Published online Feb 26, 2025. doi: 10.12998/wjcc.v13.i6.98111
Revised: September 12, 2024
Accepted: November 12, 2024
Published online: February 26, 2025
Processing time: 160 Days and 1.2 Hours
Food protein-induced enterocolitis syndrome (FPIES) is the most serious type of non-immunoglobulin E (IgE)-mediated food allergic reaction manifesting as sepsis-like symptom, which can lead to shock. Saccharomyces boulardii (S. boulardii), a probiotic prescribed frequently in clinical settings, has been reported to trigger FPIES in an infant with soy-triggered FPIES. In this report, we describe a new clinical FPIES in which S. boulardii was the sole triggering factor of acute FPIES adverse reaction in seven healthy infants.
Seven FPIES cases triggered by only S. boulardii were gathered from 2011 to the present. None of the patients had previously experienced any allergic reaction to cow’s milk, soy, or complementary food. The age of the patients was 4-10-months old, and the symptoms of FPIES developed after ingestion of S. boulardii, which is mostly prescribed for the treatment of gastroenteritis or antibiotic-associated diarrhea. All patients experienced severe repetitive vomiting 1-3 hours after S. boulardii ingestion. Extreme lethargy, marked pallor, and cyanosis were also observed. No IgE-mediated hypersensitivity developed in any patient. Diarrhea was followed by initial intense vomiting in approximately 5-10 hours after S. boulardii ingestion, and only one case showed bloody, purulent, and foul-smelling diarrhea. The patients stabilized quickly, mostly within 6 hours. Symptoms got all improved within 24 hours after discontinuation of S. boulardii.
S. boulardii can be the sole trigger of acute FPIES and be prescribed cautiously even in healthy children without FPIES.
Core Tip: Saccharomyces boulardii (S. boulardii), one of the most commonly prescribed and generally safe probiotic for children, can trigger food protein-induced enterocolitis syndrome (FPIES) in patients with non-immunoglobulin E (IgE)-mediated or IgE-mediated food allergic reaction. Here, a new FPIES entity of healthy infants is described, in which acute FPIES is triggered solely by S. boulardii without any hypersensitivity to cow’s milk, soy, or complementary foods during follow-up. Those seven cases suggest that S. boulardii should be prescribed cautiously even in healthy children without FPIES to other foods.
- Citation: Hwang JB, Jang HJ. Saccharomyces boulardii as a single trigger of food protein-induced enterocolitis syndrome: Seven case reports. World J Clin Cases 2025; 13(6): 98111
- URL: https://www.wjgnet.com/2307-8960/full/v13/i6/98111.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i6.98111
Food protein-induced enterocolitis syndrome (FPIES) is the most serious type of non- immunoglobulin E (IgE)-mediated food allergy, which manifests as sepsis-like symptoms that can lead to shock[1-4]. FPIES patients can have a single or multiple trigger, which are reported to be various and mostly to be cow’s milk (CM), soy, and grains[5-8].
Hwang et al[9], in 2009, described for the first time, a case of an acute FPIES reaction with the ingestion of the probiotic Saccharomyces boulardii (S. boulardii). The patient was diagnosed with soy-induced FPIES before being diagnosed with S. boulardii-induced FPIES. In the report, the authors suggested caution when prescribing S. boulardii to a patient previously diagnosed with FPIES to other foods.
Here, we report a new FPIES entity in seven healthy infants for the first time ever, which is that S. boulardii can be the sole trigger of acute FPIES in children who does not have any allergic history before. S. boulardii should be prescribed with caution even in healthy children without FPIES to other foods.
Vomiting, lethargy, pallor, and diarrhea after ingestion of S. boulardii (case 1-7) (Table 1).
| Case No. | Age/sex | Feeding at diagnosis | Previous FPIES-like episode including this diagnosis | Vomiting beginning time after S. boulardii ingestion (hour) | Lethargy/pallor | Skin/respiratory symptom | Diarrhea | Increase of absolute neutrophil count above baseline count (cells/mL) | |
| ‘Confirmed’ isolated S. boulardii-FPIES | 1 | 4 months/F | Breast | 5 | 2 | +/+ | -/- | - | 13346 |
| 2 | 10 months/M | Breast/CM | 2 | 3 | +/+ | -/- | + | 6575 | |
| 3 | 6 months/M | Breast | 1 | 1.5 | +/+ | -/- | + | 3162 | |
| ‘Possible’ isolated S. boulardii-FPIES | 4 | 9 months/M | Breast/CM | 2 | 1.5 | +/+ | -/- | + | Nd |
| 5 | 4 months/F | Breast | 4 | 2 | +/+ | -/- | + | Nd | |
| 6 | 7 months/F | Breast | 2 | 1 | +/+ | -/- | + | Nd | |
| 7 | 6 months/F | CM | 3 | 1 | +/+, cyanosis | -/- | +, bloody and purulent | Nd |
We defined isolated S. boulardii-induced FPIES as follows: (1) FPIES symptoms developed after ingestion of S. boulardii in a patient who had not been previously diagnosed with FPIES; (2) Oral food challenge (OFC) with CM and/or soy[1,4,10], which are generally needed at the age to complement breastfeeding or to proceed with complementary food did not result in FPIES symptoms at the age of 6–12 months; and (3) FPIES symptoms did not manifest with the introduction of various solid foods, such as dairy products, legumes (including soybean food), and grains[1,4,10].
Total of seven cases solely induced by S. boulardii were collected for recent twelve years from 2011 at the Dongsan Medical Center, a tertiary university hospital. Informed consent was waived because of the retrospective nature of the study according to the ethics committee and institutional review board of Keimyung University Dongsan Medical Center (No. 2022-05-103).
In our earlier experience, three isolated S. boulardii-induced FPIES patients, who had not been reported anywhere yet, were gone through OFC and reached a diagnosis of ‘confirmed’ S. boulardii-induced FPIES (case 1-3) (Table 1). The latter four cases, based on our earlier experience and the FPIES international consensus guidelines recently published in 2017[1], were diagnosed clinically as “possible” S. boulardii-induced FPIES (case 4-7) (Table 1).
The age of the patients was 4–10-months old, and symptoms of FPIES developed after ingestion of S. boulardii, which was prescribed for the treatment or prevention of infectious gastroenteritis and/or antibiotic-associated diarrhea. At the time of FPIES symptom onset, four patients were exclusively breastfed, two were breastmilk and formula milk-fed, and only one was exclusively formula milk-fed.
Importantly, before the diagnosis of S. boulardii-induced FPIES at our hospital, six of seven patients were found to have one to four FPIES-like reactions. Only one patient was diagnosed with S. boulardii-induced FPIES with the first episode of an FPIES-like reaction. In most cases, S. boulardii-induced FPIES is misdiagnosed as viral gastroenteritis, and this error is repeated and unrecognized by clinicians before reaching the final diagnosis.
No patient had a significant personal or family history of non-IgE-mediated or IgE-mediated food hypersensitivity.
All patients developed intense, repetitive vomiting 1-3 hours after taking S. boulardii, either with OFC or for treatment purposes. All of the cases showed extreme lethargy and marked pallor also, and one case even cyanosis. IgE-mediated allergic reaction such as skin or respiratory hypersensitivity was not developed in any patient. Six patients developed diarrhea in approximately 5-10 hours after S. boulardii ingestion, and only one showed bloody, purulent, and foul-smelling diarrhea (case 1-7) (Table 1).
The positive result of increment in absolute neutrophil count more than 1500 (cells/mL) compared to the baseline[1,10] was observed in three OFC cases.
Abdominal roentgenography and ultrasonography did not reveal any abnormal findings, such as intestinal malrotation.
S. boulardii as a single trigger of FPIES in seven healthy children.
All cases were treated with administration of 20 mL/kg boluses of normal saline and with following maintenance infusion of dextrose saline[1,10]. Patients were stabilized fast mostly within 6 hours.
All symptoms got improved within 24 hours after discontinuation of S. boulardii ingestion.
It could be difficult to clinically assess FPIES quickly and directly if a clinician does not have enough knowledge and experience about the characteristics of FPIES, i.e., the serial FPIES symptoms, the timing of symptom onset, and rapid clinical improvement after excluding triggering food[11,12]. FPIES does not yet have a diagnostic biomarker and is clinically very similar to acute infectious gastroenteritis because it is a non-IgE-mediated allergic reaction without the typical symptoms of the IgE-mediated pathway[1,10,13]. Moreover, FPIES hypersensitivity is not readily assessable as a final diagnosis, especially when the trigger factor is a medication, such as S. boulardii[14], and not food.
To our knowledge, only three cases of hypersensitivity reactions after ingestion of S. boulardii have been reported. One was S. boulardii-induced FPIES that developed in an 8-month-old infant with previously diagnosed soy-induced FPIES[9], and the second was S. boulardii-induced FPIES that developed in a 3-month-old infant with allergic colitis[15]. The third patient was a 6-year-old boy who developed an itchy skin rash after the ingestion of S. boulardii[16]. These cases suggest that caution should be exercised when prescribing S. boulardii in pediatric patients with non-IgE-mediated or IgE-mediated food hypersensitivity. Moreover, our cases are worth emphasizing because they report that S. boulardii alone can trigger FPIES in healthy infants.
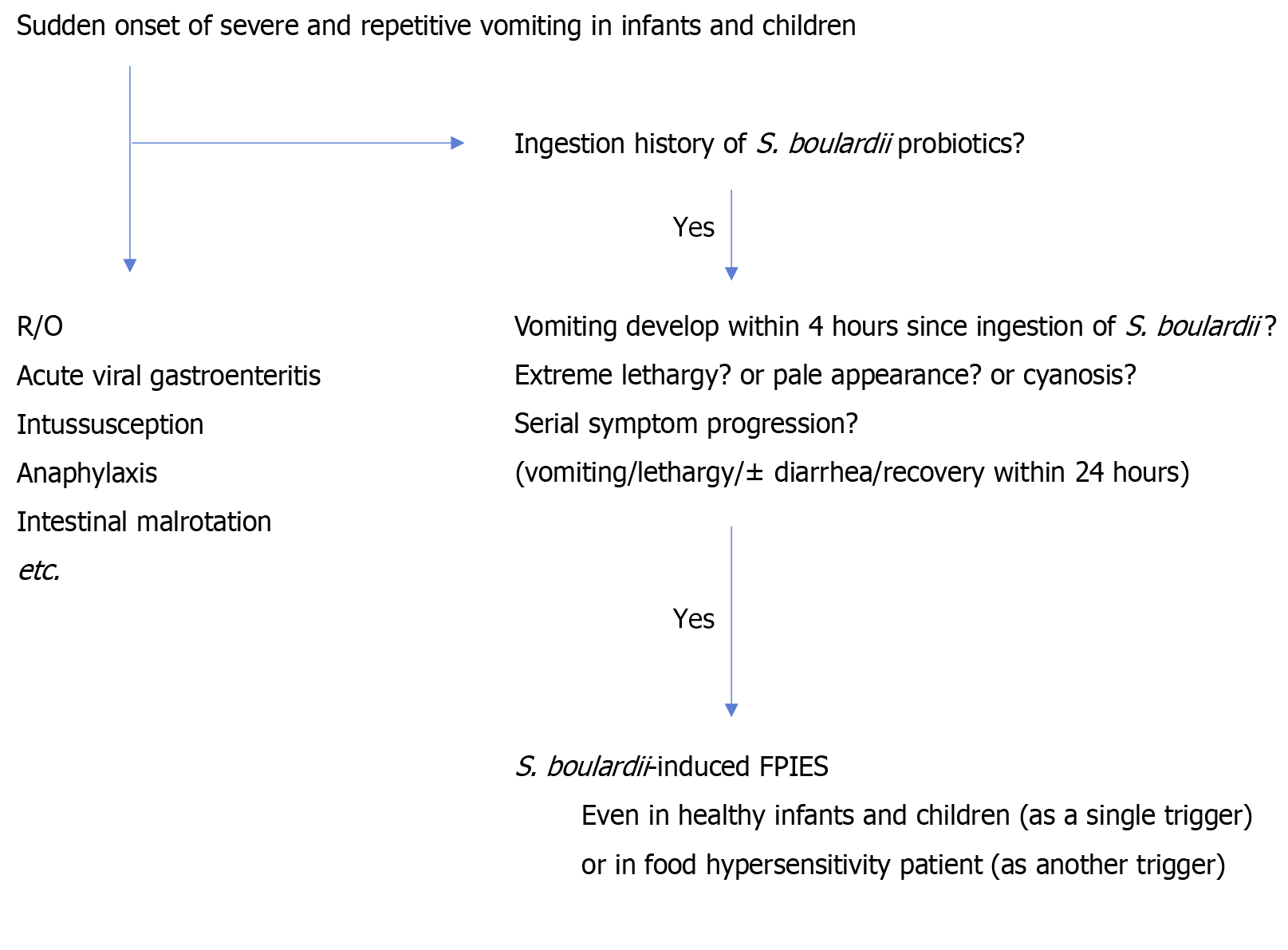
Sudden onset of severe and repetitive vomiting suggests anaphylaxis, viral gastroenteritis, and intussusception etc. in infants and children. If these episodes recur, inborn errors of metabolism or cyclic vomiting syndrome etc. should be considered[1]. To differentiate between these diseases and reach a final diagnosis[17-20] of isolated S. boulardii-induced FPIES, authors suggest using the flow of questions below (Figure 1): (1) Did the patient develop severe vomiting within 4 hours since the initial S. boulardii intake (suggesting the kickoff symptom in serial allergic reactions in FPIES); (2) Did the patient develop extreme lethargy and/or pallor in the presence of uncontrolled vomiting; (3) Did the patient get im
If the answer to the above questions is “yes,” then S. boulardii-induced FPIES should be considered.
It should be in mind that S. boulardii can be a single trigger for FPIES even in healthy infants, as well as in non-IgE-mediated or IgE-mediated food hypersensitivity patient. Caution is needed to prescribe S. boulardii probiotics for both of the patients with food allergy or not.
| 1. | Nowak-Węgrzyn A, Chehade M, Groetch ME, Spergel JM, Wood RA, Allen K, Atkins D, Bahna S, Barad AV, Berin C, Brown Whitehorn T, Burks AW, Caubet JC, Cianferoni A, Conte M, Davis C, Fiocchi A, Grimshaw K, Gupta R, Hofmeister B, Hwang JB, Katz Y, Konstantinou GN, Leonard SA, Lightdale J, McGhee S, Mehr S, Sopo SM, Monti G, Muraro A, Noel SK, Nomura I, Noone S, Sampson HA, Schultz F, Sicherer SH, Thompson CC, Turner PJ, Venter C, Westcott-Chavez AA, Greenhawt M. International consensus guidelines for the diagnosis and management of food protein-induced enterocolitis syndrome: Executive summary-Workgroup Report of the Adverse Reactions to Foods Committee, American Academy of Allergy, Asthma & Immunology. J Allergy Clin Immunol. 2017;139:1111-1126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 437] [Article Influence: 54.6] [Reference Citation Analysis (0)] |
| 2. | Baker MG, Cecilia Berin M, Sicherer S. Update on Food Protein-Induced Enterocolitis Syndrome (FPIES). Curr Allergy Asthma Rep. 2022;22:113-122. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 3. | Baker MG, Sampson HA. Recent trends in food protein-induced enterocolitis syndrome (FPIES). J Allergy Clin Immunol. 2023;151:43-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 4. | Calvani M, Anania C, Bianchi A, D'Auria E, Cardinale F, Votto M, Martelli A, Tosca M, Chiappini E, Brambilla I, Miraglia Del Giudice M, Caffarelli C. Update on Food protein-induced enterocolitis syndrome (FPIES). Acta Biomed. 2021;92:e2021518. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 5. | Caubet JC, Cianferoni A, Groetch M, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome. Clin Exp Allergy. 2019;49:1178-1190. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 6. | Agyemang A, Nowak-Wegrzyn A. Food Protein-Induced Enterocolitis Syndrome: a Comprehensive Review. Clin Rev Allergy Immunol. 2019;57:261-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 7. | Bingemann TA, Sood P, Järvinen KM. Food Protein-Induced Enterocolitis Syndrome. Immunol Allergy Clin North Am. 2018;38:141-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 8. | Pecora V, Valluzzi R, Dahdah L, Fierro V, Mennini M, Fiocchi A. Food protein-induced enterocolitis syndrome epidemiology, diagnosis, and treatment. Curr Opin Allergy Clin Immunol. 2020;20:316-322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Hwang JB, Kang KJ, Kang YN, Kim AS. Probiotic gastrointestinal allergic reaction caused by Saccharomyces boulardii. Ann Allergy Asthma Immunol. 2009;103:87-88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 16] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 10. | Feuille E, Nowak-Węgrzyn A. Definition, etiology, and diagnosis of food protein-induced enterocolitis syndrome. Curr Opin Allergy Clin Immunol. 2014;14:222-228. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 33] [Cited by in RCA: 31] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Mehr S, Campbell DE. Food protein-induced enterocolitis syndrome: guidelines summary and practice recommendations. Med J Aust. 2019;210:94-99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 12. | Barni S, Vazquez-Ortiz M, Giovannini M, Liccioli G, Sarti L, Cianferoni A, Mori F. 'Diagnosing food protein-induced enterocolitis syndrome'. Clin Exp Allergy. 2021;51:14-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 13. | Bird JA, Barni S, Brown-Whitehorn TF, du Toit G, Infante S, Nowak-Wegrzyn A. Food protein-induced enterocolitis syndrome oral food challenge: Time for a change? Ann Allergy Asthma Immunol. 2021;126:506-515. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 14. | Kelesidis T, Pothoulakis C. Efficacy and safety of the probiotic Saccharomyces boulardii for the prevention and therapy of gastrointestinal disorders. Therap Adv Gastroenterol. 2012;5:111-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 226] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 15. | Alves C, Afonso I, Leiria-Pinto P. Is Saccharomyces boulardii Really Safe? J Investig Allergol Clin Immunol. 2017;27:384-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 16. | Kartal O, Demirel F, Baysan A, Gulec M, Yesillik S, Uyanýk M, Musabak U and Sener O. An unexpected allergic reaction with Saccharomyces boulardii: a case report. Clin Transl All. 2014;4. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 17. | Mulé A, Prattico C, Al Ali A, Mulé P, Ben-Shoshan M. Diagnostic and Management Strategies of Food Protein-Induced Enterocolitis Syndrome: Current Perspectives. Pediatric Health Med Ther. 2023;14:337-345. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 18. | Mathew M, Leeds S, Nowak-Węgrzyn A. Recent Update in Food Protein-Induced Enterocolitis Syndrome: Pathophysiology, Diagnosis, and Management. Allergy Asthma Immunol Res. 2022;14:587-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 19. | Nowak-Wegrzyn A, Berin MC, Mehr S. Food Protein-Induced Enterocolitis Syndrome. J Allergy Clin Immunol Pract. 2020;8:24-35. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 82] [Article Influence: 20.5] [Reference Citation Analysis (0)] |
| 20. | Anvari S, Ruffner MA, Nowak-Wegrzyn A. Current and future perspectives on the consensus guideline for food protein-induced enterocolitis syndrome (FPIES). Allergol Int. 2024;73:188-195. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 13.0] [Reference Citation Analysis (0)] |