Published online Nov 16, 2014. doi: 10.12998/wjcc.v2.i11.705
Revised: September 23, 2014
Accepted: October 14, 2014
Published online: November 16, 2014
Processing time: 194 Days and 17.3 Hours
AIM: To evaluate the effect of chemotherapy to the acute toxicity of a hypofractionated radiotherapy (HFRT) schedule for breast cancer.
METHODS: We retrospectively analyzed 116 breast cancer patients with T1, 2N0Mx. The patients received 3-D conformal radiotherapy with a total physical dose of 50.54 Gy or 53.2 Gy in 19 or 20 fractions according to stage, over 23-24 d. The last three to four fractions were delivered as a sequential tumor boost. All patients were monitored for acute skin toxicity according to the European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group criteria. The maximum monitored value was taken as the final grading score. Multivariate analysis was performed for the contribution of age, chemotherapy and 19 vs 20 fractions to the radiation acute skin toxicity.
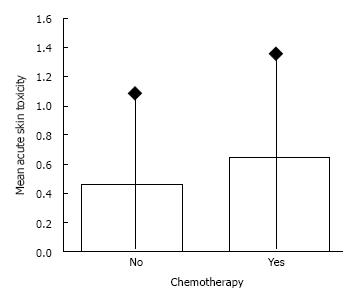
RESULTS: The acute radiation induced skin toxicity was as following: grade I 27.6%, grade II 7.8% and grade III 2.6%. No significant correlation was noted between toxicity grading and chemotherapy (P = 0.154, χ2 test). The mean values of acute toxicity score in terms of chemotherapy or not, were 0.64 and 0.46 respectively (P = 0.109, Mann Whitney test). No significant correlation was also noted between acute skin toxicity and radiotherapy fractions (P = 0.47, χ2 test). According to univariate analysis, only chemotherapy contributed significantly to the development of acute skin toxicity but with a critical value of P = 0.05. However, in multivariate analysis, chemotherapy lost its statistical significance. None of the patients during the 2-years of follow-up presented any locoregional relapse.
CONCLUSION: There is no clear evidence that chemotherapy has an impact to acute skin toxicity after an HFRT schedule. A randomized trial is needed for definite conclusions.
Core tip: The adjuvant radiotherapy for early breast cancer after lumpectomy is an established treatment. Hypofractionation is an attractive approach and the trend nowadays towards new techniques involving hypofractionation is huge, mainly due to the long waiting lists, patients’ desire for fast treatment, better planning of radiotherapy with computed tomography-based target definition and better dose homogeneity assured by 3D conformal planning. The aim of the current study is to evaluate the potential effect of previous chemotherapy to the acute skin toxicity and the local control followed for 2 years in patients with breast cancer, treated with hypofractionated radiotherapy regimen.
- Citation: Kouloulias V, Zygogianni A, Kypraiou E, Georgakopoulos J, Thrapsanioti Z, Beli I, Mosa E, Psyrri A, Antypas C, Armbilia C, Tolia M, Platoni K, Papadimitriou C, Arkadopoulos N, Gennatas C, Zografos G, Kyrgias G, Dilvoi M, Patatoucas G, Kelekis N, Kouvaris J. Adjuvant chemotherapy and acute toxicity in hypofractionated radiotherapy for early breast cancer. World J Clin Cases 2014; 2(11): 705-710
- URL: https://www.wjgnet.com/2307-8960/full/v2/i11/705.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v2.i11.705
The adjuvant radiotherapy (RT) for early breast cancer after lumpectomy is an established treatment. The most widely used schedule for whole breast irradiation is 50 Gy in 25 fractions (conventional), while randomized trials comparing conventional radiotherapy schedules to different hypofractionation, have shown equivalent results[1]. A lot of shorter (accelerated hypofractionated) RT schedules have been already used in clinical practice[2-6]. Hypofractionation is an attractive approach and the trend nowadays towards new techniques involving hypofractionation is huge, mainly due to the long waiting lists, patients’ desire for fast treatment, better planning of radiotherapy with computed tomography (CT)-based target definition and better dose homogeneity assured by 3D conformal planning.
However it is quite difficult to compare the treatment outcome due to the variation of clinical parameters, such as patient selection, chemo/hormonotherapy, differences in breast size, radiation dosimetry and RT techniques[2-6].
The aim of the current paper is to evaluate the potential effect of previous chemotherapy to the acute skin toxicity and to the local control followed for 2 years in breast cancer patients irradiated with this hypofractionated regimen.
One hundred sixteen patients were retrospectively selected, between May 2004 and December 2010. Patients characteristics are shown in details in Table 1. All patients received radiotherapy with a total prescription dose of 50.54 Gy or 53.2 Gy by 2.66 Gy per fraction, in 19 or 20 fractions, over 23-24 d. The decision of giving either 19 or 20 fractions was made in terms of stage (T1, 2) or in case maxima in dose distributions more than 108%. The last three to four fractions were delivered as a sequential tumor boost. The patients were irradiated either at the Radiotherapy unit of the 1st Department of Radiology in ATTIKON University Hospital or at the Radiotherapy Unit of the 2nd Department of Radiology in Aretaieion University Hospital[7,8]. However, the follow-up was realized in several departments either in Athens or Larisa.
| Age median (range) | 58.5 (35-86) |
| T1 | 81 |
| T2 | 35 |
| Chemotherapy | |
| Yes | 83 |
| No | 33 |
Inclusion criteria in this study were breast cancer patients with stage I-II invasive carcinoma after conservative surgery and axillary lymph node dissection. Any adjuvant chemotherapy had to be completed before the start of RT.
The exclusion criteria were: mastectomy, presence of Paget’s disease, presence of autoimmune conditions, previous thoracic neoplasia (cancer, sarcoma, lymphoma), previous breast cancer operated with bad cosmesis, diagnosis of previous or concomitant malignancies or skin disease, breast size in craniocaudal dimension more than 20 cm (or alternatively less than 2500 mL) and presence of psychiatric or addictive disorders[7].
All patients were monitored for acute skin toxicity according to the European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group (EORTC/RTOG) criteria, during radiotherapy schedule once per week and one month thereafter[9]. The maximum monitored value was taken as the final grading score. The primary outcome measure was radiation induced acute skin toxicity. The secondary end point was the local recurrence free survival. Clinical and laboratory tests suggested recurrent disease were investigated, while the criterion for local disease recurrence was recurrent tumor within the treated irradiated field. Hormonal therapy, if prescribed according to indications, was administered after the completion of radiotherapy.
Patients underwent standard CT simulation in the supine position. The ipsilateral breast and tumor bed with surgical clips were contoured for the delineation of Clinical Target Volumes (CTV), while contralateral breast, left and right lung and heart were contoured as organs at risk (OARs)[10]. When surgical clips were not present, preoperative mammography and ultrasound data were used for tumor bed definition. The planning target volume of the tumor bed (PTVt) was a 1-2 cm expansion around the clinical target volume (CTV). The ipsilateral breast volume was the planning target volume (PTVB), excluding the chest wall and 0.5 cm from the skin[10].
We used linear-quadratic (LQ) model in order to assess the equivalent of hypofractionation schedules to the Normalised Total Dose (NTD) if delivered in conventional scheme of 2 Gy per fraction[11-16]:
NTD = Dnew [(dnew + α/β)/(2 + α/β)]
where Dnew and dnew are the total dose and dose per fraction for the hypofractionated schedule, respectively. Normalized Total Dose - NTD has been calculated and tabulated for both breast (α/β = 4 Gy) and acute reacting tissues (α/β = 10 Gy)[11-16]. When considering that α/β = 4, the NTD was 56.10 Gy and 59.05 Gy for 19 and 20 fractions, respectively. When considering that α/β = 10, the NTD was 53.3 Gy and 56.13 Gy for 19 and 20 fractions, respectively.
We used the QUANTEC trial for the dose constrains, as described below, concerning NTD values for an α/β = 3 (late reacting tissues)[17,18]: (1) Ipsilateral lung: < 15% of lung should receive less than 30% of prescribed dose; (2) Heart (left sided breast): Volume of heart getting 5% of dose (V5) should be less than 40%; (3) Heart (right sided breast): < 5% of heart should receive less than 5% of the prescribed dose; (4) Contralateral breast: should receive less than < 3% of prescribed dose to any point; and (5) Contralateral lung: < 15% of lung should receive less than 5% of prescribed dose.
Patients with axillary nodal metastases received adjuvant systemic treatment. Concerning the premenopausal women, two schedules were used: 62 patients received 4 cycles of epirubicin and endoxan every 2 wk, 3 wk brake and then 4 cycles taxotere every 3 wk; 54 patients received 6 cycles of cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) chemotherapy iv every 21 d. Postmenopausal patients received also tamoxifen 20 mg daily for 5 years, after the completion of radiotherapy. All patients received irradiation in a time post chemotherapy ranged 25-45 d.
The comparison of mean value of toxicity score between patients undergone adjuvant chemotherapy vs no chemotherapy was done with the Mann Whitney non-parametric test. The correlation of the incidence of toxicity grading with either the administration of chemotherapy or the prescribed schedule of 19 vs 20 radiotherapy fractions was performed with the χ2 test. The impact of age, chemotherapy and total dose to the radiation induced acute skin toxicity was performed with the logistic linear regression analysis in two steps: first all variables were entered in the equation as a univariate analysis; second only variables with a statistical significance were entered in a multivariate model. The significance level was set at 0.05. All the analysis was performed using the SPSS ver. 10 software (IL, United States).
Thirty three patients underwent a radiotherapy schedule of 19 fractions while 83 underwent schedule of 20 fractions. Overall, acute radiation induced skin toxicity, according to European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group criteria, was as following: grade I 27.6%, grade II 7.8% and grade III 2.6%. The acute radiation induced toxicity score in details is shown in Table 2. No treatment interruption was occurred since no skin toxicity more than grade 3 was noted. No significant correlation was noted between toxicity grading and chemotherapy (P = 0.154, χ2 test). The mean values of acute toxicity score in terms of chemotherapy or not, were 0.64 and 0.46 respectively (Figure 1). No significant difference was noted (P = 109, Mann Whitney test). No significant correlation was also noted between acute skin toxicity and radiotherapy fractions (P = 0.47, χ2 test). The logistic regression analysis performed in two steps is shown in Table 3. According to univariate analysis, only chemotherapy contributed significantly to the development of acute skin toxicity but with a critical value of 0.05. However, in multivariate analysis chemotherapy lost its statistical significance.
| EORTC/RTOG radiation induced acute skin toxicity grade | Total | |||||
| 0 | 1 | 2 | 3 | |||
| Chemotherapy | No | 56/83 (67.5%) | 18/83 (21.7%) | 7/83 (8.4%) | 2/83 (2.4%) | 83 |
| Yes | 16/33 (48.5%) | 14/33 (42.4%) | 2/33 (6.0%) | 1/33 (3.0%) | 33 | |
| Total | 72/116 (62.1%) | 32/116 (27.6%) | 9/116 (7.8%) | 3/116 (2.6%) | 116 | |
| Univariate analysis | Multivariate analysis | |||||
| P | RR | 95%CI | P | RR | 95%CI | |
| Age | 0.31 | - | - | 0.41 | - | - |
| Chemotherapy | 0.05 | 2.35 | 1.01-5.52 | 0.057 | - | - |
| 19 vs 20 fractions | 0.55 | - | - | 0.66 | - | - |
None of the patients during the 2-years of follow-up presented with any locoregional relapse. The acute radiation skin toxicity decreased rapidly after the completion of radiotherapy. Three months post irradiation, 107 out of 116 (92.2%) patients presented grade 0 of skin toxicity, while 9 out of 116 (7.7%) presented only grade I acute skin toxicity.
The linear quadratic (LQ) is a well established model that provides assessments of equivalent doses to both tumor and normal tissues[11-17].
Biological factors related to proliferation (overall time and delayed after irradiation), and the effect of dose per fraction, are basic knowledge necessary for the planning of new irradiation schedules which are effective in practice[11-16].
In our institution we have already reported on the efficacy of the certain hypofractionated schedule for breast cancer[7,8]. Moreover we have made a thorough dosimetric analysis for the dose deposited at the contralateral breast[19]. However, this is the first study according to our knowledge, evaluating the impact of adjuvant chemotherapy to the skin toxicity for a hypofractionated irradiation schedule for breast cancer. In univariate analysis the parameter of chemotherapy seems to have a significant impact to the radiation induced skin toxicity with a critical value of 0.05. However, in multivariate analysis chemotherapy lost its statistical significance. Thus eventually neither chemotherapy, nor the age and the total dose seemed to have any impact to acute skin toxicity.
Sanguineti et al[20] investigated whether chemotherapy administered at earlier or concomitant with radiotherapy, has an impact either to the RT duration or to the hematological profile. The RT schedule was consisted of 50 Gy in five weeks. The investigators concluded that there is no correlation in terms of toxicity between chemotherapy dose-density and dose-intensity of RT. However, the concomitant administration of chemotherapy and RT decreases the ability of prescribing a full irradiation scheme. The only toxicity observed was in white blood cells (WBC). The toxicity on late responding tissues with the combination of hypofractionation and chemotherapy was not investigated. In terms of multivariate analysis, no significant correlation was assessed between skin toxicity and weekly dose rate, while the analysis of potential factors associated with skin toxicity was not a subject of this study[20].
According to current literature concerning clinical guidelines and randomized trails, Hypofractionated RT in breast cancer patients offers equivalent outcome to the standard conventional schedule, in terms of tumor control and normal tissue damage[21-24]. In clinical practice the most commonly used schedule of 2.66Gy in 16 fractions is equivalent to 50 in 2.0Gy fractions, when the α/β value is equal to 3Gy. Any potential loss of therapeutic ratio (2.9 Gy loss of anti-tumor dose) would be compensating with the shorted treatment time due to reduced tumor repopulation and either adjuvant or neo-adjuvant chemotherapy[21].
One of the trials, published in 2002 by Whelan et al[23], compared a schedule of of 42.5 Gy in 16 fractions over 22 d (accelerated arm 266 Gy/fraction) with conventional breast irradiation consisted of 50 Gy in 25 fractions over 35 d (2 Gy/fraction). No boost was added. The randomized women had invasive breast cancer, free resection margins, uninvolved axillary lymph nodes. After 69 mo (more than 5 years) follow up the randomized trial determined that the accelerated arm was as effective as the conventional arm concerning the two outcomes- local control and cosmetic results. As it was obvious in the long term results, published in 2010, the local recurrence rate at 10 years was 7.5% in the conventional group as compared with 7.4% in the accelerated group[24]. The survival rate at 10 years was equivalent in both arms by means of 84.4% in the conventional group vs 84.6% in the accelerated group, while cosmetic outcomes were also similar concerning a rate of 4% or less grade 3 radiation induced toxicity[24].
The 5 year results of two big randomized trials - the United Kingdom Standardisation of Breast Radiotherapy (START) Trial A and START Trial B have been also reported[25,26]. START Trial A[25] compared each of two schedules of hypofractionation 41.6 Gy or 39 Gy in 13 fractions of 3.2 Gy or 3.0 Gy over 5 wk with conventional whole-breast irradiation and START Trial B[26] compared 40 Gy in 15 fractions of 2.67 Gy over 3 wk with conventional irradiation 50 Gy in 25 fractions of 2 Gy. The interpretations of the results from the two trials were that the hypofractionation schedules offered similar rates of tumour control and normal tissue damage as the international standard fractionation schedule of 50 Gy in 25 fractions. The endpoints of both studies at term of tumor relapse, late normal tissue effects, and quality of life were at least as favorable as the standard schedule. Boost irradiation was according to protocol guidelines in both START trials, while adjuvant chemotherapy was used more widely than in Whelan et all trial[23], while up to nowadays follow-up, no significant increase in toxicity has been reported. Zygogianni et al[8] in a previous study, at the end of RT reported 24.1% of grade I and 9.3% of grade II acute skin toxicity, while 66.7% of the patients showed no radiation induced skin morbidity. In this study the results are equivalent with 27.6% and 7.8% of grade I and II skin toxicity, respectively.
Dorn et al[27] studied the skin toxicity in large breasts by an hypofractionated schedule of 42.56 Gy in 2.66 Gy per fraction. Of the 80 treated patients with large breasts, the maximum acute skin toxicity was mild erythema or hyperpigmentation in 70.0%, dry desquamation in 21.25% and focal moist desquamation in 8.75%. Maximum acute toxicity occurred after the completion of radiation in 31.9% of patients. Breast volume was the only patient-related factor significantly associated with moist desquamation on multivariable analysis (P = 0.01). Patients with breast volume > 2500 mL showed focal moist desquamation in 27.2% of cases vs 6.34% in patients with breast volume < 2500 mL (P = 0.03). In our case, according to eligibility criteria, all patients had a breast volume less than 2500 mL.
In another study referring to 44 patients with primary stage breast cancer after adjuvant chemotherapy and hypofractionated RT, Zygogianni et al[28] reported a significantly acute skin toxicity when the intermediate time between chemotherapy and RT was less than 20 d (P < 0.05). All patients in this study received irradiation 25 d at the minimum after chemotherapy.
Although, all the above mentioned trials studied skin toxicity according to the hypofractionated schedule, none of them explored the impact of chemotherapy on acute skin morbidity. According to our results, the hypofractioned radiotherapy for breast cancer is safe in terms of mild toxicity, independently with the sequential chemotherapy, if administered. Our acute toxicity is in accordance with the reported values in all previous published studies. Obviously, the maximum grade of skin toxicity was noted during the whole breast irradiation and not during the boost radiotherapy. On the other hand, it seems that chemotherapy might not be a major factor affecting the radiation induced morbidity. However, due to the retrospective nature of our study, it is difficult to extract safe conclusions, while a randomized prospective study is needed to answer the question: has chemotherapy a definite impact to radiation induced morbidity if a hypofractionated schedule is used? Consequently, the question is still open.
We want to thank the following technologists: Nazos J, Aivalioti A, Tsioumas J, Bakouras G, Darakis G, Dempegioti A, Vergetidou M, Proiskas C and Christogiannis N.
The Hypofractionated irradiation for breast cancer patients has been involved in the routine clinical practice for several years. However, the impact of previous chemotherapy to radiation induced toxicity has not been studied thoroughly.
No clear evidence that chemotherapy has an impact to radiation induced skin toxicity has been noted.
This is a retrospective study documenting the absence of clear impact of previous chemotherapy to Hypofractionated radiotherapy for breast cancer.
Clinicians that decide to use hypofractionated irradiation for breast cancer may prescribe this schedule independently to previous chemotherapy.
Hypofractionated: irradiation schedule with more than 200 cGy per fraction.
In this study, the authors evaluated the impact of chemotherapy to the acute toxicity of a hypofractionated irradiation schedule for breast cancer. Delivering postoperative radiotherapy in a shorter time could effectively be much more convenient for patients and several clinical randomized trials have shown that hypofractionated adjuvant radiotherapy in breast cancer offers similar rates of tumour control and normal tissue damage as the standard schedule.
P- Reviewer: Arcangeli S, Gehmert S S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ
| 1. | Owen JR, Ashton A, Bliss JM, Homewood J, Harper C, Hanson J, Haviland J, Bentzen SM, Yarnold JR. Effect of radiotherapy fraction size on tumour control in patients with early-stage breast cancer after local tumour excision: long-term results of a randomised trial. Lancet Oncol. 2006;7:467-471. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 409] [Cited by in RCA: 414] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 2. | Holloway CL, Panet-Raymond V, Olivotto I. Hypofractionation should be the new ‘standard’ for radiation therapy after breast conserving surgery. Breast. 2010;19:163-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 3. | Khan A, Haffty BG. Hypofractionation in adjuvant breast radiotherapy. Breast. 2010;19:168-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 4. | Shelley W, Brundage M, Hayter C, Paszat L, Zhou S, Mackillop W. A shorter fractionation schedule for postlumpectomy breast cancer patients. Int J Radiat Oncol Biol Phys. 2000;47:1219-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 78] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 5. | Yarnold J, Bloomfield D, LeVay J. Prospective randomized trial testing 5.7 Gy and 6.0 Gy fractions of whole breast radiotherapy in women with early breast cancer (FAST) trial. Clin Oncol. 2004;16:S30. |
| 6. | Martin S, Mannino M, Rostom A, Tait D, Donovan E, Eagle S, Haviland J, Yarnold J. Acute toxicity and 2-year adverse effects of 30 Gy in five fractions over 15 days to whole breast after local excision of early breast cancer. Clin Oncol (R Coll Radiol). 2008;20:502-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 7. | Zygogianni AG, Kouvaris JR, Kouloulias V, Armpilia C, Antypas C, Vlachos L. Hypofractionated accelerated irradiation for stage I-II breast carcinoma: a phase II study. Breast J. 2010;16:337-338. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 8. | Zygogianni AG, Kouloulias V, Armpilia C, Balafouta M, Antypas C, Kouvaris JR. The potential role of hypofractionated accelerated radiotherapy to cosmesis for stage I-II breast carcinoma: a prospective study. J BUON. 2011;16:58-63. [PubMed] |
| 9. | Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys. 1995;31:1341-1346. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3186] [Cited by in RCA: 3499] [Article Influence: 116.6] [Reference Citation Analysis (0)] |
| 10. | International Commission on Radiation Units and Measurements (ICRU) - Report 62. Prescribing, recording, and reporting photon beam therapy (Supplement to ICRU Report 50). Bethesda, MD: ICRU 1999; Available from: http://www.icru.org/home/reports/prescribing-recording-and-reporting-photon-beam-therapy-report-62. |
| 11. | Fowler JF. The linear-quadratic formula and progress in fractionated radiotherapy. Br J Radiol. 1989;62:679-694. [PubMed] |
| 12. | Fowler JF, Tomé WA, Fenwick JD, Mehta MP. A challenge to traditional radiation oncology. Int J Radiat Oncol Biol Phys. 2004;60:1241-1256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 220] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 13. | Barendsen GW. Dose fractionation, dose rate and iso-effect relationships for normal tissue responses. Int J Radiat Oncol Biol Phys. 1982;8:1981-1997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 819] [Cited by in RCA: 771] [Article Influence: 17.9] [Reference Citation Analysis (0)] |
| 14. | Dale RG. The application of the linear-quadratic dose-effect equation to fractionated and protracted radiotherapy. Br J Radiol. 1985;58:515-528. [PubMed] |
| 15. | Thames HD. An ‘incomplete-repair’ model for survival after fractionated and continuous irradiations. Int J Radiat Biol Relat Stud Phys Chem Med. 1985;47:319-339. [PubMed] |
| 16. | Thames HD, Bentzen SM, Turesson I, Overgaard M, Van den Bogaert W. Time-dose factors in radiotherapy: a review of the human data. Radiother Oncol. 1990;19:219-235. [PubMed] |
| 17. | Bentzen SM, Constine LS, Deasy JO, Eisbruch A, Jackson A, Marks LB, Ten Haken RK, Yorke ED. Quantitative Analyses of Normal Tissue Effects in the Clinic (QUANTEC): an introduction to the scientific issues. Int J Radiat Oncol Biol Phys. 2010;76:S3-S9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 902] [Cited by in RCA: 784] [Article Influence: 52.3] [Reference Citation Analysis (0)] |
| 18. | Marks LB, Yorke ED, Jackson A, Ten Haken RK, Constine LS, Eisbruch A, Bentzen SM, Nam J, Deasy JO. Use of normal tissue complication probability models in the clinic. Int J Radiat Oncol Biol Phys. 2010;76:S10-S19. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1352] [Cited by in RCA: 1171] [Article Influence: 78.1] [Reference Citation Analysis (1)] |
| 19. | Tolia M, Platoni K, Foteineas A, Kalogeridi MA, Zygogianni A, Tsoukalas N, Caimi M, Margari N, Dilvoi M, Pantelakos P. Assessment of contralateral mammary gland dose in the treatment of breast cancer using accelerated hypofractionated radiotherapy. World J Radiol. 2011;3:233-240. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 4] [Cited by in RCA: 6] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 20. | Sanguineti G, Del Mastro L, Guenzi M, Ricci P, Cavallari M, Canavese G, Stevani I, Venturini M. Impact of chemotherapy dose-density on radiotherapy dose-intensity after breast conserving surgery. Ann Oncol. 2001;12:373-378. [PubMed] |
| 21. | Yarnold J, Ashton A, Bliss J, Homewood J, Harper C, Hanson J, Haviland J, Bentzen S, Owen R. Fractionation sensitivity and dose response of late adverse effects in the breast after radiotherapy for early breast cancer: long-term results of a randomised trial. Radiother Oncol. 2005;75:9-17. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 360] [Cited by in RCA: 363] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 22. | Whelan TJ, Kim DH, Sussman J. Clinical experience using hypofractionated radiation schedules in breast cancer. Semin Radiat Oncol. 2008;18:257-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 65] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 23. | Whelan T, MacKenzie R, Julian J, Levine M, Shelley W, Grimard L, Lada B, Lukka H, Perera F, Fyles A. Randomized trial of breast irradiation schedules after lumpectomy for women with lymph node-negative breast cancer. J Natl Cancer Inst. 2002;94:1143-1150. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 493] [Cited by in RCA: 460] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 24. | Whelan TJ, Pignol JP, Levine MN, Julian JA, MacKenzie R, Parpia S, Shelley W, Grimard L, Bowen J, Lukka H. Long-term results of hypofractionated radiation therapy for breast cancer. N Engl J Med. 2010;362:513-520. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1204] [Cited by in RCA: 1275] [Article Influence: 85.0] [Reference Citation Analysis (0)] |
| 25. | Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bliss JM, Brown J, Dewar JA, Dobbs HJ, Haviland JS. The UK Standardisation of Breast Radiotherapy (START) Trial A of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet Oncol. 2008;9:331-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 893] [Cited by in RCA: 778] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 26. | Bentzen SM, Agrawal RK, Aird EG, Barrett JM, Barrett-Lee PJ, Bentzen SM, Bliss JM, Brown J, Dewar JA, Dobbs HJ. The UK Standardisation of Breast Radiotherapy (START) Trial B of radiotherapy hypofractionation for treatment of early breast cancer: a randomised trial. Lancet. 2008;371:1098-1107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 950] [Cited by in RCA: 821] [Article Influence: 48.3] [Reference Citation Analysis (0)] |
| 27. | Dorn PL, Corbin KS, Al-Hallaq H, Hasan Y, Chmura SJ. Feasibility and acute toxicity of hypofractionated radiation in large-breasted patients. Int J Radiat Oncol Biol Phys. 2012;83:79-83. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 28. | Zygogianni A, Kouloulias V, Antypas C, Armpilia C, Kyrgias G, Kouvaris J. The impact of intermediate time between chemotherapy and hypofractionated radiotherapy to the radiation induced skin toxicity for breast adjuvant treatment. Breast J. 2014;20:74-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 0.4] [Reference Citation Analysis (0)] |