Published online Mar 26, 2025. doi: 10.12998/wjcc.v13.i9.99421
Revised: October 15, 2024
Accepted: November 26, 2024
Published online: March 26, 2025
Processing time: 142 Days and 23.6 Hours
Small cell lung cancer (SCLC) is the most malignant type of lung cancer. Even in the latent period and early stage of the tumor, SCLC is prone to produce distant metastases with complex and diverse clinical manifestations. SCLC is most closely related to paraneoplastic syndrome, and some cases present as paraneoplastic peripheral neuropathy (PPN). PPN in SCLC appears early, lacks specificity, and often occurs before diagnosis of the primary tumor. It is easy to be misdiagnosed as a primary disease of the nervous system, leading to missed diagnosis and delayed diagnosis and treatment.
This paper reports two cases of SCLC with limb weakness as the first symptom. The first symptoms of one patient were rash, limb weakness, and abnormal ele
The two cases had PPN and abnormal electromyography, highlighting its cor
Core Tip: Paraneoplastic peripheral neuropathy is more common in patients with small cell lung cancer (SCLC), but the exact role of peripheral nerve symptoms and electromyography (EMG) in SCLC is still the subject of debate and research. At present, there are few reports of SCLC with peripheral nerve injury as the first symptom and EMG results. This article reports two cases of SCLC with limb numbness and weakness as the initial symptoms, highlighting the potential role of paraneoplastic peripheral nerve status and EMG in SCLC screening and the possible clinical correlation between them.
- Citation: Luo M, Lu XX, Meng DY, Hu J. Small cell lung cancer with peripheral neuropathy as the first symptom: Two case reports. World J Clin Cases 2025; 13(9): 99421
- URL: https://www.wjgnet.com/2307-8960/full/v13/i9/99421.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i9.99421
Lung cancer is the second most common malignancy in the world and has the highest mortality rate, with small cell lung cancer (SCLC) accounting for 15%–20% of all lung cancers[1,2]. SCLC is a poorly differentiated neuroendocrine mali
At present, there are few studies on peripheral neuropathy in paraneoplastic syndromes, and most of them are electromyographic manifestations after diagnosis. In electromyographic studies of PPN patients diagnosed with lung cancer or other cancers, PPN often is axonal degeneration, which can be accompanied by Waller’s degeneration and demyelination. In the current literature, PPN has rarely been reported as an initial symptom of SCLC, and it is even rarer to obtain electromyography data before diagnosis and at an early stage. Strengthening the understanding of PPN has important guiding significance in clinical diagnosis of primary tumors. This paper reports two cases of SCLC. The patients were diagnosed with whole body rash, limb soreness and lifting difficulty for 1 year, aggravated for 1 month, and numbness and weakness of limbs for several months, facial edema for > 1 month, ineffective antiallergy treatment, were the first symptoms. The nonrespiratory symptoms were the first symptoms, and the EMG results were positive. PPN as the initial symptom of SCLC is rare. This report highlights the clinical correlation between PPN and SCLC, which is conducive to oncology and neurological research and the diagnosis of SCLC patients.
Case 1: A 74-year-old woman was admitted to hospital with whole body rash, limb soreness and lifting difficulty for 1 year, significantly aggravated for 1 mo. The lifting difficulty was not serious at the beginning and did not affect daily life, but the limbs were only slightly lifted at admission.
Case 2: A 70-year-old Chinese man was admitted to hospital with facial edema for > 1 mo.
Case 1: One year ago, the patient presented with patchy skin rash on the frontal face, periorbital region and whole body, accompanied by distension of limbs, which gradually worsened, difficulty in lifting limbs, the degree of which did not affect life, and the rash did not improve after antiallergy treatment. The above symptoms worsened 1 mo ago, with facial edema and inability to walk and hold.
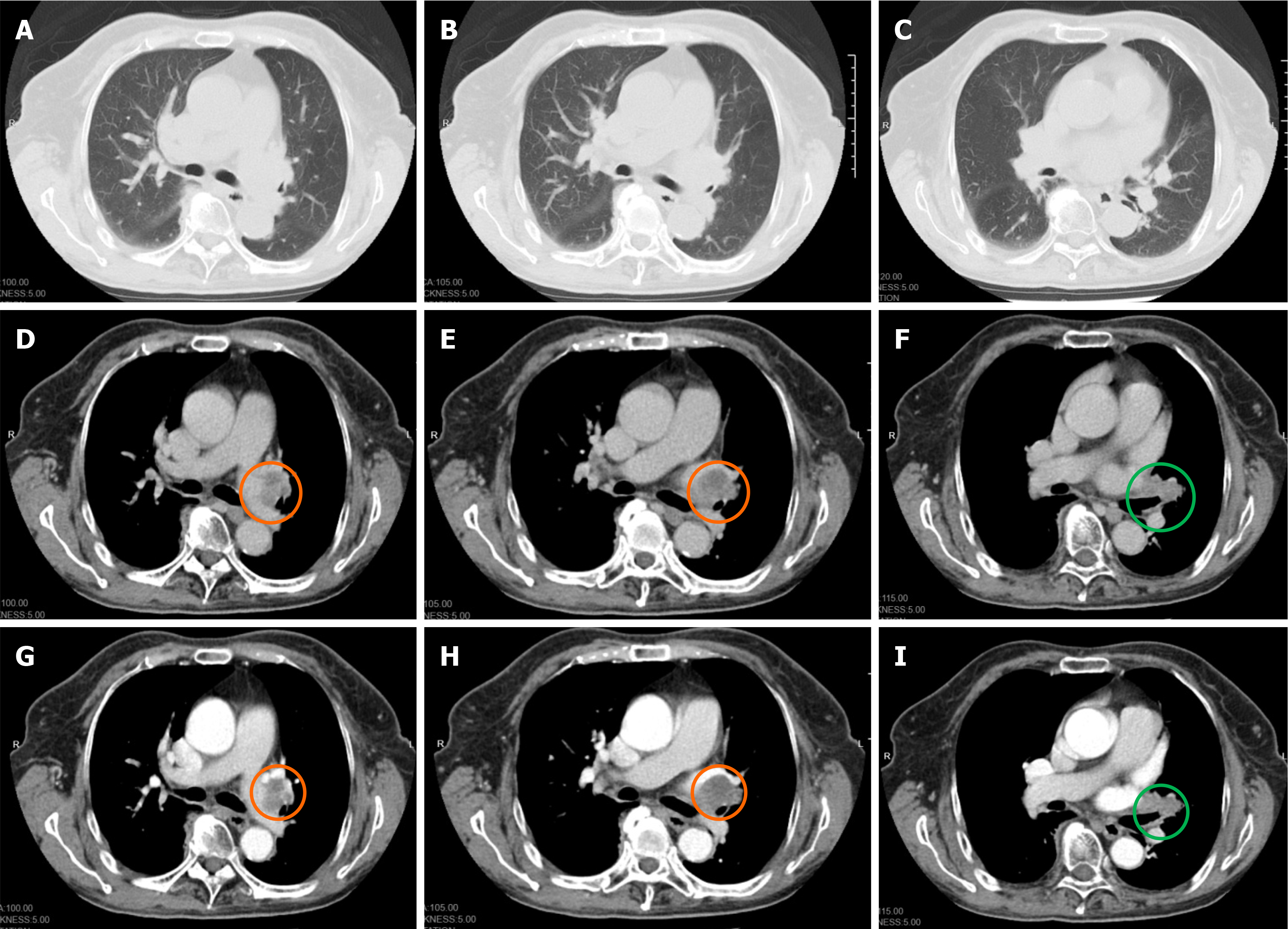
Case 2: One month prior, the patient presented with facial edema, which failed to respond to treatment at the local hospital. Later, the patient went to our hospital for treatment. Chest computed tomography (CT) showed that the anterior superior mediastinum was occupied (about 5.6 cm × 3.2 cm in cross-section) and mediastinal lymph nodes were enlarged. On January 1, 2021, chest enhanced CT (Figure 1) showed multiple mediastinal and bilateral axillary enlargement, fusion of lymph nodes, invasion and narrowing of superior vena cava, and cancerous embolism of the right brachial vein.
Case 1: The patient had a history of hypertension history of > 10 years.
Case 2: The patient had no previous history of similar conditions.
The two patients denied any family history of similar conditions.
Case 1: Vital signs were stable and facial edema was mild. Cardiopulmonary examination showed no abnormalities. Muscle tenderness in the extremities was not abnormal. Muscle strength in the extremities was grade 3 near the extremities. Skin tightness and poor elasticity were observed.
Case 2: There were no abnormalities except facial edema.
Case 1: Bronchoscopic diagnosis on April 4, 2023: immunohistochemical results suggested a poorly differentiated neuroendocrine carcinoma (small cell type). Immunohistochemical results showed: thyroid transcription factor 1(+), cytokeratin 7(+), napsin A(), cytokeratin 5/6(), P40(), chromogranin A(+), synaptophysin(+), Ki67(+, 50%), CD56(+).
Case 2: Pathological report of lymph node transbronchial needle aspiration biopsy specimens in group 4R and group 7 on January 18, 2022: a small number of heterogeneous cell nests were significantly compressed and deformed, and immunohistochemical results suggested that it was poorly differentiated neuroendocrine carcinoma (small cell type): thyroid transcription factor 1(+), cytokeratin 7(), napsin A(), cytokeratin 5/6(), P40(), chromogranin A (+), synaptophysin(+), Ki67(+, 40%), CD3(), and CD20().
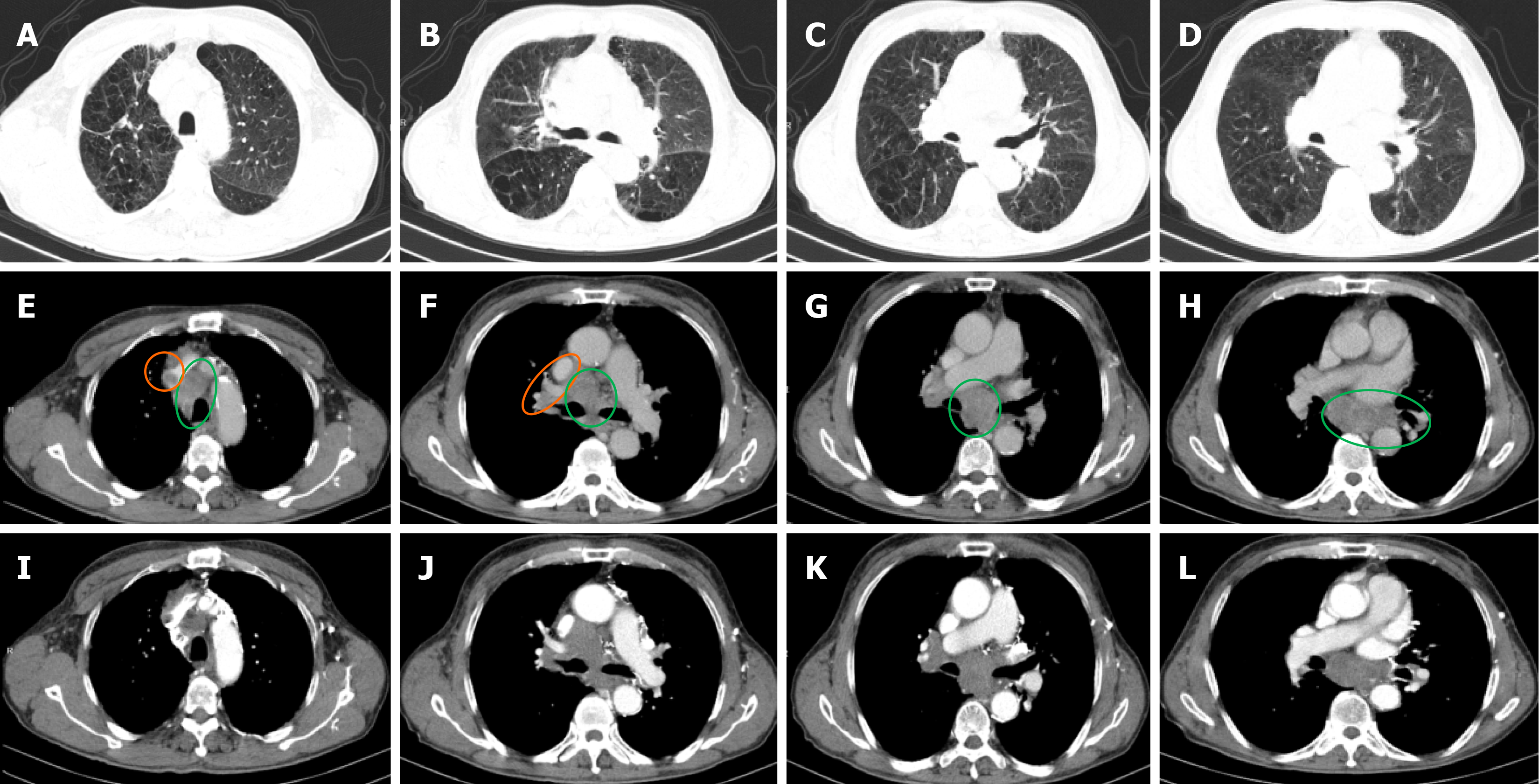
Case 1: Lung CT on April 11, 2023: small nodules in the middle lobe of the right lung. No tumor lesions were observed. Abnormal EMG on April 12, 2023 (Tables 1, 2 and 3): multiple peripheral neuropathies involving axon injury of motor sensory nerve in upper limb. After considering whether the above symptoms were paraneoplastic syndrome, on April 16, 2023 further enhanced lung CT (Figure 2) showed left central lung cancer with left hilar lymph node enlargement.
| Nerve/position | Latent period, ms | Amplitude, μV | Conduction velocity, m/s | Distance, mm |
| L median nerve | ||||
| Finger 3 | 3.23 | 5.8 | 44 | 141 |
| Finger 4 | 3.39 | 7.8 | ||
| R median nerve | ||||
| Finger 3 | 3.18 | 5.5 | 41 | 130 |
| Finger 4 | 3.28 | 4.2 | ||
| L ulnar nerve | ||||
| Finger 5 | 1.93 | 6.5 | 60 | 116 |
| Finger 4 | 1.98 | 6.5 | ||
| R ulnar nerve | ||||
| Finger 5 | 1.93 | 5.3 | 57 | 109 |
| Finger 4 | 2.03 | 6.9 | ||
| R nervi suralis | ||||
| Gastrocnemius | 1.93 | 3.9 | 49 | 95 |
| L nervi suralis | ||||
| Gastrocnemius | 2.55 | 4.3 | 50 | 127 |
| R superficial peroneal nerve | ||||
| Calf | 2.19 | 4.8 | 46 | 100 |
| L superficial peroneal nerve | ||||
| Calf | 2.08 | 4.6 | 43 | 90 |
| Nerve/position | Recording position | Latent period, ms | Amplitude, μV | Conduction velocity, m/s | Distance, mm |
| L median nerve | |||||
| Wrist | Abductor pollicis brevis muscle | 3.8 | 4.26 | ||
| Elbow | Abductor pollicis brevis muscle | 8.07 | 4.03 | 51 | 217 |
| R median nerve | |||||
| Wrist | Abductor pollicis brevis muscle | 4.01 | 4.15 | ||
| Elbow | Abductor pollicis brevis muscle | 8.02 | 3.98 | 50 | 200 |
| L ulnar nerve | |||||
| Wrist | Abductor digiti minimi | 2.97 | 7.24 | ||
| Below the elbow | Abductor digiti minimi | 6.56 | 6.47 | 54 | 195 |
| R ulnar nerve | |||||
| Wrist | Abductor digiti minimi | 2.97 | 7.58 | ||
| below the elbow | Abductor digiti minimi | 6.77 | 7.16 | 53 | 200 |
| R peroneal nerve | |||||
| Ankle | Extensor digitorum brevis | 3.23 | 1.06 | ||
| Under the capitulum of fibula | Extensor digitorum brevis | 10.57 | 0.97 | 41 | 300 |
| L peroneal nerve | |||||
| Ankle | Extensor digitorum brevis | 3.44 | 2.44 | ||
| Under the capitulum of fibula | Extensor digitorum brevis | 10.10 | 1.95 | 44 | 295 |
| R tibial nerve | |||||
| Ankle | Abductor hallucis | 3.59 | 4.17 | ||
| Popliteal fossa | Abductor hallucis | 12.92 | 4.15 | 40 | 370 |
| L tibial nerve | |||||
| Ankle | Abductor hallucis | 3.59 | 4.16 | ||
| Popliteal fossa | Abductor hallucis | 12.66 | 3.05 | 41 | 376 |
| Nerve | Min M reaction time, ms | Mean M reaction time, ms | Min F reaction time, ms | Mean F reaction time, ms | F-wave occurrence rate, % |
| L ulnar nerve | 2.9 | 2.9 | 28.2 | 28.9 | 100 |
| R ulnar nerve | 2.9 | 3.0 | 28.8 | 29.5 | 100 |
| R tibial nerve | 3.6 | 3.7 | 55.0 | 57.8 | 100 |
| L tibial nerve | 3.8 | 3.8 | 53.3 | 57.2 | 100 |
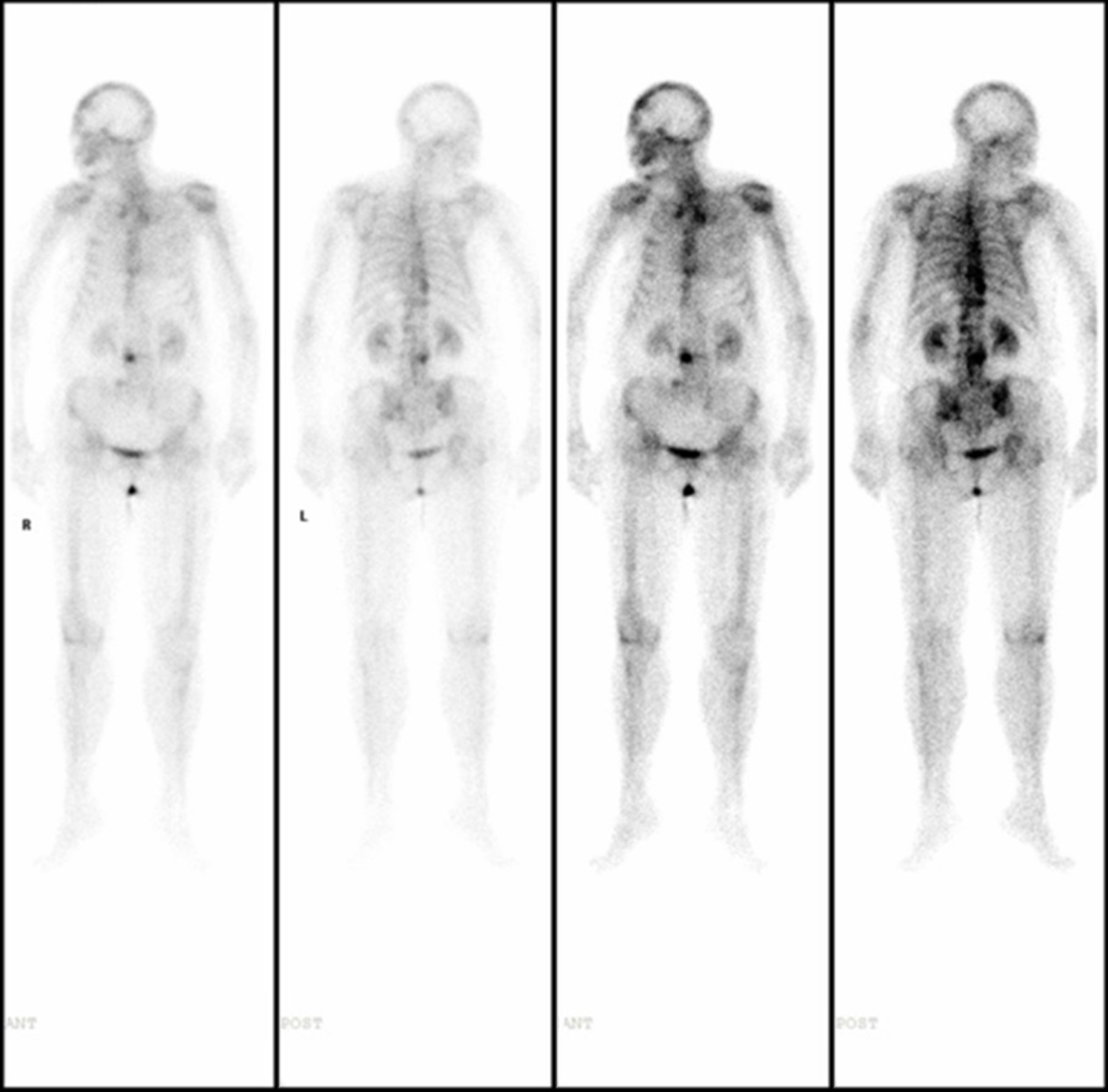
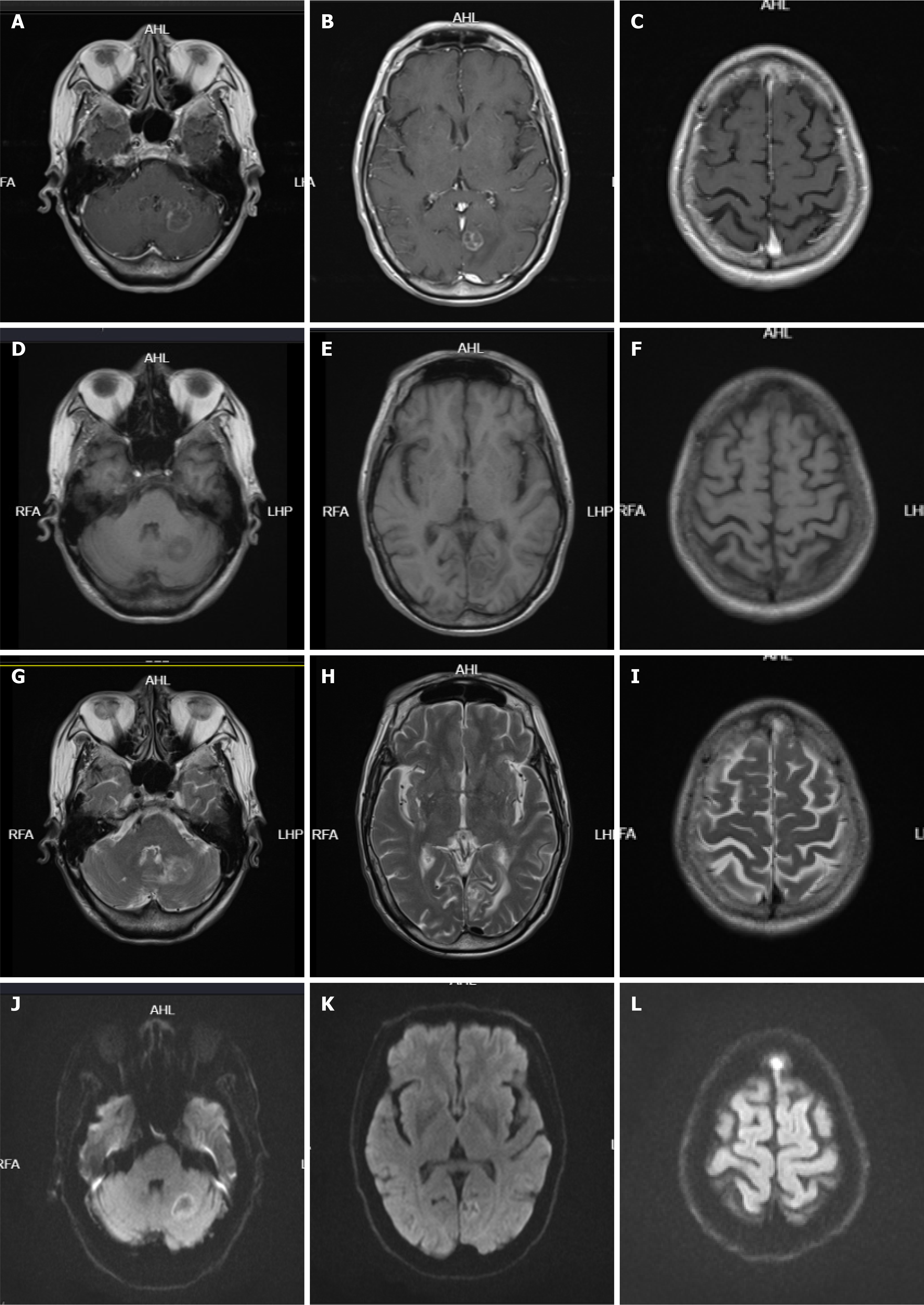
Case 2: On January 1, 2021, chest enhanced CT (Figure 1) showed multiple mediastinal and bilateral axillary enlargement, fusion of lymph nodes, invasion and narrowing of superior vena cava, and cancerous embolism of right brachial vein. No metastatic lesions were found on whole body bone imaging and skull magnetic resonance plain scan + diffusion-weighted imaging.
Differential diagnosis: there was no history of diabetes, rheumatoid arthritis, central nervous system related diseases, and heavy alcohol consumption that could cause numbness and weakness. Both patients’ paraneoplastic antibody profile was sent to other institutions, but the data are now lost, but the PPN was evaluated by a specialist.
The final diagnosis was SCLC (T4N1M0) with PPN.
The final diagnosis was SCLC (T4N3M0) with PPN.
Carboplatin injection 450 mg once and etoposide injection 0.1 g for 3 d antitumor therapy, every 3 wk. The chest lesion radiotherapy started on October 2023.
Carboplatin injection 400 mg once and etoposide injection 0.12 g for 3 d antitumor therapy, every 3 wk. Radical ra
On December 20, 2023, whole-body 18F-fluorodeoxyglucose positron emission tomography/CT imaging (non-dis
This patient had developed numbness and weakness in the extremities several months before the diagnosis and had no previous history of diabetes or lumbar disc herniation. However, the patient did not pay attention. After diagnosis, the numbness and weakness of the limbs were significantly aggravated, and the patient could not walk. On January 18, 2022, EMG showed multiple peripheral nerve damage, sensory nerve accumulation and demyelination damage in upper and lower limbs (Tables 4 and 5). The patient abandoned treatment and died a few days later in January 2022.
| Nerve/position | Latent period, ms | Amplitude, μV | Conduction velocity, m/s | Distance, mm |
| R median nerve | ||||
| Finger 3 | 2.97 | 5.5 | 39 | 117 |
| R ulnar nerve | ||||
| Finger 5 | 2.34 | 5.0 | 44 | 103 |
| L nervi suralis | ||||
| Gastrocnemius | 2.19 | 7.7 | 32 | 70 |
| R nervi suralis | ||||
| Gastrocnemius | 2.34 | 6.0 | 38 | 90 |
| R superficial peroneal nerve | ||||
| Calf | 2.34 | 5.8 | 36 | 85 |
| L superficial peroneal nerve | ||||
| Calf | 2.34 | 5.5 | 34 | 80 |
| Nerve/position | Recording position | Latent period, ms | Amplitude, μV | Conduction velocity, m/s | Distance, mm |
| R median nerve | |||||
| Wrist | Abductor pollicis brevis muscle | 3.33 | 6.0 | ||
| Elbow | Abductor pollicis brevis muscle | 7.50 | 5.5 | 54 | 225 |
| R ulnar nerve | |||||
| Wrist | Abductor digiti minimi | 2.34 | 10.2 | ||
| Below the elbow | Abductor digiti minimi | 6.51 | 9.2 | 54 | 225 |
| R peroneal nerve | |||||
| Ankle | Extensor digitorum brevis | 3.23 | 4.4 | ||
| Under the capitulum of fibula | Extensor digitorum brevis | 9.32 | 3.6 | 42 | 257 |
| L peroneal nerve | |||||
| Ankle | Extensor digitorum brevis | 3.33 | 2.7 | ||
| Under the capitulum of fibula | Extensor digitorum brevis | 9.74 | 2.3 | 41 | 260 |
| L tibial nerve | |||||
| Ankle | Abductor hallucis | 3.44 | 12.6 | ||
| Popliteal fossa | Abductor hallucis | 12.24 | 8.4 | 40 | 350 |
| R tibial nerve | |||||
| Ankle | Abductor hallucis | 3.59 | 14.4 | ||
| Popliteal fossa | Abductor hallucis | 11.82 | 9.8 | 42 | 345 |
SCLC is the most malignant subtype of lung cancer[5,6]. PPN of SCLC appears early, lacks specific clinical manifestations, and often appears before the diagnosis of the primary tumor, which is easy to be misdiagnosed as a primary disease of the nervous system, resulting in missed diagnosis of the tumor[7,8]. The incidence of PPN is high in paraneoplastic syndrome of the nervous system. EMG plays an important role in the diagnosis of PPN. Analysis of nerve electrophysiological characteristics is helpful to distinguish from peripheral neuropathy caused by other causes, and can provide an objective basis for early clinical diagnosis. PPN is seen in a variety of tumors; the most common of which is lung cancer, especially SCLC[9], which starts with subacute onset. The clinical manifestations are numbness, pain, paresthesia, limb weakness, and muscular atrophy at the distal extremity, which progress rapidly, and sensory disorders are more prominent. According to neuro-electrophysiological characteristics, it can be divided into axon injury type, demyelinating type, axon injury type and demyelinating co-existing type.
Prior to the diagnosis of SCLC, both patients presented with clinical manifestations of peripheral nerve injury. Case 1 presented with whole body rash, limb soreness and lifting difficulty, and SCLC was found earlier than in case 2. After antitumor treatment, the patient’s disease progressed but currently has a survival time of 18 mo and she is still alive. After antitumor therapy combined with hormone therapy, dermatomyositis and limb weakness were improved and controlled to a certain extent, which increased the survival. Dermatomyositis has also been shown to be a manifestation of a paraneoplastic syndrome, and the excellent response to treatment in this patient supports this view. Therefore, for patients with unexplained limb numbness and weakness and poor treatment effect of dermatomyositis, we can improve the EMG examination. If the patient has multiple peripheral nerve injury, we can further screen for lung cancer, es
PPN is characterized by a lack of specificity in clinical manifestations and low incidence, and the symptoms of pe
SCLC is the most malignant subtype of lung cancer. PPN of SCLC appears early, lacks specific clinical manifestations, and often appears before the diagnosis of the primary tumor, which is easy to be misdiagnosed as a primary disease of the nervous system, resulting in missed diagnosis of the tumor. Among paraneoplastic neurological syndromes, the incidence of PPN is high. EMG plays an important role in the diagnosis of PPN. It is suggested that more patients with unexplained limb numbness and weakness and risk factors of lung cancer should be further screened by enhanced CT and other examinations for lung cancer, especially SCLC, in order to achieve early detection and diagnosis. Routine EMG screening in patients with suspected SCLC can improve the early detection of PPN and may affect the prognosis of patients.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 64681] [Article Influence: 16170.3] [Reference Citation Analysis (177)] |
| 2. | Huang J, Deng Y, Tin MS, Lok V, Ngai CH, Zhang L, Lucero-Prisno DE 3rd, Xu W, Zheng ZJ, Elcarte E, Withers M, Wong MCS. Distribution, Risk Factors, and Temporal Trends for Lung Cancer Incidence and Mortality: A Global Analysis. Chest. 2022;161:1101-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 114] [Article Influence: 38.0] [Reference Citation Analysis (33)] |
| 3. | Wang Q, Gümüş ZH, Colarossi C, Memeo L, Wang X, Kong CY, Boffetta P. SCLC: Epidemiology, Risk Factors, Genetic Susceptibility, Molecular Pathology, Screening, and Early Detection. J Thorac Oncol. 2023;18:31-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 116] [Article Influence: 58.0] [Reference Citation Analysis (0)] |
| 4. | Alexandrov LB, Ju YS, Haase K, Van Loo P, Martincorena I, Nik-Zainal S, Totoki Y, Fujimoto A, Nakagawa H, Shibata T, Campbell PJ, Vineis P, Phillips DH, Stratton MR. Mutational signatures associated with tobacco smoking in human cancer. Science. 2016;354:618-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 787] [Cited by in RCA: 758] [Article Influence: 84.2] [Reference Citation Analysis (0)] |
| 5. | Wang S, Zimmermann S, Parikh K, Mansfield AS, Adjei AA. Current Diagnosis and Management of Small-Cell Lung Cancer. Mayo Clin Proc. 2019;94:1599-1622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 201] [Cited by in RCA: 197] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 6. | Gazdar AF, Bunn PA, Minna JD. Small-cell lung cancer: what we know, what we need to know and the path forward. Nat Rev Cancer. 2017;17:725-737. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 359] [Cited by in RCA: 528] [Article Influence: 66.0] [Reference Citation Analysis (0)] |
| 7. | Dumansky YV, Syniachenko OV, Stepko PA, Yehudina YD, Stoliarova OY. Paraneoplastic syndrome in lung cancer. Exp Oncol. 2018;40:239-242. [PubMed] |
| 8. | Ernani V, Du L, Ross HJ, Yi JE, Wampfler JA, Schild SE, Xie H, Swanson KL, Tazelaar HD, Yang P. Gastroesophageal reflux disease and paraneoplastic neurological syndrome associated with long-term survival in limited stage small-cell lung cancer. Thorac Cancer. 2022;13:925-933. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 9. | Vogrig A, Gigli GL, Segatti S, Corazza E, Marini A, Bernardini A, Valent F, Fabris M, Curcio F, Brigo F, Iacono D, Passadore P, Rana M, Honnorat J, Valente M. Epidemiology of paraneoplastic neurological syndromes: a population-based study. J Neurol. 2020;267:26-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 109] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 10. | Hill CL, Zhang Y, Sigurgeirsson B, Pukkala E, Mellemkjaer L, Airio A, Evans SR, Felson DT. Frequency of specific cancer types in dermatomyositis and polymyositis: a population-based study. Lancet. 2001;357:96-100. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 757] [Cited by in RCA: 676] [Article Influence: 28.2] [Reference Citation Analysis (0)] |
| 11. | Chen YJ, Wu CY, Huang YL, Wang CB, Shen JL, Chang YT. Cancer risks of dermatomyositis and polymyositis: a nationwide cohort study in Taiwan. Arthritis Res Ther. 2010;12:R70. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 124] [Cited by in RCA: 151] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 12. | Shalata W, Zolnoorian J, Meirovitz A, Sheva K, Jama AA, Saleh OA, Yakobson A. Dermatomyositis Associated with Lung Cancer: A Brief Review of the Current Literature and Retrospective Single Institution Experience. Life (Basel). 2022;13:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 13. | Wang Y, Zou S, Zhao Z, Liu P, Ke C, Xu S. New insights into small-cell lung cancer development and therapy. Cell Biol Int. 2020;44:1564-1576. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 89] [Article Influence: 17.8] [Reference Citation Analysis (0)] |