Published online Jan 26, 2025. doi: 10.12998/wjcc.v13.i3.97737
Revised: October 1, 2024
Accepted: October 22, 2024
Published online: January 26, 2025
Processing time: 158 Days and 17.3 Hours
In a recent case report in the World Journal of Clinical Cases, emphasized the crucial role of rapidly and accurately identifying pathogens to optimize patient treatment outcomes. Laboratory-on-a-chip (LOC) technology has emerged as a transfor
Core Tip: Laboratory-on-a-chip (LOC) technology revolutionizes microorganism identification, offering rapid diagnostics with high sensitivity and portability. Integrating microfluidics, biosensors, and artificial intelligence, LOC devices enhance clinical decision-making, accelerate outbreak response, and enable personalized treatments. Overcoming technical chal
- Citation: Ardila CM. Advancing healthcare through laboratory on a chip technology: Transforming microorganism identification and diagnostics. World J Clin Cases 2025; 13(3): 97737
- URL: https://www.wjgnet.com/2307-8960/full/v13/i3/97737.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i3.97737
A laboratory-on-a-chip (LOC) device integrates one or several laboratory functions into a single chip of only millimeters to a few square centimeters in size. It employs microfluidics, the science of manipulating and controlling fluids in tiny channels with dimensions of tens to hundreds of micrometers, to replicate and miniaturize the processes typically performed in a full-scale laboratory[1,2].
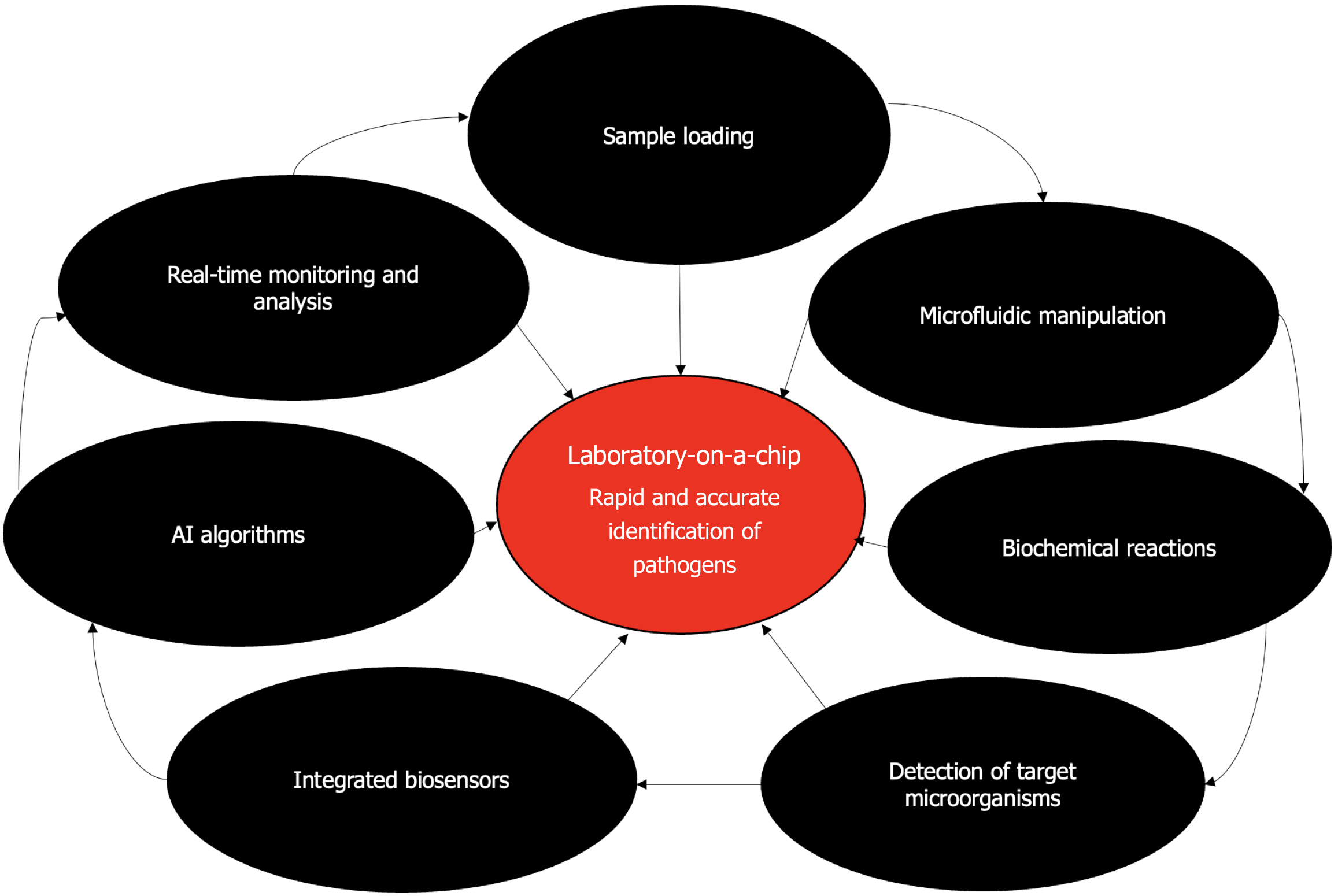
In contrast, LOC technology can significantly reduce the time required for microbial detection and identification to mere minutes or hours. This is critical in clinical diagnostics, outbreak responses, and bioterrorism defenses. LOC devices streamline complex laboratory processes, allowing for the simultaneous analysis of multiple parameters with minimal sample volumes, thus conserving resources and reducing waste[1,4]. The compact and integrated nature of LOC devices makes them highly portable, enabling on-site and point-of-care testing (POCT) in various settings, including remote areas, field hospitals, and epidemiological surveys. These devices can achieve high sensitivity and specificity by integrating advanced detection methods, such as optical, electrochemical, or magnetic sensing, enhancing the accuracy of microorganism identification[1,3]. By reducing the need for extensive laboratory infrastructure and laboratory, LOC technology can lower the costs of microbial diagnostics, making advanced testing more accessible and affordable. Figure 1 depicts the step-by-step process of microorganism identification using this technology. The workflow includes sample loading, microfluidic manipulation, biochemical reactions, and target microorganism detection. Integrated biosensors, artificial intelligence (AI) algorithms, and data analytics enable real-time monitoring and analysis, leading to rapid and accurate pathogen identification. The compact and portable nature of LOC devices allows for POCT in various settings, revolutionizing diagnostics and healthcare delivery.
Traditional methods often require significant time for sample preparation, incubation, and analysis. These are typically laboratory-intensive and require highly trained personnel. The significant necessary laboratory infrastructure and resources may not be available in all settings, particularly in low-resource or remote areas. Traditional methods may also sometimes yield false positives or negatives, leading to diagnostic uncertainty and potentially inappropriate treatments[2,3].
The ability of LOC technology to address these challenges positions it as a transformative tool in microbiology and clinical diagnostics, promising faster, more accurate, and more accessible microorganism identification. Given the recent case report published in the World Journal of Clinical Cases by Liang et al[5], which highlighted the criticality of rapid and accurate pathogen identification to optimize patient treatment outcomes, this editorial offers a thorough overview of LOC technology, detailing its principles, benefits, applications, challenges, and prospects.
The concept of miniaturizing laboratory processes began in the 1960s with the development of microelectromechanical systems (MEMSs). These systems laid the groundwork for integrating mechanical elements, sensors, and electronics on silicon chips. In the 1980s, the field of microfluidics emerged, driven by advances in MEMSs technology and the need to handle small volumes of fluids in biomedical applications. The 1990s saw significant advancements in microfabrication techniques, enabling the creation of intricate microfluidic channels and structures[6,7].
Early LOC devices were developed for specific applications such as DNA analysis, protein separation, and cell sorting, demonstrating the potential of miniaturized laboratory processes. The 2000s witnessed a rapid expansion of LOC applications across various fields, including genomics, proteomics, diagnostics, and environmental monitoring. Technological innovations such as the integration of optical and electrochemical sensors enhanced the functionality and sensitivity of LOC devices. Subsequently, researchers began to explore the potential of LOC for POCT, aiming to bring advanced diagnostic capabilities to bedside and remote locations[6,7].
Recent years have seen remarkable progress in the design and fabrication of LOC devices, driven by advances in materials science, nanotechnology, and biotechnology. Modern LOC systems are highly integrated, incorporating multiple laboratory functions such as sample preparation, reaction, separation, and detection on a single chip. Their applications have broadened to include personalized medicine, real-time monitoring of biological processes, and high-throughput screening for drug discovery[6,7].
Microfluidics is the science of manipulating and controlling fluids at a microscale, typically involving channels with dimensions ranging from tens to hundreds of micrometers. In LOC devices, fluids (e.g., samples and reagents) are transported through microchannels using various methods such as capillary action, electrokinetic flow, or pressure-driven flow. The microscale dimensions allow for precise control over fluid dynamics, enabling efficient mixing, separation, and reaction processes within the chip[8,9].
LOC devices often include modules for sample collection, filtration, concentration, and dilution, ensuring that the sample is ready for analysis. Miniaturized reactors on the chip facilitate biochemical reactions such as PCR, enzyme assays, and immunoassays, allowing for rapid amplification and detection of target molecules. Techniques like electrophoresis, chromatography, and dielectrophoresis are employed within the chip to separate and purify analytes based on their physical and chemical properties. LOC devices integrate various detection methods to identify and quantify microorganisms[2,8].
Common methods include optical detection, electrochemical detection and magnetic detection. Optical detection, utilizing fluorescence, absorbance, or chemiluminescence to detect the presence of target molecules. Electrochemical detection measuring electrical signals generated by redox reactions or changes in conductivity. Magnetic detection using magnetic particles and sensors to identify target analytes[3,8].
The integration of multiple laboratory processes on a single chip reduces the need for bulky equipment and extensive manual handling, leading to increased automation and efficiency. Miniaturization also decreases the volume of reagents and samples required, reducing costs and enabling high-throughput analysis[2,3].
Overall, the evolution of LOC technology has transformed it from a novel concept to a powerful tool with broad applications. By leveraging the principles of microfluidics and the integration of various laboratory functions, LOC devices offer a compact, efficient, and versatile solution for rapid microorganism identification and beyond.
LOC technology integrates multiple laboratory functions on a single chip, enabling seamless and rapid progression from sample preparation to detection. This integration eliminates the need for time-consuming steps that are typical in traditional methods. Many LOC devices can perform multiple analyses simultaneously on a single chip, further accelerating the overall process[2,9]. For example, a single LOC can conduct multiple PCR reactions in parallel, vastly speeding up the identification of various microorganisms. LOC devices often provide real-time monitoring of reactions and processes, allowing for immediate interpretation of results. This capability is crucial in clinical diagnostics where timely decision-making can significantly impact patient outcomes[3,10]. Traditional microbial identification methods, such as culturing, can take several hours to days. In contrast, LOC devices can reduce this time to minutes or a few hours, which is particularly beneficial in acute clinical situations, such as sepsis, where rapid identification and treatment of pathogens are critical[8,11].
LOC devices incorporate sophisticated detection technologies, including optical, electrochemical, and magnetic sensors. These advanced methods enhance the ability to detect microorganisms with high sensitivity and specificity. The enclosed microenvironment within LOC devices reduces the risk of cross-contamination, which is a common issue in traditional laboratory settings. This containment leads to more accurate and reliable results[2,3].
The precise control over fluid handling in microchannels ensures that even minute quantities of microorganisms can be efficiently captured and analyzed, increasing the sensitivity of the detection process. LOC devices can integrate powerful molecular techniques such as PCR, which can amplify tiny amounts of microbial DNA to detectable levels. This integration significantly boosts the sensitivity of detection compared to traditional methods that may miss low-abundance pathogens. The use of integrated data analytics and machine learning algorithms in some LOC devices can further improve specificity by accurately distinguishing between similar microbial species based on their unique signatures[2,11].
The small size and lightweight nature of LOC devices make them highly portable. They can be easily transported to various locations, including remote and resource-limited areas where traditional laboratory infrastructure may not be available. LOC technology enables POCT, allowing healthcare providers to conduct tests at the patient's bedside, in field hospitals, or even in the patient's home. This capability is particularly valuable in rural or underserved regions. Unlike traditional laboratory methods that require extensive infrastructure and skilled personnel, LOC devices are designed to operate with minimal equipment and training. This simplicity makes them accessible to a wider range of users, including non-specialist healthcare workers. Many LOC devices are designed to operate on battery power, making them suitable for use in areas without reliable access to electricity. This feature further enhances their applicability in field settings and during emergency response situations. In the event of an outbreak or public health emergency, LOC devices can be rapidly deployed to the affected areas, providing timely and accurate diagnostic capabilities that are crucial for controlling the spread of infectious diseases[8,10].
Overall, the advantages of LOC technology in terms of speed, efficiency, sensitivity, specificity, portability, and accessibility make it a transformative tool for the rapid identification of microorganisms. These benefits position LOC as a superior alternative to traditional methods, particularly in scenarios where timely and accurate microbial identification is critical. Table 1 succinctly outlines the key differences between LOC technology and traditional methods of microorganism identification, highlighting the advantages of LOC in terms of speed, sensitivity, portability, automation, integration with other technologies, and accessibility.
| Aspect | Laboratory-on-a-chip | Traditional methods |
| Speed of identification | Rapid results within minutes to hours | Longer turnaround time (hours to days) |
| Sensitivity and specificity | High sensitivity and specificity | Variable sensitivity and specificity depending on method and sample quality |
| Portability | Compact and portable devices | Laboratory-based equipment requiring specialized facilities |
| Sample size | Minimal sample volume required | Larger sample volume often needed |
| Automation | Automated processes for streamlined workflow | Manual handling of samples with potential for human error |
| Cost | Initial investment may be higher but cost-effective over time | Lower initial cost but higher operational costs |
| Integration with other technologies | Easily integrated with biosensors, artificial intelligence, and data analytics | Limited integration capabilities with other technologies |
| Accessibility | Suitable for use in remote or resource-limited settings | Reliant on centralized laboratories, limiting accessibility |
LOC devices enable POCT, which allows healthcare providers to perform diagnostic tests at the patient's bedside. This capability is crucial in emergency and critical care settings where time is of the essence[2,8]. Traditional diagnostic methods often require samples to be sent to centralized laboratories, resulting in delays. LOC devices eliminate this need by performing the necessary tests on-site, thus reducing turnaround times and accelerating clinical decision-making. LOC devices can conduct a variety of tests, including blood glucose monitoring, detection of infectious agents (e.g., bacteria, viruses), and measuring biomarkers for chronic diseases (e.g., heart disease, diabetes)[3,8]. This versatility makes them invaluable tools in diverse clinical scenarios. The integration of multiple diagnostic steps into a single device simplifies the workflow, reducing the potential for human error and improving the overall efficiency of the diagnostic process. By eliminating the need for extensive laboratory infrastructure and skilled personnel, LOC devices can significantly reduce the costs associated with diagnostic testing, making advanced diagnostics more accessible and affordable[2,11].
LOC devices can quickly identify pathogens responsible for infectious diseases, enabling rapid response to outbreaks[12,13]. This swift identification is critical for implementing effective containment measures and preventing the spread of disease. The portability of LOC devices allows them to be used in field settings, such as during epidemiological surveys or in regions with limited laboratory facilities[2,13]. This capability is essential for real-time surveillance and monitoring of infectious diseases in various populations. LOC devices can provide real-time data on pathogen presence and pre
LOC devices enable the rapid identification of pathogens at the point-of-care, providing critical information that can guide the selection of appropriate antimicrobial therapies. This personalized approach ensures that patients receive the most effective treatments promptly. LOC technology can detect specific biomarkers associated with individual patients' responses to infections or treatments[14,15]. This capability allows for the customization of therapeutic strategies based on the patient’s unique biological profile. LOC devices can be used to monitor patients’ responses to treatments in real-time, allowing for timely adjustments to therapeutic regimens based on how well the patient is responding[2,15]. This dynamic monitoring is essential for optimizing treatment outcomes. By enabling precise and targeted treatments, LOC technology helps to minimize the adverse effects associated with broad-spectrum antibiotics and other generalized therapies. Personalized medicine aims to achieve maximum therapeutic efficacy with minimal side effects. Early and accurate identification of pathogens and relevant biomarkers through LOC devices can facilitate early intervention in disease progression, improving patient outcomes and reducing the overall burden of disease[14,15].
Overall, the applications of LOC technology in healthcare and diagnostics are vast and transformative. From enhancing clinical diagnostics through POCT to playing a pivotal role in public health surveillance and advancing personalized medicine, LOC devices are poised to revolutionize how we detect, monitor, and treat infectious diseases and other health conditions.
LOC devices are often integrated with various biosensors (e.g., optical, electrochemical, and magnetic) to improve the detection capabilities. These biosensors can provide highly sensitive and specific measurements of microbial presence and activity. The integration of biosensors allows for real-time monitoring of biological processes, offering immediate feedback on the presence and concentration of pathogens[16]. This capability is crucial for timely clinical interventions.
AI algorithms can process the complex data generated by LOC devices, identifying patterns and correlations that may not be apparent through traditional analysis[17]. This integration enhances the accuracy and reliability of diagnostics. Machine learning models can be trained on large datasets to predict disease outbreaks, patient responses to treatments, and potential complications, thereby supporting proactive healthcare management. AI can automate the entire diagnostic process, from sample handling to data interpretation, reducing the need for human intervention and minimizing the risk of errors[17,18].
The vast amount of data generated by LOC devices can be integrated with electronic health records and other data sources to provide a comprehensive view of patient health. Advanced data analytics can help identify personalized treatment plans based on individual patient data, including genetic, environmental, and lifestyle factors. Data analytics can aggregate and analyze data from multiple LOC devices deployed in the field, providing valuable insights into disease prevalence and transmission dynamics[19].
During the coronavirus disease 2019 (COVID-19) pandemic, LOC technology was adapted to develop rapid and portable diagnostic tests for severe acute respiratory syndrome-coronavirus 2 (SARS-CoV-2). For example, Abbott's ID NOW COVID-19 test uses isothermal nucleic acid amplification on a microfluidic chip to deliver results in 15 minutes. Researchers have developed LOC devices capable of multiplex testing, allowing simultaneous detection of multiple respiratory pathogens, including SARS-CoV-2, influenza, and respiratory syncytial virus, from a single sample[20,21].
LOC devices have been designed to rapidly identify antibiotic-resistant bacteria by detecting specific resistance genes or by analyzing phenotypic resistance patterns. For instance, devices that combine microfluidics with clustered regularly interspaced short palindromic repeats (CRISPR)-based detection systems can quickly identify resistant strains. Recent advances include LOC devices that can perform aspartate aminotransferase at the point of care, determining the susceptibility of bacteria to different antibiotics within hours instead of days, thus guiding appropriate antibiotic use[2,8].
LOC technology has been utilized to develop devices that can isolate and analyze circulating tumor cells from blood samples, providing a non-invasive method for cancer diagnosis and monitoring. Researchers have developed LOC platforms capable of detecting cancer-specific biomarkers, such as microRNAs and proteins, with high sensitivity and specificity, facilitating early cancer detection and personalized treatment plans[14].
LOC devices have been deployed in field settings to monitor infectious disease outbreaks, such as Ebola virus and Zika virus. These devices provide rapid, on-site diagnostics that are crucial for controlling the spread of disease in affected regions[22].
LOC technology is being used in global health initiatives to improve diagnostic capabilities in low-resource settings, where traditional laboratory infrastructure is lacking. For example, portable LOC devices have been developed for the rapid detection of malaria and tuberculosis (TB) in remote areas[23].
LOC technology is being integrated with wearable devices to enable continuous monitoring of biomarkers, providing real-time health data. This integration is particularly useful for managing chronic diseases and monitoring patients' health status remotely. Innovations include LOC-based wearable patches that can analyze sweat or interstitial fluid to monitor glucose levels, electrolytes, and other critical biomarkers non-invasively[24].
Overall, the integration of LOC technology with biosensors, AI, and data analytics, along with recent breakthroughs in diagnostics and monitoring, demonstrates the transformative potential of LOC devices in healthcare. These innovations not only enhance the capabilities of LOC technology but also expand its applications, making it a vital tool for modern diagnostics and personalized medicine.
Creating the intricate microfluidic channels and integrated components of LOC devices requires advanced microfabrication techniques, such as photolithography, soft lithography, and etching. These processes can be technically challenging and require specialized equipment and expertise.
The choice of materials for LOC devices must balance biocompatibility, chemical resistance, and mechanical stability. Common materials include glass, silicon, and polymers like polydimethylsiloxane, each with its own set of fabrication challenges. Scaling up the production of LOC devices from prototypes to commercial quantities while maintaining quality and consistency is a significant challenge. Variations in fabrication can affect the performance and reliability of the devices[2,25].
Biological samples such as blood, urine, or saliva contain various components that can interfere with the analysis. Efficiently preparing these samples for processing on a microfluidic chip, including separation of target microorganisms from other components, is complex and often requires additional steps. Incorporating all necessary sample preparation steps (e.g., lysis, filtration, concentration) into the LOC device without compromising its compactness and functionality is a major technical hurdle[8,25].
The diversity of LOC devices and their applications leads to a lack of standard protocols for fabrication, operation, and data interpretation. This variability can hinder the reproducibility and comparability of results across different devices and studies. Ensuring consistent quality control during the manufacturing process is crucial for the reliability of LOC devices. Establishing standardized quality control measures is challenging due to the bespoke nature of many LOC applications[2,8].
The development of LOC technology involves significant initial investment in research and development, microfabrication facilities, and specialized equipment. This high cost can be a barrier for startups and smaller companies. Achieving cost-effective production often requires economies of scale, which can be difficult to attain during the early stages of commercialization. The initial high cost of LOC devices can limit their adoption, particularly in resource-limited settings[2,3].
LOC devices, especially those intended for clinical use, must undergo rigorous regulatory approval processes to ensure their safety, efficacy, and reliability. This process can be time-consuming and costly, often requiring extensive clinical trials and validation studies. Regulatory bodies such as the Food and Drug Administration in the United States and the European Medicines Agency in Europe have stringent standards for medical devices. Ensuring compliance with these standards is essential but can be challenging due to the innovative and often unconventional nature of LOC technology. Once approved, LOC devices must be continually monitored for performance and safety in real-world settings. Establishing robust post-market surveillance systems adds to the regulatory burden and requires ongoing resources[3,25].
Convincing healthcare providers and laboratories to adopt new LOC technology involves overcoming inertia and skepticism. Demonstrating the cost-effectiveness, reliability, and superiority of LOC devices compared to traditional methods is crucial for widespread adoption. Effective use of LOC devices requires training healthcare professionals and technicians. Developing comprehensive training programs and ensuring widespread education about the benefits and operation of LOC technology is necessary but resource intensive[3,8].
Securing reimbursement from insurance companies and healthcare systems for diagnostics performed using LOC devices can be challenging. Establishing the economic value and demonstrating improved patient outcomes are key factors in influencing reimbursement policies[3,25].
Overall, while LOC technology holds immense potential for revolutionizing microorganism identification and diagnostics, several technical, economic, and regulatory challenges must be addressed. Overcoming these hurdles will require collaborative efforts from researchers, manufacturers, regulatory bodies, and healthcare providers to realize the full benefits of LOC technology in healthcare and diagnostics.
Further research into innovative detection mechanisms, such as advanced optical sensors, nanomaterials, and quantum dots, could significantly improve the sensitivity and specificity of LOC devices. Combining LOC devices with genomics, proteomics, and metabolomics technologies could enhance their diagnostic capabilities by providing comprehensive molecular profiles of microorganisms[26-28].
Research into new materials that offer better biocompatibility, chemical resistance, and mechanical properties will be crucial for improving LOC device performance. Exploring three-dimensional printing and other advanced micro-nanofabrication techniques could enable more complex and efficient designs, potentially reducing production costs and improving scalability[27-29].
Developing fully integrated LOC systems that combine all necessary functions, from sample preparation to detection and data analysis, will streamline workflows and enhance usability. Incorporating AI and machine learning algorithms directly into LOC devices for real-time data analysis and decision-making could improve diagnostic accuracy and speed[28-30].
Establishing standardized protocols for the fabrication, operation, and validation of LOC devices will be essential for ensuring reproducibility and reliability across different settings and applications. Conducting extensive clinical trials and working with regulatory bodies to streamline approval processes will be crucial for the widespread adoption of LOC technology in clinical diagnostics.
LOC devices can be used to monitor water quality by detecting pathogens, toxins, and chemical contaminants in real-time, ensuring safe drinking water and identifying pollution sources[31-33]. Portable LOC devices could monitor air quality by detecting airborne pathogens, pollutants, and allergens, providing critical data for public health and environmental protection[25-27].
LOC technology can be applied to detect foodborne pathogens such as Salmonella, Escherichia coli, and Listeria, ensuring food safety from farm to table. LOC devices can monitor the presence of contaminants and ensure compliance with safety standards during food processing and packaging[2,3,8].
LOC technology can be used for single-cell analysis, enabling researchers to study cellular heterogeneity and discover new insights into cell biology and disease mechanisms[34-36]. Developing organ-on-a-chip models that mimic human organ systems can revolutionize drug testing and disease modeling, providing more accurate and ethical alternatives to animal testing[27-29].
LOC devices can assess soil health by detecting pathogens, nutrient levels, and chemical residues, helping farmers optimize crop management practices. Portable LOC devices can monitor the health of livestock by detecting pathogens and biomarkers associated with diseases, ensuring animal welfare and food safety[3,8,25].
The demand for POCT is expected to drive significant growth in the LOC market, especially in remote and resource-limited settings where access to traditional laboratories is limited[37-39]. As personalized medicine gains traction, the need for rapid and precise diagnostic tools like LOC devices will increase, driving further innovation and market expansion[26-28].
Major healthcare companies and diagnostic laboratorys are likely to invest heavily in LOC technology, integrating it into their existing services to offer rapid and accurate diagnostics. Companies in environmental monitoring and food safety sectors will adopt LOC technology to enhance their testing capabilities and compliance with regulatory standards[27-29].
The integration of LOC devices with digital health platforms, such as mobile health apps and telemedicine, will enable remote monitoring and data sharing, improving patient care and public health surveillance. The incorporation of Internet of Things technology will allow LOC devices to be connected to broader data networks, facilitating real-time data collection, analysis, and decision-making[28-30].
As regulatory bodies become more familiar with LOC technology, approval processes are expected to become more streamlined, facilitating quicker market entry. Establishing clear reimbursement strategies for LOC-based diagnostics will be essential for widespread adoption in clinical settings, ensuring that these technologies are financially accessible to healthcare providers and patients[2,25].
In conclusion, the future of LOC technology is bright, with numerous opportunities for research, innovation, and market expansion. By addressing current challenges and leveraging technological advancements, LOC devices are poised to revolutionize diagnostics, environmental monitoring, food safety, and beyond, ultimately contributing to improved health outcomes and sustainable practices.
Table 2 provides an overview of the diverse applications of LOC technology across various sectors, emphasizing its potential for addressing critical challenges in healthcare, public health, environmental protection, food safety, biomedical research, and agriculture.
| Application | Description |
| Clinical diagnostics | Rapid point-of-care testing for infectious diseases, biomarker analysis, and chronic disease monitoring |
| Epidemiology and public health | Real-time monitoring of infectious disease outbreaks, tracking of pathogen transmission, and mass screening in remote or resource-limited settings |
| Personalized medicine | Tailored treatments based on rapid identification of pathogens, biomarkers, and patient-specific responses to therapies |
| Environmental monitoring | Detection of pathogens, toxins, and pollutants in water and air, ensuring safety and environmental protection |
| Food safety | Monitoring of foodborne pathogens and contaminants, quality control in food processing, and compliance with safety standards |
| Biomedical research | Single-cell analysis, organ-on-a-chip models, and disease modeling for drug testing and understanding disease mechanisms |
| Agriculture | Soil and crop health monitoring, livestock disease detection, and ensuring food security through improved agricultural practices |
During the COVID-19 pandemic, Abbott's ID NOW COVID-19 test, a LOC device, demonstrated the technology's capabilities by providing rapid, reliable testing at the point of care. The device uses isothermal nucleic acid amplification to detect SARS-CoV-2, delivering results in 15 minutes. This rapid testing was crucial in settings such as airports, nursing homes, and emergency rooms, significantly enhancing the ability to manage and contain the spread of the virus. The CRISPR-based DETECTR assay, developed by Mammoth Biosciences, utilized LOC technology to detect SARS-CoV-2 with high accuracy. The portable, easy-to-use device provided results within 30 minutes and was particularly beneficial in low-resource settings where traditional laboratory infrastructure was lacking[13,20,21].
The GeneXpert system by Cepheid is a LOC-based device that has revolutionized TB diagnosis. It can detect Mycobacterium TB and resistance to rifampicin within two hours. This rapid diagnostic capability has been transformative in regions with high TB prevalence, allowing for timely initiation of appropriate treatment and improving patient outcomes[23,40,41].
During the Ebola outbreak in West Africa, the ReEBOV Antigen Rapid Test, a LOC device, was used to quickly identify Ebola virus infections. The test provided results in minutes, facilitating rapid isolation and treatment of infected individuals and helping to control the outbreak more effectively than traditional methods[22,42].
A comparative study evaluated the effectiveness of a LOC device for detecting bacterial infections against traditional culture methods. The LOC device incorporated PCR and microfluidics to identify bacterial DNA directly from clinical samples[2,3,25].
The LOC device provided results in under an hour, whereas traditional culture methods took 24-48 hours. The sensitivity and specificity of the LOC device were comparable to culture methods, demonstrating its reliability. The significant reduction in time to diagnosis allowed for earlier intervention and appropriate antibiotic treatment, improving patient outcomes and reducing the spread of infection[3,8,25].
LOC vs ELISA for Viral Load Monitoring. This study compared a LOC device with traditional enzyme-linked immu
Overall, these case studies and comparative examples highlight the significant advantages of LOC technology over traditional methods. The rapid, accurate, and portable nature of LOC devices has demonstrated improved diagnostic capabilities, faster intervention times, and enhanced outcomes in various settings, from clinical diagnostics to public health and food safety.
LOC technology integrates various laboratory functions onto a single microchip, enabling rapid, sensitive, and specific identification of microorganisms. This innovation holds significant promise for transforming diagnostics in healthcare and beyond. LOC technology has significantly evolved, with notable progress in microfluidics, biosensors, and integration capabilities. These developments enhance its ability to miniaturize laboratory processes, allowing for efficient and comprehensive analysis on a small scale.
LOC devices drastically reduce the time required for microorganism identification, delivering results in minutes to hours. They offer highly accurate detection, thus improving diagnostic reliability. The compact and portable nature of LOC devices makes them ideal for use in remote and resource-limited settings. This portability facilitates POCT, reducing reliance on centralized laboratorys and accelerating clinical decision-making.
LOC technology is crucial for tracking and controlling infectious disease outbreaks by providing real-time data and enabling rapid response. Furthermore, LOC devices aid in personalizing treatments by quickly identifying pathogens and relevant biomarkers. The integration of LOC technology with biosensors, AI, and data analytics, along with recent breakthroughs in rapid testing and pathogen detection, showcases the transformative potential of LOC devices in diagnostics and beyond.
| 1. | Zimina TM, Pinchuk OA, Kaplun DI, Kraeva LA, Sitkov NO. Study of Laser Light Scattering Methods in Rapid Viability Assessment of Microorganisms under Antibiotics Exposure for Adaptation in Lab-on-A-Chip Format. Diagnostics (Basel). 2023;13:1130. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 2. | Ardila CM, Zuluaga-Gómez M, Vivares-Builes AM. Applications of Lab on a Chip in Antimicrobial Susceptibility of Staphylococcus aureus: A Systematic Review. Medicina (Kaunas). 2023;59:1719. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 3. | Ardila CM, Jiménez-Arbeláez GA, Vivares-Builes AM. A Systematic Review of In Vitro Studies Using Microchip Platforms for Identifying Periodontopathogens from the Red Complex. Dent J (Basel). 2023;11:245. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 4. | Kotsiri Z, Vidic J, Vantarakis A. Applications of biosensors for bacteria and virus detection in food and water-A systematic review. J Environ Sci (China). 2022;111:367-379. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 52] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 5. | Liang ZC, Ouyang H, Song XJ, Liang JX, Zheng WH, Chen JJ, Yin ZG, Chen SY. Eikenella corrodens isolated from pleural effusion: A case report. World J Clin Cases. 2024;12:3596-3602. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (2)] |
| 6. | Maia R, Carvalho V, Lima R, Minas G, Rodrigues RO. Microneedles in Advanced Microfluidic Systems: A Systematic Review throughout Lab and Organ-on-a-Chip Applications. Pharmaceutics. 2023;15:792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 7. | Carvalho V, Gonçalves I, Lage T, Rodrigues RO, Minas G, Teixeira SFCF, Moita AS, Hori T, Kaji H, Lima RA. 3D Printing Techniques and Their Applications to Organ-on-a-Chip Platforms: A Systematic Review. Sensors (Basel). 2021;21:3304. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 55] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 8. | Ardila CM, Jiménez-Arbeláez GA, Vivares-Builes AM. The Potential Clinical Applications of a Microfluidic Lab-on-a-Chip for the Identification and Antibiotic Susceptibility Testing of Enterococcus faecalis-Associated Endodontic Infections: A Systematic Review. Dent J (Basel). 2023;12:5. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 3] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 9. | Nguyen VVT, Gkouzioti V, Maass C, Verhaar MC, Vernooij RWM, van Balkom BWM. A systematic review of kidney-on-a-chip-based models to study human renal (patho-)physiology. Dis Model Mech. 2023;16:dmm050113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 10. | Wen P, Yang F, Zhao H, Xu Y, Li S, Chen L. Novel Digital SERS-Microfluidic Chip for Rapid and Accurate Quantification of Microorganisms. Anal Chem. 2024;96:1454-1461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 17] [Article Influence: 17.0] [Reference Citation Analysis (0)] |
| 11. | Aryal P, Hefner C, Martinez B, Henry CS. Microfluidics in environmental analysis: advancements, challenges, and future prospects for rapid and efficient monitoring. Lab Chip. 2024;24:1175-1206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 9] [Reference Citation Analysis (0)] |
| 12. | Zhang Q, Rawal G, Qian J, Ibrahim H, Zhang J, Dong L, Lu M. An integrated magneto-opto-fluidic biosensor for rapid on-chip assay of respiratory viruses of livestock. Lab Chip. 2022;22:3236-3244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 13. | Xing W, Wang J, Zhao C, Wang H, Bai L, Pan L, Li H, Wang H, Zhang Z, Lu Y, Chen X, Shan S, Wang D, Pan Y, Weng D, Zhou X, Huang R, He J, Jin R, Li W, Shang H, Zhong N, Cheng J. A Highly Automated Mobile Laboratory for On-site Molecular Diagnostics in the COVID-19 Pandemic. Clin Chem. 2021;67:672-683. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Zhai J, Liu Y, Ji W, Huang X, Wang P, Li Y, Li H, Wong AH, Zhou X, Chen P, Wang L, Yang N, Chen C, Chen H, Mak PI, Deng CX, Martins R, Yang M, Ho TY, Yi S, Yao H, Jia Y. Drug screening on digital microfluidics for cancer precision medicine. Nat Commun. 2024;15:4363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 20] [Reference Citation Analysis (0)] |
| 15. | Perez-Toralla K, Pereiro I, Garrigou S, Di Federico F, Araya-Farias M, Proudhon C, Bidard FC, Viovy JL, Taly V, Descroix S. On-Chip Magnetic Extraction of Circulating Cell-Free DNA from Biological Samples. Methods Mol Biol. 2024;2804:65-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 16. | Liu B, Cheng Y, Pan X, Yang W, Li X, Wang L, Ye H, Pan T. Multicolor-Assay-on-a-Chip Processed by Robotic Operation (MACpro) with Improved Diagnostic Accuracy for Field-Deployable Detection. Anal Chem. 2024;96:6634-6642. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 17. | Goda K, Lu H, Fei P, Guck J. Revolutionizing microfluidics with artificial intelligence: a new dawn for lab-on-a-chip technologies. Lab Chip. 2023;23:3737-3740. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Zare Harofte S, Soltani M, Siavashy S, Raahemifar K. Recent Advances of Utilizing Artificial Intelligence in Lab on a Chip for Diagnosis and Treatment. Small. 2022;18:e2203169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 24] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 19. | Sadasivuni S, Saha M, Bhanushali SP, Banerjee I, Sanyal A. In-Sensor Artificial Intelligence and Fusion With Electronic Medical Records for At-Home Monitoring. IEEE Trans Biomed Circuits Syst. 2023;17:312-322. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 20. | Exner HM, Gregorchuk BSJ, Castor A-G, Crisostomo L, Kolsun K, Giesbrecht S, Dust K, Alexander DC, Bolaji A, Quill Z, Head BM, Meyers AFA, Sandstrom P, Becker MG. Post-market surveillance of six COVID-19 point-of-care tests using pre-Omicron and Omicron SARS-CoV-2 variants. Microbiol Spectr. 2024;12:e0016324. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 21. | Arakawa Y, Nishida Y, Sakanashi D, Nakamura A, Ota H, Tokuhiro S, Mikamo H, Yamagishi Y. Clinical evaluation of a modified SARS-CoV-2 rapid molecular assay, ID NOW ™ COVID-19 2.0. J Infect Chemother. 2024;30:955-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 22. | Ataide VN, Pradela-Filho LA, Ameku WA, Negahdary M, Oliveira TG, Santos BG, Paixão TRLC, Angnes L. Paper-based electrochemical biosensors for the diagnosis of viral diseases. Mikrochim Acta. 2023;190:276. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 23. | Lehnert T, Gijs MAM. Microfluidic systems for infectious disease diagnostics. Lab Chip. 2024;24:1441-1493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (1)] |
| 24. | Röckendorf N, Ramaker K, Gaede K, Tappertzhofen K, Lunding L, Wegmann M, Horbert P, Weber K, Frey A. Parallel detection of multiple biomarkers in a point-of-care-competent device for the prediction of exacerbations in chronic inflammatory lung disease. Sci Rep. 2024;14:12830. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Ardila CM, Jiménez-Arbeláez GA, Vivares-Builes AM. Potential Clinical Application of Organs-on-a-Chip in Periodontal Diseases: A Systematic Review of In Vitro Studies. Dent J (Basel). 2023;11:158. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 5] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 26. | Cinti S, Singh S, Covone G, Tonietti L, Ricciardelli A, Cordone A, Iacono R, Mazzoli A, Moracci M, Rotundi A, Giovannelli D. Reviewing the state of biosensors and lab-on-a- chip technologies: opportunities for extreme environments and space exploration. Front Microbiol. 2023;14:1215529. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Reference Citation Analysis (0)] |
| 27. | Sabaté Del Río J, Ro J, Yoon H, Park TE, Cho YK. Integrated technologies for continuous monitoring of organs-on-chips: Current challenges and potential solutions. Biosens Bioelectron. 2023;224:115057. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 28. | Kawakita S, Mandal K, Mou L, Mecwan MM, Zhu Y, Li S, Sharma S, Hernandez AL, Nguyen HT, Maity S, de Barros NR, Nakayama A, Bandaru P, Ahadian S, Kim HJ, Herculano RD, Holler E, Jucaud V, Dokmeci MR, Khademhosseini A. Organ-On-A-Chip Models of the Blood-Brain Barrier: Recent Advances and Future Prospects. Small. 2022;18:e2201401. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 37] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 29. | Liu J, Du Y, Xiao X, Tan D, He Y, Qin L. Construction of in vitro liver-on-a-chip models and application progress. Biomed Eng Online. 2024;23:33. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 30. | Dou J, Yang Z, Singh B, Ma B, Lu Z, Xu J, He Y. Discussion: Embracing microfluidics to advance environmental science and technology. Sci Total Environ. 2024;937:173597. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 31. | Mitrogiannopoulou AM, Tselepi V, Ellinas K. Polymeric and Paper-Based Lab-on-a-Chip Devices in Food Safety: A Review. Micromachines (Basel). 2023;14:986. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 17] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 32. | Bourguignon N, Kamat V, Perez M, Mathee K, Lerner B, Bhansali S. New dynamic microreactor system to mimic biofilm formation and test anti-biofilm activity of nanoparticles. Appl Microbiol Biotechnol. 2022;106:2729-2738. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 33. | Buja I, Sabella E, Monteduro AG, Rizzato S, Bellis L, Elicio V, Formica L, Luvisi A, Maruccio G. Detection of Ampelovirus and Nepovirus by Lab-on-a-Chip: A Promising Alternative to ELISA Test for Large Scale Health Screening of Grapevine. Biosensors (Basel). 2022;12:147. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 34. | Huh J, Parra JPRLL, Copus JS, Kang HW, Bishop CE, Soker S, Murphy S, Shupe TD, Yoo JJ, Lee SJ, Atala A. 3D Bioprinted Liver-on-a-Chip for Drug Cytotoxicity Screening. Tissue Eng Part A. 2024;30:333-341. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 35. | Bettenfeld R, Claudel J, Kourtiche D, Nadi M, Schlauder C. Design and Modeling of a Device Combining Single-Cell Exposure to a Uniform Electrical Field and Simultaneous Characterization via Bioimpedance Spectroscopy. Sensors (Basel). 2023;23:3460. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 36. | Özyurt C, Uludağ İ, İnce B, Sezgintürk MK. Lab-on-a-chip systems for cancer biomarker diagnosis. J Pharm Biomed Anal. 2023;226:115266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 37. | Khanfar MF, Abu Eisheh NJ, Al-Ghussain L, Al-Halhouli AT. Lab on a Chip for the Colorimetric Determination of Nitrite in Processed Meat Products in the Jordanian Market. Micromachines (Basel). 2019;10:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 38. | Ismayilzada N, Tarar C, Dabbagh SR, Tokyay BK, Dilmani SA, Sokullu E, Abaci HE, Tasoglu S. Skin-on-a-chip technologies towards clinical translation and commercialization. Biofabrication. 2024;16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 39. | Wang Y, Yang F, Fu Y, He X, Tian H, Yang L, Wu M, Cao J, Liu J. A point-of-care testing platform for on-site identification of genetically modified crops. Lab Chip. 2024;24:2622-2632. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 40. | Ventimiglia G, Pesaturo M, Malcolm A, Petralia S. A Miniaturized Silicon Lab-on-Chip for Integrated PCR and Hybridization Microarray for High Multiplexing Nucleic Acids Analysis. Biosensors (Basel). 2022;12:563. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 41. | Thacker VV, Dhar N, Sharma K, Barrile R, Karalis K, McKinney JD. A lung-on-chip model of early Mycobacterium tuberculosis infection reveals an essential role for alveolar epithelial cells in controlling bacterial growth. Elife. 2020;9:e59961. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 46] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 42. | Romao VC, Martins SAM, Germano J, Cardoso FA, Cardoso S, Freitas PP. Lab-on-Chip Devices: Gaining Ground Losing Size. ACS Nano. 2017;11:10659-10664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |