a diagnostic sequence that can be useful to confirm the diagnosis of unilateral peripheral neuropathic pain (Figure 3).
Figure 3 Suggested diagnostic sequences for the confirmation of a peripheral nerve lesion sustaining a definite, peripheral, unilateral neuropathic pain.
EPIDERMAL INNERVATION SYMMETRY RATIO
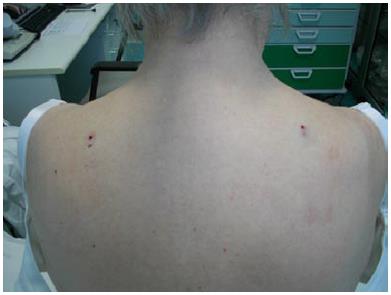
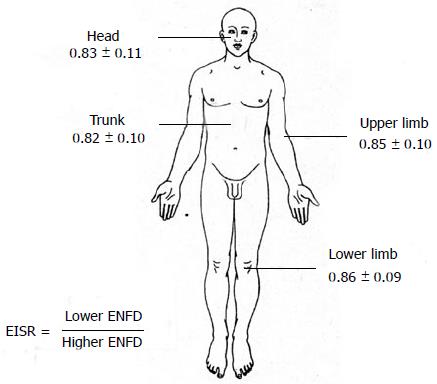
One of the main disadvantages of NSB is that it is very difficult to obtain robust normative data for any part of the body because of their different epidermal innervation patterns. This problem can be elegantly solved in cases of unilateral peripheral nerve lesions by comparing the neuropathic skin ENFD with the contralateral, normal side ENFD. To this aim, a bilateral biopsy is necessary (Figure 4). It is well known that the two sides of the body of a normal subject are only theoretically symmetrical and a significant asymmetry can be frequently found in several types of biological measures. NSB is not an exception. For this reason, a ratio (the Epidermal Innervation Symmetry Ratio) was developed to compare the epidermal innervation of two symmetrical, mirror parts of the body in normal subjects. Preliminary data were obtained from 133 normal subjects[33]. In particular, when comparing the ENFD of the right with the left side, the ratio showed a normal distribution (mean 1.02; median 1.01; standard deviation 0.21; asymmetry 1.86, kurtosis -0.97). Moreover, when confronting the lower with the higher (contralateral) ENFD, the ratio was quite constant and surprisingly reproducible, also considering different parts of the body (Figure 5).
Figure 4 Figure exemplifies the bilateral biopsy needed for comparing the epidermal nerve fiber density of two symmetrical, mirror skin parts.
Figure 5 Figure shows the values of epidermal innervation symmetry ratio obtained for different body parts in 133 normal subjects.
ENFD: Epidermal Nerve Fiber Density.
LIMITATIONS
As with any other diagnostic method, the use of NSB for investigation of the peripheral nerve system has some limitations. First of all, it is important to mention that NSB is surely not useful for the diagnosis of entrapment neuropathies, particularly in their early phases when only large diameter fibers are involved. Another important limitation is that NSB is a time-consuming method; calculating the time for specimen processing and the manual counting of two blinded investigators and the time for obtaining a reliable medical report, it is usually not less than two weeks. Finally, NSB is currently confined to a small number of specialized diagnostic units.
CONCLUSION
The demonstration of a lesion or a disease involving the somatosensory system is mandatory for the diagnosis of definite neuropathic pain and objective diagnostic tools play an important role to reach the aim. To this end, several methods are currently available but none is suitable for every disease (or lesion). NSB can be an important diagnostic method for the demonstration of peripheral nervous system involvement, with a special reference to small fiber neuropathies and to peripheral nerves not evaluated by other tests. For these characteristics, NSB can be considered a precious instrument for the diagnosis of peripheral unilateral neuropathic pain.
ACKNOWLEDGMENTS
The author would like to thank the neuropathophysiology technicians Michela Canti and Rosa Bagnasco for their essential role in drafting the paper and Dr. Anna Maria Gatti for helping with the preparation of figures.
P- Reviewers: Alimehmeti R, Cho SY, Trohman RG S- Editor: Qi Y L- Editor: Roemmele A E- Editor: Wu HL