Published online Mar 16, 2025. doi: 10.12998/wjcc.v13.i8.100889
Revised: October 29, 2024
Accepted: November 22, 2024
Published online: March 16, 2025
Processing time: 97 Days and 0.4 Hours
Acute hyperglycemia due to insulin resistance is common in critically ill patients, typically managed with insulin infusion. However, the occurrence of transient extreme insulin resistance (EIR) requiring exceptional high-dose insulin is rare.
We present the case of a 68-year-old woman with pneumonia who suffered an out-of-hospital cardiac arrest, subsequently developing transient EIR following a new episode of sepsis. Remarkably, insulin resistance rapidly reversed when the insulin infusion rate peaked at 960 units/hour (a total of 18224 units on that day), and it was promptly titrated down to zero upon achieving the target glucose level.
Exceptional high-dose insulin infusion may be required in critically ill patients with stress-related EIR, which is typically transient. Clinicians should be aware of the phenomenon and cautious to avoid hypoglycemia and fluid overload during the steep titration of high-dose insulin infusion.
Core Tip: The occurrence of transient extreme insulin resistance requiring exceptional high-dose insulin is rare, which is typically transient. Clinicians should be aware of the phenomenon and cautious to avoid hypoglycemia and fluid overload during the steep titration of high-dose insulin infusion.
- Citation: Wei XY, Shen HN. Transient extreme insulin resistance in a critically ill patient: A case report. World J Clin Cases 2025; 13(8): 100889
- URL: https://www.wjgnet.com/2307-8960/full/v13/i8/100889.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i8.100889
Hyperglycemia is common in critically ill patients due to a high prevalence of insulin resistance[1]. Current guidelines recommend insulin infusion for critically ill patients with hyperglycemia, targeting blood sugar below 180 mg/dL to optimize recovery[2]. Daily insulin requirement can be used as an indicator of insulin resistance. In healthy individuals, the typical insulin requirement ranges from 0.5 unit/kg/day to 1 unit/kg/day. When an insulin requirement surpasses 3 units/kg/day, it signals extreme insulin resistance (EIR)[3]. In extremely rare instances, patients may require exceptional doses of insulin (> 100 units/hour)[4-6]. However, this phenomenon is typically transient in critically ill patients, and the insulin dosage may be rapidly tapered down once insulin resistance improves[6]. Herein, we present a case of a critically ill patient with transient EIR, receiving a maximum insulin infusion rate of 960 units/hour, to illustrate the rapid reversal of EIR and the associated management.
A 68-year-old woman with hypertension and type 2 diabetes presented with progressive dyspnea and collapsed on the road to the emergency department.
Upon arrival, the initial rhythm showed pulseless electrical activity (PEA). Sustained return of spontaneous circulation was noted after 6 minutes of cardiopulmonary resuscitation. She was then treated with targeted temperature manage
Prior to this admission, she was prescribed with Glimepiride 2 mg per day, Sitagliptin 100 mg per day and Repaglinide 1 mg three times per day for diabetes, but did not take these medications regularly. Her latest HbA1c was 9.3%, and her weight was 65.5 kg, with a body mass index of 27.2 kg/m2.
Personal and family history was unremarkable.
On admission, physical examination revealed purulent tracheal secretions and right lung crackles, without jugular vein distention, cardiac murmur or leg edema.
Initial laboratory tests revealed elevated white blood cell count (30100 per μL), bandemia (10.3%), and markedly elevated procalcitonin (82.21 ng/mL) along with signs of impaired kidney function (blood urea nitrogen: 27 mg/dL, creatinine: 1.37 mg/dL), severe hyperglycemia (glucose: 574 mg/dL), and suspected cardiac injury (high-sensitivity troponin I: 240 pg/mL). Additionally, a mixed respiratory and metabolic acidosis was indicated by elevated lactate (19.0 mmol/L), low potential of hydrogen (6.87), elevated PaCO2 (58.7 mmHg) and low bicarbonate (10.8 mmol/L). Potassium and ketone levels were within normal range. Repeat troponin I levels were elevated and peaked at 26066 pg/mL about 31 hours later. All initial cultures and tests for potential pathogens were negative.
Chest radiograph showed infiltration in the right lung. Electrocardiography showed sinus tachycardia with diffuse ST depression. Echocardiography showed a left ventricular ejection fraction of 47.6% with hypokinesis in the inferior wall.
A diagnosis of pneumonia leading to sepsis, hyperglycemic hyperosmolar state, non-ST elevation myocardial infarction, acute respiratory failure and severe respiratory/metabolic acidosis with PEA was made.
After fluid, insulin and empirical antibiotic therapy, her hemodynamics and hyperglycemia improved in the first few days. However, azotemia progressed and her consciousness did not recover after rewarming from TTM. Hypoxic brain injury was suspected after evaluation through brain images and electroencephalography. Enteral feeding was started. The daily insulin requirement decreased from 108 units on day 1 unit to 22 units on day 3, maintaining a glucose level of 130-200 mg/dL as monitored by finger-stick glucose testing. However, recurrent leukocytosis and hyperglycemia were observed from day 4 onwards, which was later attributed to a newly developed sepsis. Cultures of both the urine and blood grew Candida glabrata. Micafungin was administrated.
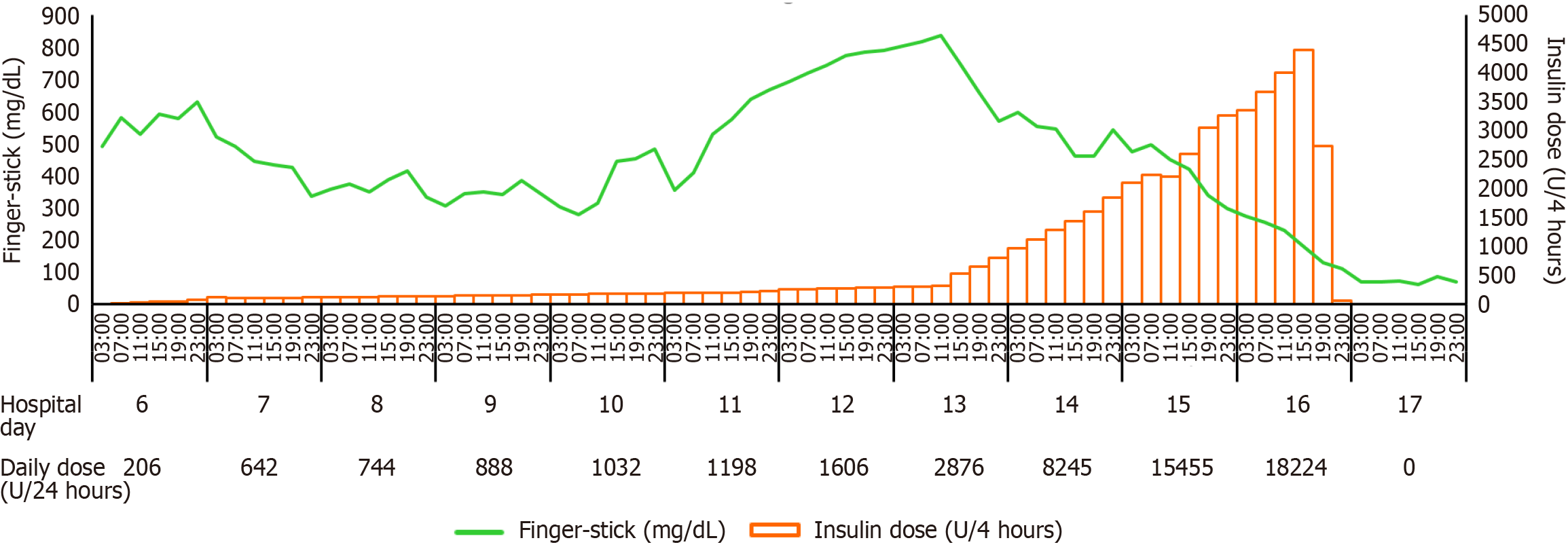
Continuous insulin infusion was started on day 6 when serum osmolality was 427 mOsm/kg (reference range: 275-295 mOsm/kg). Throughout the high-dose insulin therapy, regular monitoring of serum potassium levels was performed in accordance with our institution's insulin treatment protocol. Potassium supplementation was administered as needed to prevent hypokalemia. Hemodialysis was initiated on day 7 due to the development of oliguria and metabolic acidosis. Her glucose level fluctuated after initiating insulin infusion and then continued to rise despite an increasing dosage of insulin (Figure 1). The glucose level reached a maximum of 850 mg/dL on day 13 when the insulin infusion rate was 155 units/hour. The fluid balance from day 1 to day 13 showed a daily gain ranging from approximately 500 mL to 3000 mL, not accounting for insensible losses. A survey for possible causes of insulin resistance was done, and the results were unrevealing (Table 1). To achieve a target glucose range of 150-200 mg/dL and to avoid hypoglycemia, the infusion rate was titrated and the glucose level was monitored hourly. Moreover, the concentration of insulin was changed from 1 unit/mL to 20 units/mL to avoid fluid overload. Norepinephrine was required for unstable hemodynamics since day 15 and dialysis was switched to continuous venovenous hemofiltration since day 16. A reversal of insulin resistance was noticed on day 16 when the insulin infusion rate reached a maximum of 960 units/hour (a total dose of 18224 units on that day). Approximately 8 hours later, the target glucose level was achieved, then the insulin infusion rate was rapidly titrated down to zero (Figure 1). Between day 17 and day 20, her blood glucose levels ranged from 44 mg/dL to 144 mg/dL, with 2-9 episodes/day (total 24 episodes) of mild to moderate hypoglycemia, even when hydrocortisone 200 mg per day was given for vasopressor-dependent septic shock since day 17. Her lowest blood glucose level was 44 mg/dL on day 19, and the glucose level rose up to 180-220 mg/dL with a need of small dosages of insulin after day 21.
| Variables | Values | References |
| Antinuclear antibody | Negative | Negative |
| Peri-nuclear anti-neutrophil cytoplasmic antibody/cytoplasmic anti-neutrophil cytoplasmic antibodies (IU/mL) | 0.3/0.5 | < 5/< 3 |
| C3/C4 (mg/dL) | 60/12.5 | 83-193/15-57 |
| Erythrocyte sedimentation rate (mm/hour) | 60 | 0-20 |
| Anti-double strand DNA antibody (IU/mL) | 1.4 | < 15 |
| Others | ||
| Insulin (μIU/mL)1 | > 600 | < 17 |
| C-peptide(ng/mL)1 | 18.22 | 0.78-5.19 |
| Cortisol (8AM) (ug/dL) | 19.9 | 3.7-19.4 |
| Insulin-like growth factor 1 (ng/mL) | 70.75 | 40-225 |
| Human growth hormone (ng/mL) | 0.55 | < 6.6 |
| Thyroid-stimulating hormone (mIU/L) | 0.23 | 0.17-4.05 |
Despite the control of hyperglycemia, she eventually died of recurrent sepsis on day 25.
The presented case describes a critically ill patient with transient EIR after a new episode of sepsis. This case is note
Insulin resistance is present in 90% of critically ill patients[1]. However, the occurrence of EIR requiring exceptional high-dose insulin is rare. A recent study shows that only 0.35% of intensive care unit patients requiring intravenous insulin ≥ 35 units/hour[7]. Table 2 shows cases reports with EIR requiring a maximal insulin infusion rate > 100 units/hour. The highest insulin infusion rate, 10000 units/hour, was reported in a patient with shock associated with diabetic ketoacidosis[4-6,8-10].
| Ref. | Age/sex | Diagnoses | Highest glucose level (mg/dL) | Target glucose range (mg/dL) | Peak insulin infusion rate (U/hour) | Time to target range (from peak insulin infusion rate) | Duration of insulin infusion | Hypoglycemia | Outcome |
| Naghmi and Kaboor[4], 1988 | 70/F | DKA | > 1000 | < 250 | 200 | 9 hours1 | NA | Yes | NA |
| 40/M | DKA | > 600 | < 250 | 400 | 5 hours1 | NA | Yes | NA | |
| 40/M | DKA | > 500 | < 250 | 1000 | 6 hours1 | NA | Yes | NA | |
| 20/M | DKA | > 600 | < 250 | 200 | 9 hours1 | NA | No | NA | |
| Yokoyama et al[5], 1992 | 65/M | DKA | 1241 | < 300 | 10000 | 6 hours1 | 23 hours | Yes | Survived |
| Surani et al[8], 2012 | 62/F | Septic shock | > 400 | < 200 | 832 | 5 hours | 17 hours | No | NA |
| Oo et al[9], 2013 | 60/M | DKA and AMI | 847 | NA | 120 | NA | NA | NA | Survived |
| Illuri et al[6], 2016 | 64/F | Sepsis | 430 | 180-200 | 142 | 16 hours | 6 days | No | Deceased |
| July et al[10], 2017 | 68/M | AMI, cardiac arrest | 434 | 140-180 | 280 | 24 hours1 | 5 days | No | Deceased |
| The present case | 68/F | Cardiac arrest, sepsis | 850 | 150-200 | 960 | 8 hours | 11 days | Yes | Deceased |
The swift resolution of EIR observed on day 16 is a captivating aspect of this case, indicating a dynamic and potentially reversible process. Examining reported cases, the duration of insulin infusion for EIR varied widely, ranging from 15 hours to 25 days[7]. Consequently, it is improbable that insulin receptor antibodies or insulin autoantibodies are the root cause of EIR, considering its transient nature. Furthermore, no thiol-containing drugs (e.g., reduced glutathione), which are known to induce insulin autoimmune syndrome (IAS), were administered during treatment. As a result, it is unlikely that thiol-induced IAS contributed to the insulin resistance. In addition, low hydrocortisone was administered starting after the reversal of EIR and therefore is unlikely to have influenced its resolution.
The mechanism of EIR in critically ill patients is likely multifaceted, involving various contributing factors[5,7]. First, patient-specific elements such as severity of illness, medication adjustments, and nutritional support impact insulin requirements[7]. Second, critical illnesses induce a stress response, releasing cortisol, catecholamines, and cytokines, leading to impaired glucose metabolism and acute insulin resistance[7]. Third, sepsis and circulatory shock can impede microcirculation, restricting insulin delivery to tissues and contributing to resistance[5]. Fourth, the potential saturation of insulin effect at high infusion rates and post-insulin receptor resistance may play a role, although definitive evidence is lacking[5,7]. Finally, micronutrient deficiency, such as chromium deficiency, may also contribute. For instance, two case reports suggest that chromium infusion decreases insulin needs and improves glucose control in critically ill patients with EIR[8,11].
The optimal target glucose level for critically ill diabetic patients remains uncertain[12]. A recent study challenges the conventional tight glycemic control, proposing a "liberal" approach with a target glucose range of 180-252 mg/dL, which significantly reduces the risk of hypoglycemia compared to the conventional range of 108-180 mg/dL, without a significant difference in 90-day mortality[12]. In our case, despite setting a higher target glucose level (150-200 mg/dL vs 108-180 mg/dL), multiple hypoglycemia episodes still occurred after discontinuation of insulin infusion. In reported cases of EIR, the time to achieve target glucose levels is relatively short, ranging from 5 hours to 24 hours, when the insulin infusion rates peak (Table 2)[4-6,8-10]. In our case, the time to reach the target glucose level after peak insulin infusion rate is only 8 hours (Figure 1). To mitigate the risk of hypoglycemia, adopting a liberal approach with more frequent glucose monitoring (every 30-60 minutes) is considered more suitable[6]. However, understanding the reason behind the onset of hypoglycemia after discontinuation of insulin for over 3 days in our patient poses a challenge, particularly given the brief half-life of intravenous insulin (< 10 minutes)[4].
Fluid balance is critical during high-dose insulin infusion for EIR in critically ill patients. Guidelines suggest using a 1 unit/mL insulin solution[2], but concerns about fluid overload arise beyond 100 units/hour. Notably, prior cases of EIR lack reporting on adjustments to the insulin solution concentration. Our approach involved preemptively adjusting insulin concentration from 1 unit/mL to 20 units/mL when the infusion rate reached 155 units/hour. This adjustment enabled a significant reduction in fluid volume from 960 mL/hour to about 48 mL/hour, ensuring a more controlled administration in the event of higher insulin requirements, up to 960 units/hour.
In conclusion, exceptional high-dose insulin infusion may be required in critically ill patients with stress-related EIR, which is typically transient. Clinicians should be aware of the phenomenon and cautious to avoid hypoglycemia and fluid overload during the steep titration of high-dose insulin infusion.
| 1. | Saberi F, Heyland D, Lam M, Rapson D, Jeejeebhoy K. Prevalence, incidence, and clinical resolution of insulin resistance in critically ill patients: an observational study. JPEN J Parenter Enteral Nutr. 2008;32:227-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Jacobi J, Bircher N, Krinsley J, Agus M, Braithwaite SS, Deutschman C, Freire AX, Geehan D, Kohl B, Nasraway SA, Rigby M, Sands K, Schallom L, Taylor B, Umpierrez G, Mazuski J, Schunemann H. Guidelines for the use of an insulin infusion for the management of hyperglycemia in critically ill patients. Crit Care Med. 2012;40:3251-3276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 367] [Cited by in RCA: 383] [Article Influence: 31.9] [Reference Citation Analysis (0)] |
| 3. | Lane WS, Cochran EK, Jackson JA, Scism-Bacon JL, Corey IB, Hirsch IB, Skyler JS. High-dose insulin therapy: is it time for U-500 insulin? Endocr Pract. 2009;15:71-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 94] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 4. | Naghmi R, Kaboor M. Transient severe insulin resistance in diabetic ketoacidosis (a report of four episodes). Horm Metab Res. 1988;20:584-586. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 5. | Yokoyama H, Wasada T, Shimizu Y, Yoshino H, Hasumi S, Omori Y. Transient extreme insulin resistance in shock during diabetic ketoacidosis. Endocrinol Jpn. 1992;39:571-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.0] [Reference Citation Analysis (0)] |
| 6. | Illuri VD, Layden BT, Aleppo G. Extreme Insulin Resistance in Critically Ill Patient With Sepsis. Clin Diabetes. 2016;34:158-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 7. | Vidger AJ, Czosnowski QA. Outcomes and adverse effects of extremely high dose insulin infusions in ICU patients. J Crit Care. 2021;63:62-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 8. | Surani SR, Ratnani I, Guntupalli B, Bopparaju S. Severe insulin resistance treatment with intravenous chromium in septic shock patient. World J Diabetes. 2012;3:170-173. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 11] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Oo YH, Karam JG, Resta CA. Extreme insulin resistance in a patient with diabetes ketoacidosis and acute myocardial infarction. Case Rep Endocrinol. 2013;2013:520904. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 10. | July M, Santhanam P, Joan C, Khthir RA. Severe Insulin Resistance in the Setting of Therapeutic Hypothermia. MJM. 2017;3:29. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 11. | 11 Via M, Scurlock C, Raikhelkar J, Di Luozzo G, Mechanick JI. Chromium infusion reverses extreme insulin resistance in a cardiothoracic ICU patient. Nutr Clin Pract. 2008;23:325-328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Hermanides J, Egi M. The Optimal Glycemic Control in Patients with Diabetes in the ICU: Where Is the Sweet Spot? Am J Respir Crit Care Med. 2022;206:811-812. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |