Published online Oct 16, 2025. doi: 10.12998/wjcc.v13.i29.110948
Revised: June 30, 2025
Accepted: July 24, 2025
Published online: October 16, 2025
Processing time: 70 Days and 19.4 Hours
Postpartum depression (PPD) is a prevalent and debilitating psychiatric disorder affecting maternal mental health, infant development, and family well-being. Despite increasing global awareness, significant disparities remain in screening, diagnosis, and treatment, particularly in low-resource and culturally diverse settings. The complex interplay of biological and psychosocial determinants complicates conventional intervention models. Integrating epidemiological patterns, pathophysiological mechanisms, and sociocultural factors will inform more effective and equitable strategies for PPD screening, prevention, and treatment.
To synthesize current evidence on risk factors, underlying mechanisms, and interventions for postpartum depression and outline directions toward equitable care.
A narrative review was conducted following PRISMA 2020 guidelines. Peer-reviewed studies published from January 2010 to May 2025 were systematically searched in PubMed, Web of Science, EMBASE, and PsycINFO. Inclusion criteria comprised studies addressing PPD epidemiology, risk stratification, biological mechanisms, and intervention strategies. After screening and full-text review, 84 studies were included. Study designs primarily involved cohort studies, randomized controlled trials, and meta-analyses. Extracted data were categorized thematically and assessed for methodological quality and generalizability.
PPD arises from multifactorial interactions involving hormonal dysregulation, neurochemical changes, psychosocial stressors, and cultural influences. Primary risk factors include personal or family history of depression, antenatal anxiety, low maternal self-efficacy, and inadequate social support. Evidence-based interventions encompass Edinburgh Postnatal Depression Scale-based screening, cognitive behavioral therapy, interpersonal psychotherapy, psychoeducation, and pharmacological treatments such as brexanolone and zuranolone. Culturally adapted, community-integrated models—including stepped-care approaches and task-shifting—improve feasibility and scalability, particularly in underserved populations. Emerging evidence highlights inflammatory biomarkers (e.g., interleukin-6 and C-reactive protein), AI-assisted screening tools, and family-inclusive strategies as promising for enhanced detection and outcomes.
Effective PPD management requires integrative, culturally sensitive approaches, prioritizing scalable, personalized non-pharmacological interventions to reduce disparities and enhance maternal mental health equity across diverse populations.
Core Tip: This systematic review synthesizes recent evidence on postpartum depression, highlighting its multifactorial etiology involving hormonal, inflammatory, and psychosocial mechanisms. It evaluates emerging interventions including cognitive behavioral therapy, community-based models, and GABAergic modulation. The review proposes a stepped-care framework integrating biological risk stratification, culturally adapted tools, and digital delivery platforms, aiming to advance equitable and personalized maternal mental health care globally.
- Citation: Ji QQ, Wang MY. Epidemiology, pathophysiology, and interventions for postpartum depression: Systematic review. World J Clin Cases 2025; 13(29): 110948
- URL: https://www.wjgnet.com/2307-8960/full/v13/i29/110948.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i29.110948
Beyond maternal distress, postpartum depression (PPD) disrupts mother-infant bonding and can hinder children's cognitive and socio-emotional development, ultimately destabilizing family dynamics[1-3]. Affected mothers frequently exhibit diminished physical engagement and emotional attunement with their infants, which significantly increases the risk of emotional and behavioral challenges in early childhood[3]. As such, PPD has emerged as a critical concern in both maternal and child health domains.
Despite its considerable prevalence, the etiology of PPD remains incompletely elucidated. However, it is widely accepted as a multifactorial condition shaped by an interplay of biological, psychological, and social determinants. Key predictive factors include a personal history of psychiatric illness, antenatal anxiety, specific personality traits such as neuroticism and self-critical tendencies, and a diminished sense of maternal self-worth[4,5]. Among these, maternal self-efficacy has drawn increasing attention as a powerful psychological risk factor. Notably, reduced maternal self-efficacy is associated with a nearly six-fold increase in the likelihood of PPD and a ten-fold increase in postpartum anxiety[4].
The Edinburgh Postnatal Depression Scale (EPDS) remains the most extensively adopted and rigorously assessed instrument for identifying PPD symptoms in a variety of clinical contexts and cultural backgrounds. Its diagnostic validity and consistency have been substantiated by a broad body of research spanning multiple populations[6-8]. While pharmacological treatments, including standard antidepressants, are commonly prescribed, their application is often constrained by delayed therapeutic onset, variable efficacy, and concerns regarding side effects and safety in the perinatal context. These limitations have spurred interest in non-pharmacological interventions including cognitive behavioral therapy (CBT), interpersonal therapy (IPT), psychoeducation, yoga, and music therapy[8-11].
This review synthesizes recent advances in PPD research and management. Specifically, it examines the global epidemiological trends, key psychosocial and biological risk factors, underlying mechanisms, and emerging non-pharmacological interventions. By pooling current evidence, we aim to guide clinical practice and improve outcomes for both mothers and infants.
We carried out a systematic review following PRISMA 2020 guidelines to ensure transparent and reproducible methods. The review protocol was not registered with PROSPERO.
A comprehensive literature search was conducted across four major electronic databases: PubMed, Web of Science, EMBASE, and PsycINFO, covering studies published from January 2010 to May 2025. Our search used MeSH terms and free-text keywords ralated to “postpartum depression and maternal mental health”, “risk factors”, “intervention”, “neurobiology”, “inflammation”, and “cultural adaptation”.
We tailored search terms to each database’s indexing system. A representative search string used in PubMed was: ("postpartum depression"[MeSH Terms] OR "postnatal depression"[Title/Abstract] OR "maternal depression"[Title/Abstract]) AND ("risk factors"[Title/Abstract] OR "pathogenesis"[Title/Abstract] OR "neurobiology"[Title/Abstract] OR "inflammation"[Title/Abstract] OR "intervention"[Title/Abstract] OR "treatment"[Title/Abstract] OR "prevention"[Title/Abstract]) AND ("2010/01/01"[Date - Publication]: "2025/05/31"[Date - Publication]) AND (English[Language]).
We imported all references into EndNote 21 to remove duplicates and manage citations.
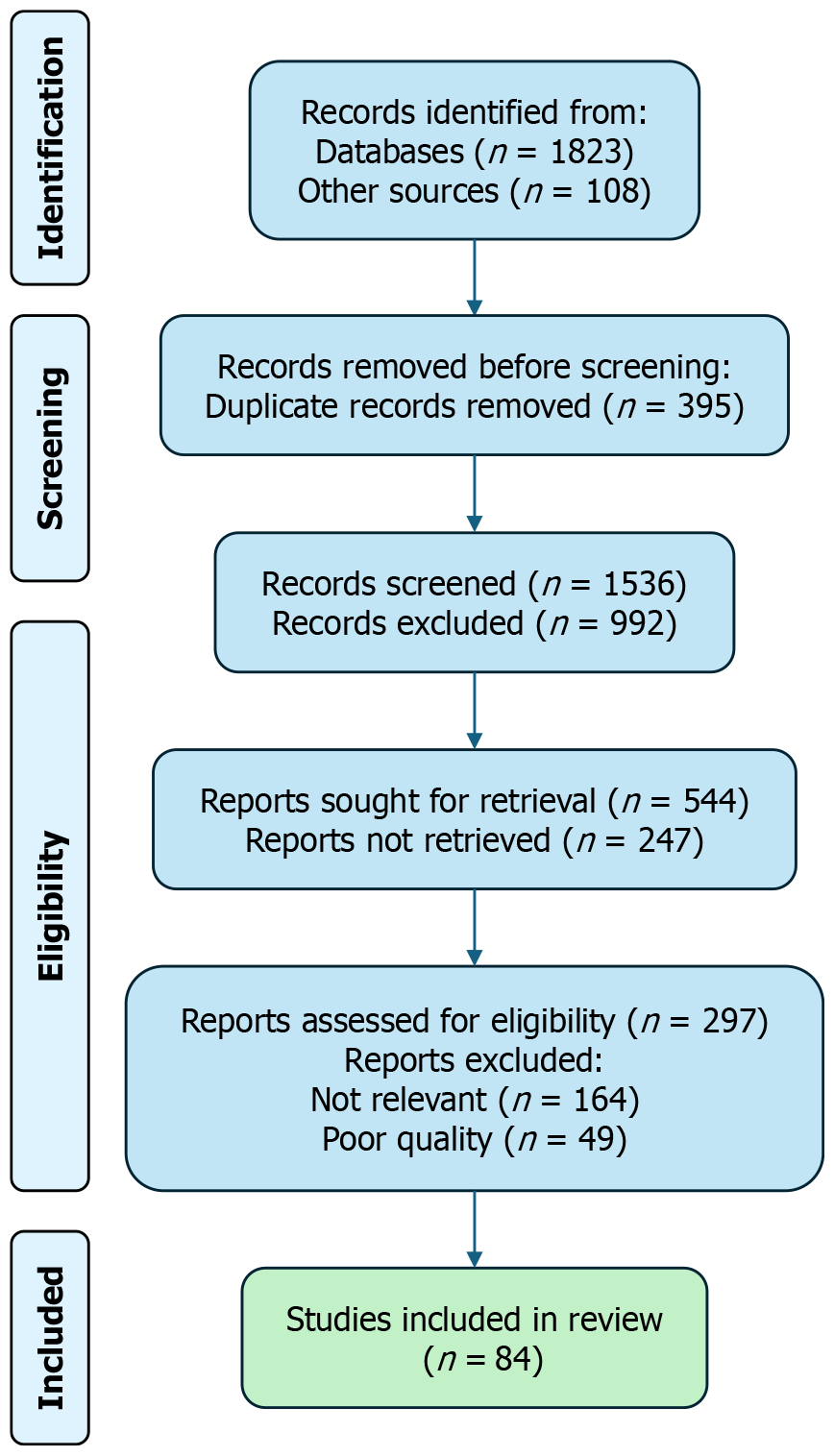
Both authors independently screened titles and abstracts against the eligibility criteria. Full-text articles were obtained for studies identified as potentially relevant. We resolved inclusion disagreements by discussion until consensus. Figure 1 (PRISMA 2020) shows the study-selection flow and reasons for full-text exclusion. In total, 84 studies met the inclusion criteria and were retained for final synthesis. Detailed characteristics of the included studies are summarized in Supplementary Table 1. PRISMA 2020 flow diagram illustrating the identification, screening, and inclusion process for the studies included in this narrative review.
We created a standardized Excel form for data extraction. The following variables were extracted independently by two reviewers: Study characteristics (authors, year, country, and design); participant characteristics (sample size, age, and parity); study focus (e.g., prevalence, biomarkers, and interventions); type of intervention (e.g., CBT, pharmacologic, and digital); outcomes measured (e.g., symptom severity, biomarkers, and adherence); and major findings and reported limitations. Discrepancies were resolved through consensus. Data were grouped thematically based on the review's core domains: Epidemiology, risk factors, biological mechanisms, and intervention strategies.
Because the included studies span randomized controlled trials (RCTs), cohort analyses, and qualitative work, we used study-specific appraisal tools rather than a single uniform checklist. Appropriate quality assessment frameworks were selected based on study type. Interventional studies were evaluated using the Joanna Briggs Institute Critical Appraisal Tools. Observational studies were reviewed using the Newcastle-Ottawa Scale, while qualitative studies were appraised for methodological rigor based on criteria such as credibility, transferability, dependability, and confirmability.
We could not perform a meta-analysis because the studies differed in participants, methods, interventions, and outcomes. Instead, a narrative synthesis approach was adopted. The included studies were thematically grouped and analyzed according to four primary domains aligned with the research objectives: (1) Epidemiological trends and prevalence estimates; (2) Risk stratification and individual-level predictors; (3) Biological mechanisms, including neuroendocrine, immune, and gut-brain pathways; and (4) Intervention strategies. We highlighted innovations—biomarker-guided care, digital platforms, and culturally tailored approaches—that signal new translational progress.
Extensive research confirms that PPD has multifactorial etiology, with psychological and biological factors consistently identified as the strongest predictors. An umbrella review encompassing over 80 systematic reviews has confirmed that a personal history of depression and antenatal anxiety represent the most powerful predictors of PPD onset[12]. These findings are supported by high-certainty evidence and underscore the enduring impact of pre-existing and antenatal mental health on postpartum outcomes.
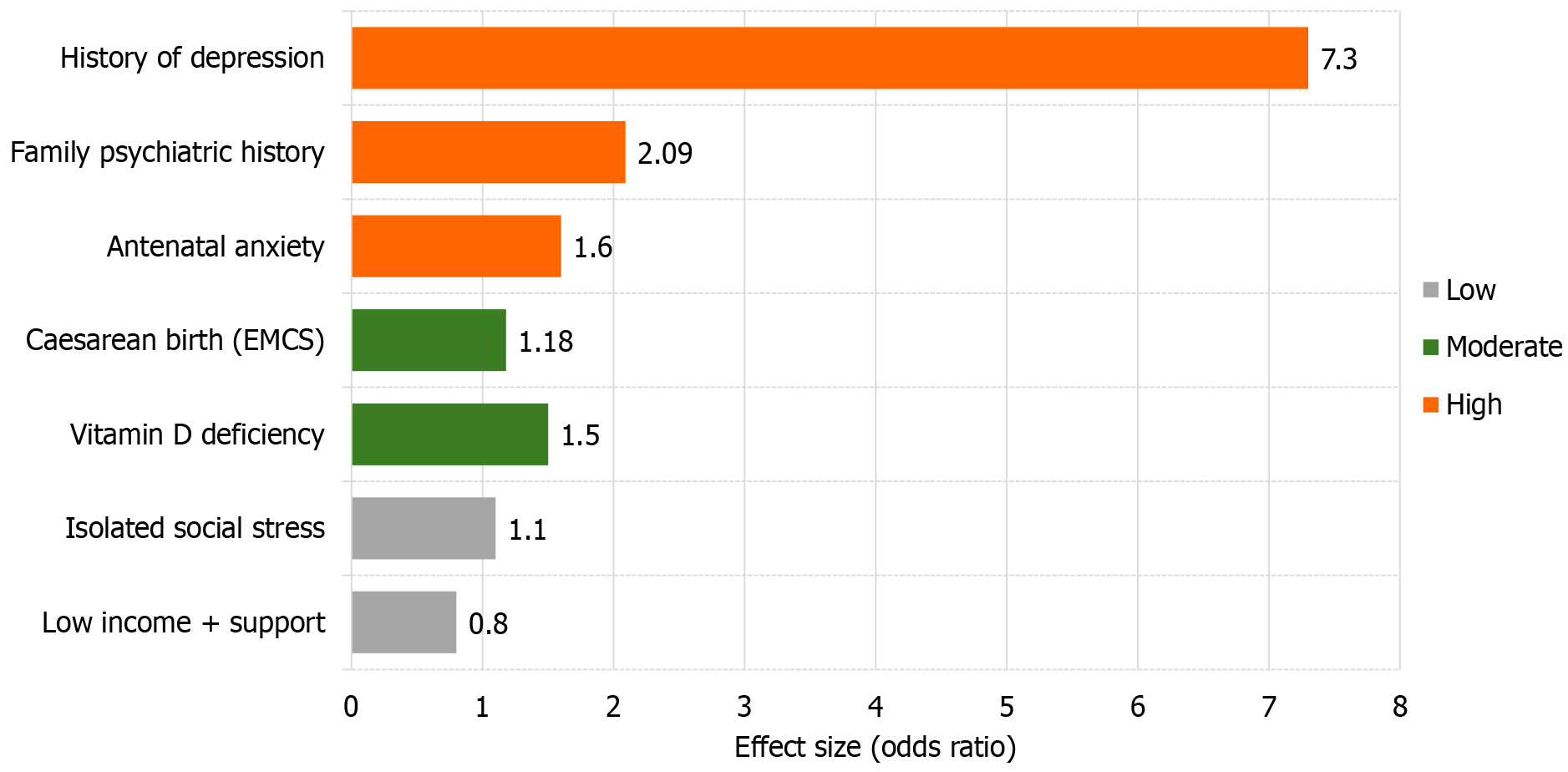
In addition to these primary predictors, a family history of psychiatric disorders has also been established as a major risk factor, with a recent systematic review and meta-analysis reporting a twofold increase in risk for women with such a history (odds ratio [OR] = 2.09;[13]).
Risk factors with moderate certainty of evidence include: (1) Emergency or unplanned caesarean section: A meta-analysis of 27 studies found an increased risk of PPD associated with this delivery mode (OR ≈ 1.18; 95% confidence interval [CI]: 1.09-1.26[14]); and (2) Low serum 25-hydroxy-vitamin D levels: Across several randomized controlled trials and meta-analyses, vitamin D deficiency was associated with a 40% to 80% increase in PPD risk, with ORs ranging from 1.4 to 1.8[15].
In contrast, low-certainty risk factors include isolated or transient psychosocial stressors, such as short-term financial hardship in the absence of a prior mental health diagnosis. These variables have demonstrated inconsistent or weak associations with PPD across studies, falling into lower tiers of evidentiary support[16].
Stratifying risk factors based on evidence strength facilitates a tiered clinical approach to prevention and early intervention. Those identified as high-risk—especially women with a personal or family history of depression—can benefit from targeted surveillance and psychosocial support during the perinatal period.
Table 1 provides a synthesized classification of leading psychosocial and biological risk factors for PPD, organized by the strength of supporting evidence from systematic reviews and large-scale cohort studies. Only variables supported by at least two high-quality meta-analyses or large cohorts (n > 1000) were designated as high-evidence factors.
| Evidence level | Risk factors (≥ 2 high-quality SRs/> 1 000-case cohorts) | Representative evidence & effect size |
| High | Previous major depression; Antenatal (pregnancy) anxiety; Family history of psychiatric disorders | OR ≈ 7.3 for prior depression[12]; OR = 2.09 for family history[13] |
| Medium | Caesarean birth, especially emergency CS (EMCS OR ≈ 1.18); 25(OH)-vitamin D deficiency (OR ≈ 1.4-1.8 in RCTs) | OR = 1.18 in pooled meta-analysis[14]; Systematic review of RCTs[15] |
| Low | Single social-stress event; Low income with adequate social support | No consistent pooled effect; evidence limited to small cross-sectional or non-replicated studies[16] |
A visual summary of these risk factors and their graded evidence levels is presented in Figure 2, facilitating rapid comparison of clinical significance and prioritization for screening and intervention. Figure 2 helps clinicians quickly spot high-priority risk factors and plan early screening or intervention.
PPD stems from intertwined biological, psychological, and sociocultural factors. Understanding these interacting domains is essential for identifying at-risk individuals and tailoring early interventions.
Biological determinants: Biological mechanisms underpinning PPD primarily involve neuroendocrine disruptions triggered by the abrupt hormonal shifts that follow childbirth. The rapid postpartum decline in estrogen and progesterone destabilizes neurotransmitter systems, especially GABAergic signaling, which is closely associated with mood regulation[1]. In addition, nutritional deficiencies—notably in vitamin D, zinc, omega-3 fatty acids, and elevated homocysteine levels—have been consistently associated with increased vulnerability to PPD[17,18].
The mode of delivery can affect both biological stress responses and maternal psychological well-being. Specifically, emergency cesarean sections have been associated with an almost twofold increase in PPD risk compared to vaginal deliveries, likely due to both physiological stress and perceived loss of control[7].
Psychological determinants: Psychological vulnerabilities are major drivers of PPD. A prior diagnosis of depression is one of the most reliable and consistently replicated risk factors across studies[4]. Additionally, certain personality traits—including neuroticism, trait anxiety, and self-critical tendencies—have been closely linked to postpartum emotional dysregulation[5]. Evidence suggests that heightened self-criticism during late pregnancy predicts depressive symptomatology as early as 8 weeks postpartum.
Another psychological dimension is maternal self-efficacy, or a mother’s confidence in her ability to care for her child. Low maternal self-efficacy has emerged as a potent predictor of both PPD and anxiety, increasing the risk by nearly six-fold and ten-fold, respectively[4].
Sociocultural and environmental determinants: Economic hardship, marital conflict, social isolation, and other sociocultural stressors amplify biological and psychological risks. Persistent financial stress, marital discord, lack of partner support, social isolation, and exposure to adverse life events are all significantly correlated with elevated PPD risk[7,17]. Among these, inadequate partner support stands out as particularly influential, with studies showing a 4.5-fold increase in PPD risk in its absence[7].
Moreover, breastfeeding behavior has also emerged as an important sociobiological marker. Failure to initiate or sustain breastfeeding by the third postpartum week has been independently linked to higher PPD risk, possibly reflecting both hormonal disruptions and psychosocial distress[4].
These findings call for risk-assessment tools that combine biological, psychological, and social factors. Early identification of high-risk mothers through such integrative screening models may allow for more targeted, timely, and effective interventions to mitigate the onset and severity of PPD.
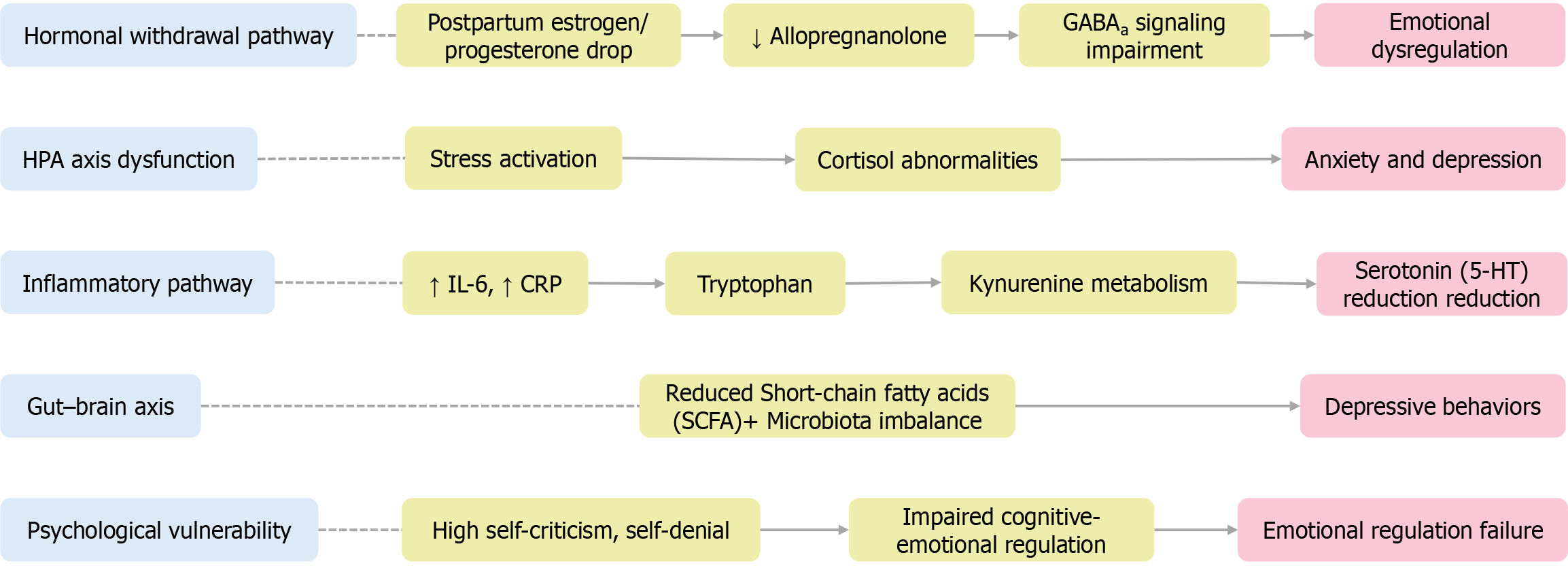
The pathophysiology of PPD is inherently complex, encompassing interactions among neuroendocrine, neurochemical, immune, and psychological systems. Rather than stemming from a single origin, PPD emerges from an intricate network of biological susceptibilities and cognitive-emotional processes triggered by the postpartum state.
Neuroendocrine and neurochemical pathways: Among the most critical biological contributors to PPD is the abrupt withdrawal of reproductive hormones—specifically estrogen and progesterone—immediately following childbirth. This hormonal decline disrupts the expression of GABAa receptor subunits, resulting in impaired inhibitory neurotransmission and decreased synthesis of allopregnanolone, a neurosteroid vital for mood stabilization[18]. Such neurochemical perturbations may be especially pronounced in individuals with pre-existing neurobiological susceptibilities.
Neuroimaging studies corroborate this mechanism. A comprehensive 2024 review by Zhang et al[19] synthesizing PET and MRS studies demonstrated that postpartum estradiol withdrawal corresponds with reduced cortical allopregnanolone levels and diminished GABAergic tone, both of which closely correlate with depressive symptom severity. These findings provide clinical support for emerging GABAa-modulating interventions, such as zuranolone. Similarly, a Swiss prospective cohort study showed that women with low or steeply declining salivary allopregnanolone trajectories had a 3.4 times greater odds of scoring ≥ 13 on the EPDS, with a notable U-shaped association between allopregnanolone levels and depression symptoms[20].
A multi-center RCT showed that a 14-day zuranolone course produced rapid, lasting symptom relief by day 3 postpartum, confirming the value of GABA-targeted therapy[21].
The hypothalamic-pituitary-adrenal axis is another key pathway. Dysregulated cortisol activity—manifesting as either blunted or exaggerated stress responses—has been consistently linked to heightened depressive and anxiety symptoms in the postpartum period[1,18].
Neuroimaging shows heightened amygdala responses to negative cues in PPD, a pattern that differs from non-perinatal depression[22]. Additionally, increased monoamine oxidase activity postpartum may accelerate the breakdown of serotonin, dopamine, and norepinephrine, exacerbating emotional instability[23].
Cognitive and emotional processing: Psychological vulnerabilities compound biological risk factors. Traits such as self-criticism, trait rumination, and poor emotional regulation have been strongly associated with PPD onset[5]. Maladaptive cognitive styles—characterized by persistent negative self-evaluation, emotional suppression, and dysfunctional interpersonal schemas—impair postpartum coping capacity and may disrupt mother-infant bonding and caregiving behaviors. Deficits in emotional awareness and cognitive flexibility further intensify psychological distress and interfere with adaptive functioning in the early postpartum period.
Immuno-inflammatory signaling: Accumulating evidence highlights the role of systemic inflammation in PPD pathophysiology. A recent study employing a machine learning approach identified interleukin-6 as a key predictive biomarker, reporting an area under the receiver operating characteristic of 0.87 for distinguishing PPD from healthy controls and 0.94 for PPD comorbid with anxiety[24]. Elevated IL-6 Levels within 24 hours postpartum have also been linked to depressive symptoms persisting up to six months, supporting its utility as a clinically relevant early indicator. These findings suggest that inflammatory dysregulation may influence mood by interacting with the kynurenine-serotonin pathway, ultimately impairing serotonergic signaling[25].
Gut-brain axis dysbiosis: Research on maternal gut microbiota has revealed compositional alterations in women with PPD, including reduced levels of short-chain fatty acid-producing bacteria and increased Firmicutes/Bacteroidetes ratios. Notably, fecal microbiota transplantation from PPD-affected mothers into germ-free mice induces depression-like behaviors, implicating gut-derived immune and neurochemical pathways in mood regulation[26,27].
Together, these findings support a multi-systemic model of PPD, in which hormonal withdrawal, neurochemical imbalances, immune activation, gut microbiome disruption, and maladaptive cognitive-emotional processing interact to produce the clinical syndrome of PPD. Understanding these interacting pathways helps design targeted prevention and treatment.
Figure 3 visually summarizes these five major mechanistic pathways—highlighting the neuroendocrine, neurochemical, immune, gut-brain, and cognitive dimensions contributing to the development of PPD, adapted from multi-systemic conceptual frameworks and updated with neuroimaging data presented by Zhang et al[19].
These findings support treatment models that unite biological, psychological, and behavioral approaches. A systems-based understanding of PPD may also facilitate the identification of novel biomarkers and support the development of personalized therapeutic strategies aimed at enhancing early detection and improving maternal outcomes.
PPD arises from complex perturbations across neuroendocrine, immune, gut-brain, and psychosocial systems. To move beyond fragmented etiological perspectives, we propose a systems biology framework that integrates emerging multi-omics biomarkers—including genomic variants, cytokine profiles, gut microbial metabolites, and plasma proteomic signatures[28]—with longitudinal clinical phenotyping and real-time digital health streams. The model echoes recent translational work showing that multimodal biomarkers can guide personalized PPD care[19].
We also emphasize the contribution of multimodal neuroimaging studies, which have revealed early structural and functional brain network alterations in women with PPD, supporting the incorporation of neural signatures into predictive models[29]. To operationalize this framework, this article outlines a cross-disciplinary collaboration model spanning psychiatry, obstetrics, immunology, digital epidemiology, and public health. This systems-level integration not only advances mechanistic insight but also facilitates the development of scalable, adaptive care models that reflect the complexity and diversity of real-world maternal mental health needs. To ensure effective coordination, this model advocates for the establishment of shared data platforms, joint training programs, and translational research consortia that foster sustained interaction among clinical practitioners, neuroscientists, public health experts, and computational modelers. Shared data platforms and joint training can connect basic discoveries with scalable interventions.
Cultural stigma and health inequity: PPD disproportionately affects rural, low-income, and ethnic-minority women, especially in low- and middle-income countries (LMICs). Women in these settings face multiple barriers to mental-health care: Scarce professional services, few culturally appropriate interventions, and persistent social stigma.
Emerging evidence from Sub-Saharan Africa underscores the effectiveness of community-based, culturally adapted mental health interventions. In Uganda and Ethiopia, structured programs such as psychoeducational home visits and community-led follow-up sessions have been shown to improve service engagement, reduce EPDS scores, and foster greater trust in formal care pathways[30]. Furthermore, studies from Kenya and Uganda highlight the critical role of non-primary caregivers, such as grandmothers and extended kin, in shaping maternal well-being and early infant care practices. These findings support the development of culturally congruent parenting interventions that involve family elders and align with local caregiving hierarchies[31].
In India, structural inequities continue to hinder access to perinatal mental health services. Contributing factors include geographic inaccessibility, poor mental health literacy, and system-level stigma. A meta-analysis revealed persistent underutilization of psychological services despite high clinical demand, largely due to sociocultural and infrastructural barriers[32].
In Vietnam, qualitative research has illuminated the impact of internalized shame, patriarchal norms, language barriers, and low partner involvement, all of which deter postpartum women from seeking psychiatric care[33].
A recent meta-analysis by Chan and Shorey[34] found that in Asian caregiving contexts, psychosocial interventions—particularly CBT and mother-infant dyadic support—effectively alleviate psychological distress. However, such distress often remains underreported due to cultural ideals of emotional restraint, maternal stoicism, and familial role expectations. These sociocultural dynamics perpetuate symptom concealment and contribute to delays in help-seeking behavior. For instance, in peri-urban Pakistani communities, PPD is frequently perceived not as a psychiatric disorder, but rather as a manifestation of personal failure, family discord, or spiritual imbalance, with religious frameworks often taking precedence over biomedical explanations[35].
Framework for inclusive intervention: Effectively addressing these disparities requires the adoption of culturally responsive, community-anchored mental health strategies. Successful interventions in marginalized populations should adhere to the following principles: (1) Be culturally tailored, reflecting indigenous beliefs, family structures, and caregiving traditions; (2) Be delivered by trusted local figures, including peer mothers, community health workers, traditional birth attendants, and religious leaders; (3) Be embedded within inclusive policy frameworks that ensure affordability, accessibility, and integration with routine maternal and child health services; (4) Involve partners and family members, promoting shared caregiving responsibilities and familial support networks; and (5) Be accompanied by anti-stigma public health campaigns, particularly within prenatal and postnatal care systems.
These multidimensional strategies are essential to reducing the global burden of PPD and ensuring equitable access to mental health resources for historically underserved communities. The insights presented here reinforce the necessity for context-specific, individualized models of care, which will be elaborated in the following section on integrative intervention frameworks.
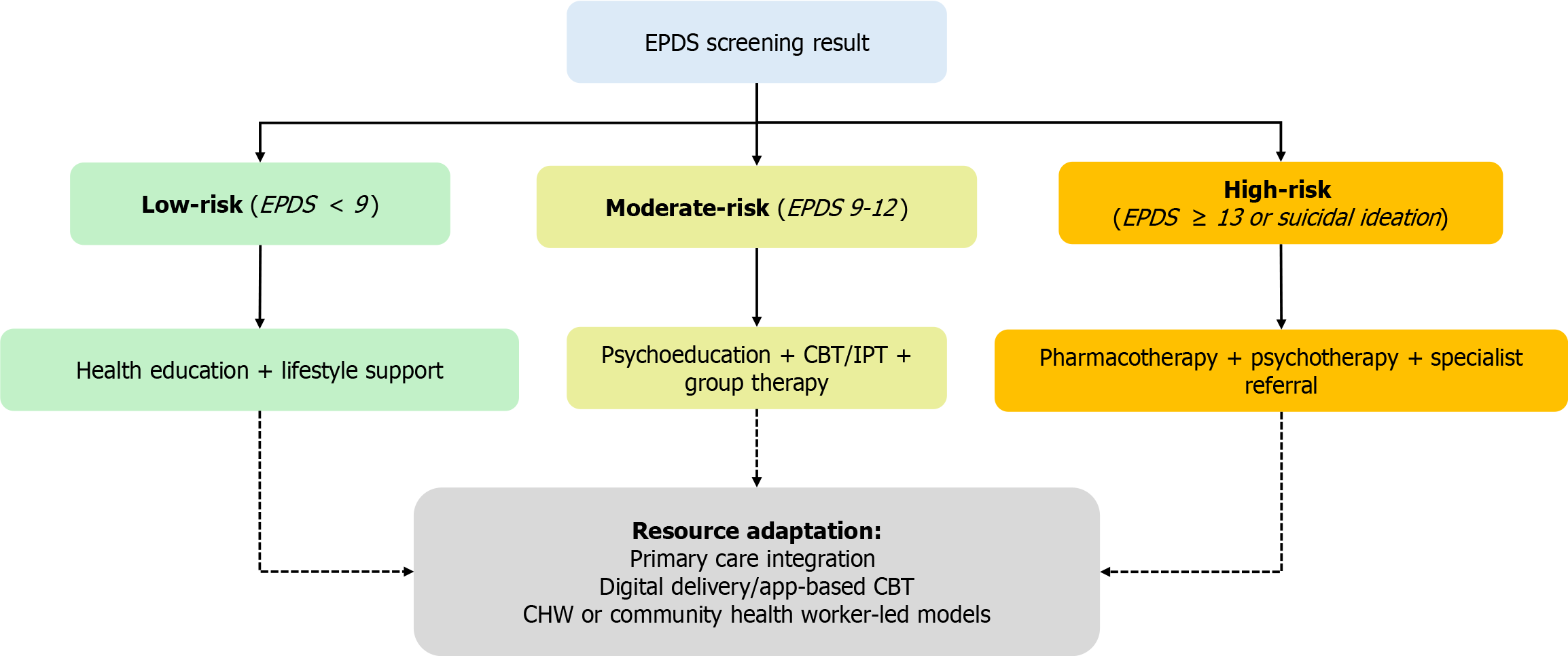
Screening and risk stratification using EPDS: Effective PPD programs begin with standardized screening followed by risk-stratified interventions. The EPDS—a validated 10-item self-report questionnaire—remains the international gold standard for identifying perinatal depressive symptoms. A threshold score of ≥ 13 is typically indicative of clinically significant depression[6].
International clinical guidelines recommend administering the EPDS at least twice: Once in late pregnancy and again between 4-6 weeks postpartum[36,37]. Early detection enables targeted, tiered intervention planning: (1) Low-risk: General psychoeducation, lifestyle counseling, and informal social support; (2) Moderate-risk: Structured psychoeducation, CBT, or facilitated peer support; and (3) High-risk: Referral to specialized mental health services, pharmacological intervention, and integrated psychological care. The EPDS is particularly advantageous in primary care and low-resource settings, given its brevity, ease of administration, and minimal training requirements. It is adaptable for use across urban and rural populations, making it a practical tool for scalable and equitable PPD management.
Yet recent studies reveal key EPDS limitations, particularly in culturally diverse or marginalized groups. Concerns include: (1) Cultural inappropriateness: Items assessing guilt, self-blame, and affective expression are often misinterpreted or underreported in collectivist or non-Western contexts[38,39]; (2) Inconsistent thresholds: Studies in LMICs report variable sensitivity and specificity across language versions, raising concerns about cut-off reliability and generalizability[40,41]; (3) Literacy and stigma barriers: In refugee and asylum-seeking populations, the EPDS is hampered by low item clarity, high literacy demands, and the potential for stigmatizing misclassification[42]; and (4) Clinician concerns: Health professionals report discomfort and perceived irrelevance when using the EPDS with immigrant or minority mothers, often due to cultural discordance[43].
These limitations underscore the need for localized validation, contextual threshold recalibration, and the development of hybrid or adaptive screening models that better capture the mental health experiences of diverse populations.
Personalized and context-sensitive intervention design: Effective intervention strategies must extend beyond standardized tools by accounting for psychological traits, social environments, and cultural worldviews. For instance, women with high neuroticism or self-critical tendencies may derive greater benefit from CBT or mindfulness-based therapies that target maladaptive cognitive patterns[5]; and those experiencing partner absence, social isolation, or family conflict may respond better to home visitation programs or community-based participatory interventions, which enhance engagement and emotional support[7].
Accounting for individual differences: Individual traits strongly influence which PPD interventions that women accept and benefit from. Systematic reviews have revealed significant heterogeneity in intervention design and outcomes—variability that may, in part, stem from inadequate consideration of individual psychological, sociodemographic, and cultural differences[44].
Personality traits, such as neuroticism, self-criticism, and trait anxiety, have been consistently associated with increased vulnerability to PPD and differential responsiveness to specific treatment modalities[5]. For instance, women with high neuroticism may benefit more from structured CBT, whereas those with high interpersonal sensitivity might respond better to relational or peer-led interventions.
Sociodemographic variables—including maternal age, parity, educational attainment, and digital literacy—also influence intervention uptake, adherence, and efficacy. Women with lower educational levels or limited technological experience often face accessibility and usability challenges when engaging with app-based or online mental health platforms, thereby limiting their utility in such populations[45].
Cultural norms and racial/ethnic identity further modulate how depressive symptoms are experienced, expressed, and acted upon. Cultural stigma, historical mistrust of healthcare systems, and language discordance may deter some women from seeking or maintaining mental health care, contributing to persistent disparities in diagnosis and treatment across racial and ethnic groups[46].
To enhance the real-world effectiveness and equity of PPD care, future research should integrate stratified analyses and culturally responsive frameworks that tailor intervention design and delivery to diverse maternal populations. Personalizing treatment approaches in this way can help close service gaps and optimize outcomes across heterogeneous settings.
Delivery mode and cultural optimization: How an intervention is delivered largely determines whether it can reach many mothers and remain effective. In resource-constrained settings, group-based interventions—such as yoga, music therapy, or peer-led psychoeducation—offer cost-effective, non-stigmatizing, and culturally congruent modalities[11,47]. Embedding these services within existing maternal care platforms enhances sustainability and increases program reach.
While digital mental health interventions (DMHIs) present scalable solutions, real-world implementation challenges persist, including: (1) Technological barriers: Poor internet access, low smartphone ownership, and digital illiteracy limit uptake in low-income and rural populations[48,49]; (2) Privacy and trust concerns: Distrust in digital systems is particularly pronounced in minority populations, where fears of surveillance, data misuse, and medical discrimination persist[49]; (3) Low engagement: Without human facilitation, dropout rates for digital CBT apps can exceed 50% within weeks[50,51]; and (4) Algorithmic inequity: Race-based correction factors embedded in some algorithms may unintentionally undermine care quality and eligibility for vulnerable groups[49].
To mitigate these risks, hybrid models—which combine digital platforms with human facilitation (e.g., via community health workers or telehealth)—have demonstrated higher engagement and effectiveness. Key design considerations for equity include: Multilingual access; offline functionality; device-sharing compatibility; and inclusive, participatory development processes.
This integrative approach supports the design of customized, resource-sensitive, and culturally responsive PPD intervention models. It emphasizes alignment between clinical risk, delivery modality, and sociocultural context.
Figure 4 provides a visual framework for mapping clinical risk levels to tailored intervention pathways, delivery formats, and support structures.
This stepped-care model accommodates both pharmacological and non-pharmacological approaches, offering a flexible and context-sensitive blueprint for PPD intervention. By aligning intervention intensity with individual risk profiles, it supports early detection, timely management, and equitable mental health care. The framework is designed to function effectively across diverse clinical, cultural, and socioeconomic environments[52], acknowledging that cultural stigma, emotional burden, and lack of support can significantly affect engagement and outcomes among vulnerable subgroups.
Overview of evidence-based modalities: Table 2 presents a structured summary of the four principal evidence-based intervention modalities for the treatment of PPD, organized by strength of evidence and clinical applicability.
| Modality | Core elements | Key evidence (illustrative) | Target population | Evidence grade |
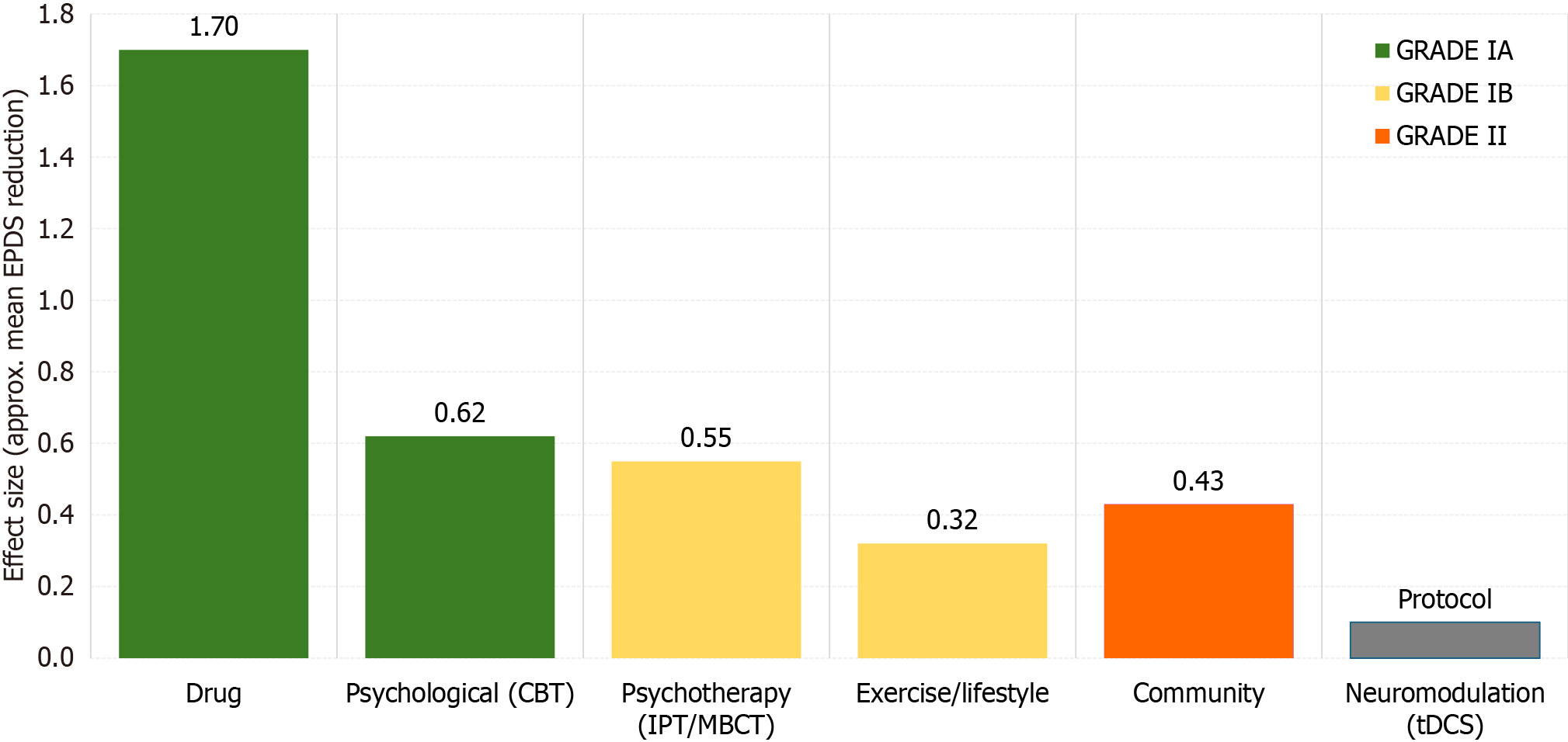
| Drug | Brexanolone 60 hours IV; zuranolone 14 days oral; conventional SSRIs | Brexanolone phaseIII (n = 246): Mean ΔPHQ19 = -17.0 (95%CI: -19.3 to -14.7) within brexanolone arm, -14.5 vs placebo; zuranolone phaseII: Rapid onset | Moderatetosevere PPD; lactating mothers with careful evaluation | IA |
| Psychological/psychotherapy | CBT (facetoface/online); IPT (partner/group); MBCT | CBT metaanalysis 2022 (31 RCTs) SMD -0.62; coupleIPT RCT ΔEPDS -4.1; MBCTPD trial: 50% relapserisk reduction | Mildtomoderate PPD or women declining drugs | IA |
| Exercise/lifestyle | ≥ 150 minutes per week moderate aerobic exercise; highfiber diet; vitaminD supplementation | Systematic review 2022 (35 studies) RR 0.77; highfiber diet improves gut microbiota & mood | Women ≥ 6 weeks postpartum who are physically able | IB |
| Integrated/community | CHW “Thinking Healthy” module; digital appbased CBT; WHO mhGAP + group IPT | WHO mhGAP + nurseled groupIPT RCT in Nepal: ΔEPDS -4.3 | Resourcelimited or lowaccess settings | II |
| Neuromodulation (pilot/protocol) | Anodal prefrontal tDCS 2 mA × 20 minutes, 10 sessions | Protocol only: Sun et al[52], 2023 RCT design; planned n = 120, primary outcome ΔEPDS at 4 weeks | Moderate PPD unresponsive to CBT/SSRI | NR |
This comparative framework highlights each modality’s core therapeutic components; level of supporting evidence (based on randomized controlled trials, meta-analyses, and clinical guidelines); and optimal target population (e.g., by severity, setting, or psychosocial profile).
Table 2 serves as a decision-support tool for clinicians, policymakers, and program designers, facilitating the alignment of intervention strategies with diverse maternal populations and varying resource settings. It also provides the foundational framework for the detailed analysis of each modality presented in the subsequent sections.
To visually compare the relative impact and certainty of these interventions, Figure 5 presents a harmonized summary of effect sizes and GRADE-based evidence levels derived from the core trials referenced in Table 2.
Aligning evidence with intervention strategy: Given the heterogeneous risk profiles of PPD, contemporary clinical guidelines increasingly advocate adopting a stepped-care model. This framework begins with universal or risk-stratified EPDS screening, followed by tailored interventions based on factors such as symptom severity, breastfeeding status, patient preferences, and resource availability[53].
Available treatments range from CBT and IPT to lifestyle programs, community care and, for moderate-to-severe cases, medication. This adaptive structure allows for both escalation and de-escalation based on clinical response and contextual fit.
Pharmacologic therapies: Brexanolone (60-hour IV infusion): In two multicenter phase III trials (n = 246), brexanolone demonstrated a significant reduction in depressive symptoms compared to placebo, with least-squares mean differences of: HAM-D17: -17.7 vs -12.9; EPDS: -19.1 vs -10.9; and PHQ-9: -14.3 vs -8.8 (all comparisons: P < 0.001)[54].
Due to risks of excessive sedation and sudden loss of consciousness, brexanolone is subject to Zulresso REMS restrictions, requiring continuous monitoring (pulse oximetry and face-to-face supervision) during the 60-hour inpatient infusion.
A multicenter RCT (n = 151) reported symptom improvements with zuranolone (14-day oral course) by Day 15: HAM-D: -17.8 vs -13.6; and EPDS: -16.9 vs -11.4 (improvements sustained through Day 45)[21] Common side effects include somnolence (15%) and dizziness (13%).
Brexanolone is indicated for moderate-to-severe cases of PPD necessitating rapid symptomatic relief under monitored inpatient settings. Zuranolone provides a home-based alternative with comparable onset but without REMS constraints.
Selective serotonin reuptake inhibitors (SSRIs; e.g., sertraline) remain first-line for mild-to-moderate PPD, especially during maintenance or when psychotherapy is insufficient.
Psychological interventions: CBT remains a cornerstone non-pharmacological intervention for perinatal depression. A 2022 meta-analysis of 79 randomized trials found that CBT significantly reduced depressive symptoms in both the short term (SMD = -0.69; 95%CI: -0.83 to -0.55) and long term (SMD = -0.59; 95%CI: -0.75 to -0.42) across diverse perinatal populations[55]. More recently, an analysis of 18 trials involving 3689 postpartum women demonstrated that online CBT also produced meaningful reductions in depressive symptoms, particularly when interventions were ≥ 9 weeks, professionally guided, and delivered via secure platforms[56].
IPT is a first-line option, especially for women facing relationship conflict, role changes, or limited support. A randomized controlled trial in China demonstrated that couple-based IPT significantly reduced depressive symptoms in both mothers and fathers at 6 weeks and 6 months postpartum compared to routine care[57]. A 2024 Campbell systematic review further confirmed IPT’s effectiveness in LMICs, showing comparable or superior outcomes to other psychological and pharmacological interventions in reducing PPD severity[58]. Clinical guidelines recommend a minimum of six structured sessions, with pharmacologic augmentation reserved for non-responders.
Recent trials support expanded modalities: A community-based group IPT trial in Uganda demonstrated > 60% remission and significant EPDS reduction using World Health Organization (WHO)-adapted formats[59].
A 2022 meta-analysis confirmed the efficacy of mindfulness-based cognitive therapy (MBCT), particularly in non-medicated women[60].
These findings affirm the effectiveness and adaptability of structured psychotherapies across varied cultural and resource settings.
Lifestyle and exercise prescriptions: Lifestyle-based interventions play a crucial role in reducing the risk and severity of PPD. A 2023 network meta-analysis of 26 randomized trials (n = 2867) found that moderate-intensity aerobic exercise—performed 3 to 4 times per week for 35-45 minutes—significantly reduced depressive symptoms compared to standard care (MD = -1.90; 95%CI: -2.58 to -1.21)[61]. Earlier meta-analytic data also support preventive effects of prenatal and postnatal physical activity, particularly when initiated within the first 12 postpartum weeks[62]. Nutritional strategies—such as high-fiber diets and vitamin D supplementation—may also alleviate depressive symptoms by modulating the gut microbiota and reducing systemic inflammation, though large-scale pragmatic trials are needed to confirm these effects[63].
Community-based and digital delivery models: In low-resource environments, the WHO-endorsed Thinking Healthy Programme—delivered by community health workers (CHWs)—has been shown to reduce EPDS scores by 4-5 points and achieve remission rates of up to 70%[64].
Digital adaptations of these models now include brief psychoeducation modules, automated CBT tools, and peer-to-peer support networks. These platforms work as well as clinic care and greatly improve access and scalability[65]. They show how task-sharing and digital tools can fill mental-health gaps where specialist care is scarce or stigmatized.
Cost-effectiveness considerations: Many PPD interventions work clinically, but their cost and infrastructure needs differ greatly, complicating large-scale adoption. High-intensity treatments such as brexanolone—which requires a 60-hour continuous intravenous infusion under close clinical monitoring—impose substantial logistical and financial burdens that limit widespread accessibility, especially outside high-resource settings[18]. Similarly, transcranial direct current stimulation (tDCS), though noninvasive, necessitates specialized equipment, trained personnel, and adherence protocols. Recent pilot trials have highlighted these real-world challenges, with some studies terminated early due to feasibility issues[66].
In contrast, low-intensity psychosocial interventions—including community-based psychoeducation and task-shifted CBT—present cost-effective and scalable alternatives. These modalities require minimal infrastructure and have shown favorable economic profiles in recent randomized implementation studies, particularly in LMICs[67].
Nevertheless, the broader literature continues to lack comprehensive comparative economic evaluations across intervention types. In particular, few studies integrate long-term outcome modeling, human resource demands, or delivery logistics into cost-effectiveness assessments. Incorporating rigorous economic frameworks into future PPD intervention research is essential to support evidence-informed policy-making and ensure equitable, efficient delivery of postpartum mental health services in diverse global contexts. Future implementation studies should incorporate cost-utility analyses and budget impact modeling to guide prioritization of resource allocation, particularly in health systems operating under fiscal constraints.
To promote consistent, high-quality care for PPD, numerous global health authorities have issued evidence-based guidelines that address screening, diagnosis, and treatment pathways. Alignment with these recommendations enhances early detection, treatment efficacy, and continuity of care—especially in low-resource settings and among vulnerable populations. Central to these frameworks are standardized screening tools; stepped-care models; and culturally sensitive, accessible interventions.
Together, these strategies improve maternal mental health for individuals and communities. Key institutional guidelines are described below.
WHO: The WHO recognizes maternal mental health as a global public health priority. Its 2022 guideline on early childhood development recommends routine screening with validated tools such as the EPDS during antenatal and postnatal care. The WHO also advocates task-shifting strategies, enabling CHWs to deliver low-intensity psychological interventions (e.g., CBT-lite and problem-solving therapy) in areas lacking specialist providers[68].
American College of Obstetricians and Gynecologists: The American College of Obstetricians and Gynecologists (ACOG) endorses universal screening for depression and anxiety at least once during pregnancy and once postpartum, using tools such as the EPDS or PHQ-9. Positive screens should be followed by structured follow-up, referral, and appropriate treatment. First-line interventions for mild to moderate cases include CBT, IPT, and peer support[69].
National Institute for Health and Care Excellence (United Kingdom): The National Institute for Health and Care Excellence (NICE) guideline CG192 (updated in 2020) recommends a stepped-care approach for identifying and managing perinatal mental health conditions. It advises initial screening using validated tools such as the EPDS, followed by clinical interviews if necessary. For moderate to severe depression, high-intensity psychological interventions such as CBT or IPT, or pharmacological treatment, are recommended. For mild symptoms, structured self-help approaches may be considered. The guideline also emphasizes the importance of shared decision-making, tailoring treatment choices to individual preferences and clinical needs[70].
United States Preventive Services Task Force: The United States Preventive Services Task Force (USPSTF)’s 2023 updated recommendation statement assigns a Grade “B” for both: (1) Routine screening of perinatal women with validated tools such as the EPDS or PHQ-9; and (2) Preventive counseling for those at elevated risk. The panel emphasizes shared decision-making, early referral pathways, and integration with primary-care workflows[71].
Canadian Task Force on Preventive Health Care: The Canadian Task Force advocates for systematic PPD screening using the EPDS within primary care during the first postpartum year. Special attention is given to ensuring access for underserved groups, particularly those facing geographic, linguistic, or economic barriers[72].
Royal Australian and New Zealand College of Psychiatrists: The Royal Australian and New Zealand College of Psychiatrists (RANZCP) recommends a stepped-care model beginning with lifestyle interventions, peer support, and low-intensity care for mild symptoms, and progression to structured psychotherapy (CBT/IPT) and pharmacologic treatment for moderate to severe cases. These guidelines emphasize partner involvement and family-centered care as crucial elements for improving maternal outcomes[73].
Synthesis and clinical integration: Across these authoritative bodies, several convergent themes emerge, forming a unified framework for optimal PPD care: (1) Routine screening using validated tools such as the EPDS during both prenatal and postnatal visits; (2) Selective task-sharing with non-specialist providers—particularly CHWs—is endorsed by the WHO to expand access to low-intensity psychological interventions; (3) Adoption of a stepped-care model, aligning treatment intensity with symptom severity; and (4) Psychological and psychosocial interventions—including CBT, IPT, psychoeducation, and structured peer support—are prioritized as first-line treatments for mild to moderate cases.
These international guidelines collectively advocate for standardized yet context-sensitive approaches that accommodate local healthcare infrastructure, cultural norms, and individual patient preferences. A global public health priority lies in scaling equitable, evidence-based interventions—especially for underserved and resource-constrained populations.
Despite significant advances in the screening, diagnosis, and treatment of PPD, critical knowledge gaps persist. Future research must extend beyond conventional paradigms to encompass biological innovations, digital technologies, family-centered approaches, and cross-cultural frameworks, enabling the design of more precise, scalable, and inclusive models of maternal mental health care.
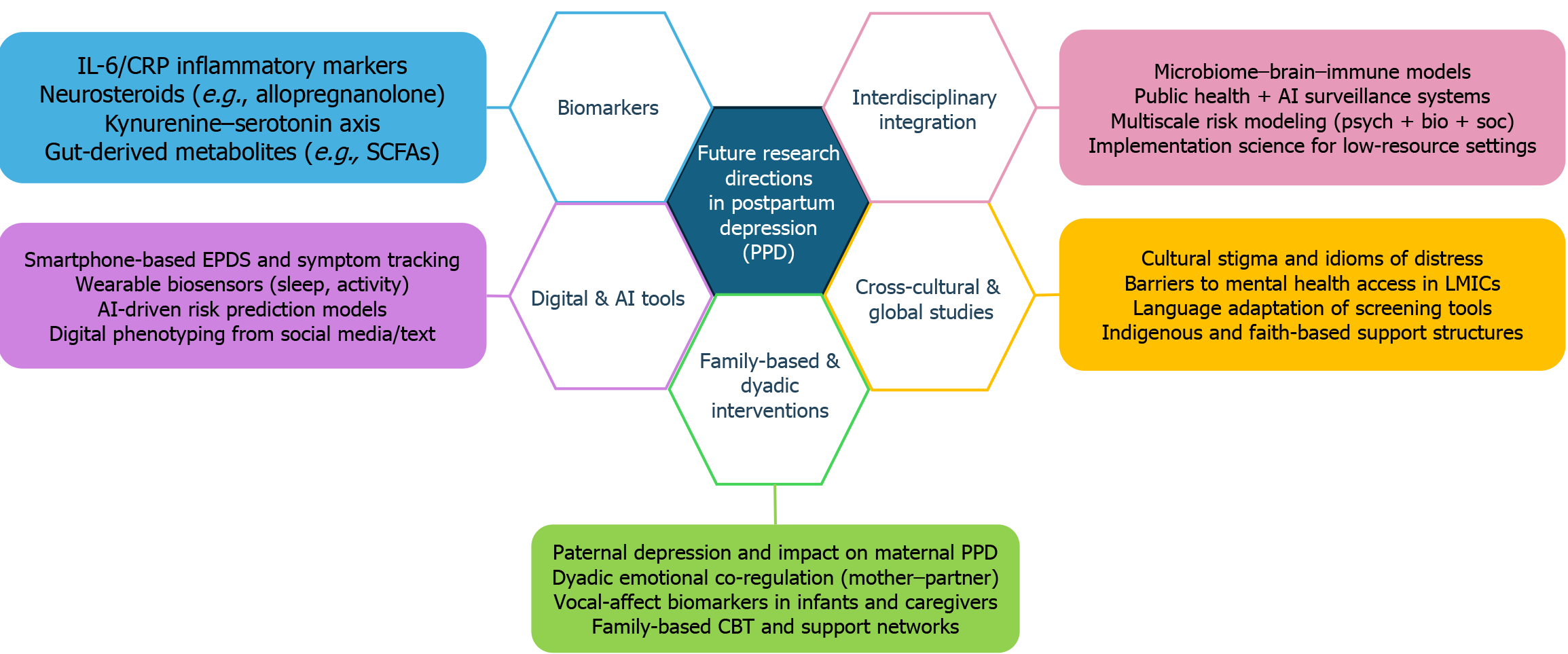
Identification of biological markers: Inflammatory markers such as C-reactive protein, IL-6, and cellular indices like the neutrophil-to-lymphocyte ratio (NLR), platelet-to-lymphocyte ratio (PLR), and monocyte-to-lymphocyte ratio (MLR) may help identify women at heightened risk for perinatal mental health disorders. A 2024 prospective observational study by La Verde et al[74] found significantly elevated levels of NLR, PLR, and MLR in postpartum women exhibiting depressive symptoms, underscoring the relevance of systemic inflammation in the pathophysiology of PPD. These findings strengthen the rationale for incorporating biomarker-guided risk stratification into early intervention strategies for perinatal mental health.
Digital technologies and AI-enabled screening: DMHIs hold promise as scalable, low-cost tools for delivering PPD care. However, implementation challenges remain substantial: Digital access and literacy gaps persist, particularly in rural or low-income regions with limited internet connectivity or device access[48,49].
Privacy and trust concerns, especially among historically marginalized communities, hinder adoption[49].
Low adherence is common in unguided applications, with dropout rates often exceeding 50% within weeks[50,51].
Algorithmic bias within AI-powered platforms—such as race-adjusted scoring—may inadvertently amplify disparities in care delivery[49].
To mitigate these challenges, hybrid models—combining human facilitation (e.g., CHW-assisted mobile CBT) with digital platforms—are gaining traction. Successful implementation depends on inclusive, co-designed interfaces, multilingual support, and offline functionality and device-sharing compatibility.
Simultaneously, AI-assisted screening tools are advancing rapidly. These include platforms capable of detecting depressive symptoms through natural language processing, voice analysis, and passive behavioral monitoring.
Coecke et al[75] reviewed emerging AI screening methods and emphasized the need for ethical oversight and data governance.
Le and Quang[76] demonstrated the utility of wearable devices that track sleep and movement patterns for early detection in low-resource contexts lacking mental health personnel.
Family and paternal mental health: While maternal outcomes have traditionally dominated the PPD literature, recent research has increasingly emphasized the interactive role of paternal mental health and broader family dynamics. Recent systematic evidence confirms that paternal PPD not only affects approximately 10%-25% of fathers globally, but is also significantly associated with an increased risk of depression in their offspring, underscoring its intergenerational impact and public health relevance[77]. More recent meta-analyses estimate prevalence rates of 8%-13%, underscoring the global relevance and clinical significance of paternal mental health[78,79].
Importantly, paternal depression has been shown to correlate with—and in some cases exacerbate—maternal PPD symptoms, suggesting a bidirectional model of psychological distress transmission within family systems.
Konrad et al[80] introduced the use of interaction-based and neural synchrony biomarkers to detect emotional dysregulation in parental dyads, offering promising avenues for real-time, family-level mental health screening and interventions.
Future priorities in this space should include: (1) The development of dyadic and family-based therapy models; (2) Research into co-regulation dynamics between parents; and (3) Exploration of extended family roles in buffering maternal distress and promoting recovery.
Cross-cultural and interdisciplinary integration: To ensure global relevance, future research must rigorously test the generalizability of PPD interventions across socioeconomic strata, cultural belief systems, and healthcare delivery infrastructures.
Progress in PPD research depends on close collaboration between clinicians, neuroscientists, public-health experts, and data scientists. Bringing together neuroscience, public-health, behavioral, and digital insights can yield care pathways that are both personalized and context-specific.
Figure 6 synthesizes these emerging directions, presenting five interlinked frontiers shaping the next generation of PPD research—spanning biological, technological, familial, systemic, and global domains.
PPD is a global public-health issue with significant intergenerational consequences. While pharmacological treatments and psychotherapy are effective, their benefits depend on cultural appropriateness, equitable access, and adequate health system support. To truly address the burden of PPD, health systems must institutionalize routine screening, strengthen non-pharmacologic pathways, and expand community-based delivery platforms—particularly in underserved settings.
Left undiagnosed or untreated, PPD can have profound long-term consequences for maternal well-being, families, healthcare systems, and future generations. Key adverse public health outcomes of untreated PPD include: (1) Compromised maternal mental health and diminished quality of life; (2) Disruption of family dynamics and parenting capacity; (3) Adverse neurodevelopmental and emotional outcomes in children[1]; and (4) Increased maternal suicide risk, a leading cause of postpartum mortality in several countries[1].
These outcomes call for affordable, scalable, and culturally adaptable models that can reach at-risk mothers in primary care and community settings.
Growing evidence supports the integration of low-cost, non-pharmacological strategies including: (1) Psychoeducation to improve mental health literacy and reduce stigma; (2) CBT to address maladaptive thinking and emotional regulation; (3) Mind-body practices such as yoga and mindfulness to enhance resilience; and (4) Family-centered support programs to strengthen caregiving skills and emotional co-regulation.
These interventions have been shown to reduce symptom burden, improve functional and interpersonal outcomes, and lessen pressure on overburdened health systems[6,9].
Importantly, these interventions are both clinically effective and cost-efficient, making them strategic investments for public health. Embedding them into maternal and child health frameworks—while also leveraging digital tools and biologically informed risk stratification—will help close the implementation gap and ensure early detection and personalized care. Global collaboration, supportive health policies, and interdisciplinary research are essential to achieve long-term sustainability and meaningful impact.
While this review synthesizes findings from a diverse body of research on PPD, several limitations must be acknowledged to support balanced interpretation and guide future research and clinical translation.
First, existing studies exhibit marked heterogeneity in sample sizes, study designs (e.g., RCTs vs observational cohorts), outcome measures (e.g., EPDS and HDRS), and socio-cultural contexts, as well as follow-up durations. This variability hampers meaningful comparison between studies and limits the global applicability of their findings[44].
Second, the long-term efficacy, safety, and feasibility of emerging interventions—such as MBCT, zuranolone, and tDCS—remain insufficiently established, as current evidence is largely based on short-term trials. Robust longitudinal studies are urgently needed to evaluate sustained outcomes across diverse clinical and resource settings[81,82].
Third, the lack of cost-effectiveness analyses poses a major barrier to assessing scalability. While community-based interventions (e.g., home-delivered psychoeducation) have demonstrated promise[67], rigorous economic evaluations—especially for high-cost or technology-intensive interventions—are essential for informed policy-making, particularly in LMICs.
Fourth, despite growing attention to personalized care, few studies systematically investigate how maternal characteristics—such as age, education, personality traits, or cultural background—influence treatment response, adherence, or acceptability. A better understanding of these psychosocial and demographic moderators may improve the real-world impact and equity of PPD interventions[83].
Finally, disciplinary silos still hinder mechanistic integration. A systems biology framework is increasingly warranted—one that synthesizes neuroendocrine, immune, microbiome, and psychosocial domains using multi-omics platforms and neuroimaging tools[19,28,29,81]. Emerging studies suggest feasibility: Transcriptomic analyses have identified gene expression profiles linked to perinatal depressive symptoms[84]; proteomic biomarkers[28], multimodal brain changes[29], and integrative biomarker panels[19] offer promising avenues for early detection and stratified care. Progress hinges on steady cross-disciplinary teamwork that turns biological insights into scalable, patient-centered care.
PPD is among the most common and debilitating mood disorders after childbirth. It exerts a profound toll on maternal mental health, undermines early mother-infant bonding, and impairs family functioning and child development[10]. The etiology of PPD is multifactorial, driven by a confluence of hormonal dysregulation, nutritional deficits, and neurotransmitter imbalances, as well as psychological vulnerabilities such as neuroticism, self-critical tendencies, and low maternal self-efficacy[1,3,5,54].
While pharmacological treatments (e.g., SSRIs and zuranolone) are essential for managing moderate-to-severe cases, their limitations—such as delayed onset, adverse effects, and restricted applicability during breastfeeding—have spurred increasing interest in non-pharmacological interventions. These include CBT, IPT, psychoeducation, mind-body therapies (e.g., yoga and music therapy), and nutritional supplementation.
These strategies demonstrate sustained efficacy, favorable safety profiles, cost-effectiveness, and significant cultural adaptability, particularly suitable for low-resource and community-based settings[9,11,23,47].
Interventions that strengthen maternal self-efficacy and social support consistently outperform symptom-only approaches, highlighting the value of empowerment-focused care[4]. Emerging modalities, such as tDCS, represent a promising frontier for innovative and scalable treatment solutions[52].
Despite a growing evidence base, implementation of non-pharmacological interventions remains inconsistent, hampered by lack of standardization, workforce shortages, and intervention discontinuity. To close these gaps, the following recommendations are proposed for clinical practice and public health policy: (1) Institutionalize routine screening: Administer the EPDS at least once during pregnancy and again at 4-6 weeks postpartum. Prioritize high-risk individuals, particularly those with a history of depression, antenatal anxiety, or high neuroticism[6,36]; (2) Tailor risk-based interventions: Customize care for individuals with low self-efficacy, poor social support, or maladaptive cognitive traits. Recommended modalities include CBT, IPT, peer-based models, and psychoeducation[5,7]; (3) Scale up community-based interventions: Expand access to home-visiting programs, group therapies, mind-body interventions, and psychoeducational sessions—particularly in primary care and community settings[3,9,11]; (4) Empower maternal self-efficacy: Integrate parenting skills training, mindfulness programs, and peer-led support groups into postpartum care to build confidence and improve emotional regulation[4,18]; (5) Strengthen workforce capacity: Train nurses, midwives, and community health workers to deliver validated non-pharmacologic interventions. Emphasize protocol standardization, skills certification, and fidelity monitoring[9]; and (6) Support clinical innovation: Promote randomized trials exploring emerging modalities such as neuromodulation (e.g., tDCS) and targeted nutritional therapies, with a focus on real-world effectiveness and scalability[23,52].
| 1. | Yu Y, Liang HF, Chen J, Li ZB, Han YS, Chen JX, Li JC. Postpartum Depression: Current Status and Possible Identification Using Biomarkers. Front Psychiatry. 2021;12:620371. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Agrawal I, Mehendale AM, Malhotra R. Risk Factors of Postpartum Depression. Cureus. 2022;14:e30898. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 3. | Saharoy R, Potdukhe A, Wanjari M, Taksande AB. Postpartum Depression and Maternal Care: Exploring the Complex Effects on Mothers and Infants. Cureus. 2023;15:e41381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 36] [Reference Citation Analysis (0)] |
| 4. | van der Zee-van den Berg AI, Boere-Boonekamp MM, Groothuis-Oudshoorn CGM, Reijneveld SA. Postpartum depression and anxiety: a community-based study on risk factors before, during and after pregnancy. J Affect Disord. 2021;286:158-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 78] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 5. | Puyané M, Subirà S, Torres A, Roca A, Garcia-Esteve L, Gelabert E. Personality traits as a risk factor for postpartum depression: A systematic review and meta-analysis. J Affect Disord. 2022;298:577-589. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 36] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 6. | Dominiak M, Antosik-Wojcinska AZ, Baron M, Mierzejewski P, Swiecicki L. Recommendations for the prevention and treatment of postpartum depression. Ginekol Pol. 2021;92:153-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 7. | Al Nasr RS, Altharwi K, Derbah MS, Gharibo SO, Fallatah SA, Alotaibi SG, Almutairi KA, Asdaq SMB. Prevalence and predictors of postpartum depression in Riyadh, Saudi Arabia: A cross sectional study. PLoS One. 2020;15:e0228666. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 40] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 8. | Suryawanshi O 4th, Pajai S. A Comprehensive Review on Postpartum Depression. Cureus. 2022;14:e32745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 9. | Mwita M, Dewey D, Konje ET, Patten S. Non-pharmacological interventions for perinatal depression and anxiety among adolescent mothers: A systematic review and meta-analysis. J Affect Disord. 2025;379:168-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 10. | Alba BM. CE: Postpartum Depression: A Nurse's Guide. Am J Nurs. 2021;121:32-43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Hoffmann CS, Hoegholt NF, Vuust P, Kringelbach M, Jespersen KV. The effect of music on pregnancy-related insomnia: A systematic review and meta-analysis. Midwifery. 2025;142:104294. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 12. | Gastaldon C, Solmi M, Correll CU, Barbui C, Schoretsanitis G. Risk factors of postpartum depression and depressive symptoms: umbrella review of current evidence from systematic reviews and meta-analyses of observational studies. Br J Psychiatry. 2022;221:591-602. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 57] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 13. | Zacher Kjeldsen MM, Bricca A, Liu X, Frokjaer VG, Madsen KB, Munk-Olsen T. Family History of Psychiatric Disorders as a Risk Factor for Maternal Postpartum Depression: A Systematic Review and Meta-analysis. JAMA Psychiatry. 2022;79:1004-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 18] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 14. | Ning J, Deng J, Li S, Lu C, Zeng P. Meta-analysis of association between caesarean section and postpartum depression risk. Front Psychiatry. 2024;15:1361604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 15. | Gould JF, Gibson RA, Green TJ, Makrides M. A Systematic Review of Vitamin D during Pregnancy and Postnatally and Symptoms of Depression in the Antenatal and Postpartum Period from Randomized Controlled Trials and Observational Studies. Nutrients. 2022;14:2300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 16. | Geng Y, Liu W, Yu Z, Zhang H, Li Y, Zhao W. Socioeconomic factors and sex effects of postpartum maternal depression on offspring internalizing symptoms: a systematic review and meta-analysis. BMC Med. 2025;23:69. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Shelke A, Chakole S. A Review on Risk Factors of Postpartum Depression in India and Its Management. Cureus. 2022;14:e29150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 18. | Meltzer-Brody S, Kanes SJ. Allopregnanolone in postpartum depression: Role in pathophysiology and treatment. Neurobiol Stress. 2020;12:100212. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 87] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 19. | Zhang K, He L, Li Z, Ding R, Han X, Chen B, Cao G, Ye JH, Li T, Fu R. Bridging Neurobiological Insights and Clinical Biomarkers in Postpartum Depression: A Narrative Review. Int J Mol Sci. 2024;25:8835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 20. | Grötsch MK, Ehlert U. Allopregnanolone and mood in the peripartum: a longitudinal assessment in healthy women. Front Behav Neurosci. 2024;18:1499416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 21. | Deligiannidis KM, Meltzer-Brody S, Gunduz-Bruce H, Doherty J, Jonas J, Li S, Sankoh AJ, Silber C, Campbell AD, Werneburg B, Kanes SJ, Lasser R. Effect of Zuranolone vs Placebo in Postpartum Depression: A Randomized Clinical Trial. JAMA Psychiatry. 2021;78:951-959. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 72] [Cited by in RCA: 143] [Article Influence: 35.8] [Reference Citation Analysis (0)] |
| 22. | Batt MM, Duffy KA, Novick AM, Metcalf CA, Epperson CN. Is Postpartum Depression Different From Depression Occurring Outside of the Perinatal Period? A Review of the Evidence. Focus (Am Psychiatr Publ). 2020;18:106-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 67] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 23. | Colombage RL, Holden S, Lamport DJ, Barfoot KL. The effects of flavonoid supplementation on the mental health of postpartum parents. Front Glob Womens Health. 2024;5:1345353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 24. | Fang P, Li GH, Rao YB, Cheng C, He WL, Wang JJ, Li XY, Lu YR. Serum Cytokines as Biomarkers for Comorbid Anxiety in Postpartum Depression: A Machine Learning Approach. Psychiat Clin Psych. 2025;1. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 25. | Silverman ME, Reichenberg A, Savitz DA, Cnattingius S, Lichtenstein P, Hultman CM, Larsson H, Sandin S. The risk factors for postpartum depression: A population-based study. Depress Anxiety. 2017;34:178-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 214] [Cited by in RCA: 257] [Article Influence: 32.1] [Reference Citation Analysis (0)] |
| 26. | Xu Q, Sun L, Chen Q, Jiao C, Wang Y, Li H, Xie J, Zhu F, Wang J, Zhang W, Xie L, Wu H, Zuo Z, Chen X. Gut microbiota dysbiosis contributes to depression-like behaviors via hippocampal NLRP3-mediated neuroinflammation in a postpartum depression mouse model. Brain Behav Immun. 2024;119:220-235. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 18] [Reference Citation Analysis (0)] |
| 27. | Zheng Q, Wang S, Tian X, Liu W, Gao P. Fecal microbiota transplantation confirmed that 919 Syrup reduced the ratio of erucamide to 5-AVAB in hippocampus to alleviate postpartum depression by regulating gut microbes. Front Immunol. 2023;14:1203015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 28. | Accortt E, Mirocha J, Zhang D, Kilpatrick SJ, Libermann T, Karumanchi SA. Perinatal mood and anxiety disorders: biomarker discovery using plasma proteomics. Am J Obstet Gynecol. 2023;229:166.e1-166.e16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 29. | Schnakenberg P, Hahn L, Stickel S, Stickeler E, Habel U, Eickhoff SB, Chechko N, Dukart J. Examining early structural and functional brain alterations in postpartum depression through multimodal neuroimaging. Sci Rep. 2021;11:13551. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Desalegn MG, Nam E. Scaling Up Maternal Mental Health Care: Evaluating Community-Based Interventions in Ethiopia: A Systematic Review. Korean J Health Promot. 2025;25:1-8. [DOI] [Full Text] |
| 31. | Aubel J. Grandmothers - a neglected family resource for saving newborn lives. BMJ Glob Health. 2021;6:e003808. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 31] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 32. | Upadhyay RP, Chowdhury R, Aslyeh Salehi, Sarkar K, Singh SK, Sinha B, Pawar A, Rajalakshmi AK, Kumar A. Postpartum depression in India: a systematic review and meta-analysis. Bull World Health Organ. 2017;95:706-717C. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 140] [Cited by in RCA: 179] [Article Influence: 22.4] [Reference Citation Analysis (0)] |
| 33. | Nguyen LT, Minh Giang L, Nguyen DB, Nguyen TT, Lin C. Unraveling reproductive and maternal health challenges of women living with HIV/AIDS in Vietnam: a qualitative study. Reprod Health. 2024;21:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 34. | Chan SH, Shorey S. Effectiveness of psychosocial interventions on the psychological outcomes of parents with preterm infants: A systematic review and meta-analysis. J Pediatr Nurs. 2024;74:23-34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 35. | Sakina R, Khan SE, Chaudhry AG. Stigma of postpartum depression: The role of lady health workers in health care-A qualitative study. Health Care Women Int. 2022;43:1095-1104. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 36. | Cheng CY, Chou YH, Chang CH, Liou SR. Trends of Perinatal Stress, Anxiety, and Depression and Their Prediction on Postpartum Depression. Int J Environ Res Public Health. 2021;18:9307. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 35] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 37. | Fan Q, Long Q, De Silva V, Gunarathna N, Jayathilaka U, Dabrera T, Lynn H, Østbye T. Prevalence and risk factors for postpartum depression in Sri Lanka: A population-based study. Asian J Psychiatr. 2020;47:101855. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 38. | Chan AW, Reid C, Skeffington P, Marriott R. A systematic review of EPDS cultural suitability with Indigenous mothers: a global perspective. Arch Womens Ment Health. 2021;24:353-365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 19] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 39. | Yang M, Seon Q, Gomez Cardona L, Karia M, Velupillai G, Noel V, Linnaranta O. Safe and valid? A systematic review of the psychometric properties of culturally adapted depression scales for use among Indigenous populations. Glob Ment Health (Camb). 2023;10:e60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 40. | Ali GC, Ryan G, De Silva MJ. Validated Screening Tools for Common Mental Disorders in Low and Middle Income Countries: A Systematic Review. PLoS One. 2016;11:e0156939. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 237] [Cited by in RCA: 208] [Article Influence: 23.1] [Reference Citation Analysis (0)] |
| 41. | Fellmeth G, Harrison S, Opondo C, Nair M, Kurinczuk JJ, Alderdice F. Validated screening tools to identify common mental disorders in perinatal and postpartum women in India: a systematic review and meta-analysis. BMC Psychiatry. 2021;21:200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 42. | Heer K, Mahmoud L, Abdelmeguid H, Selvan K, Malvankar-Mehta MS. Prevalence, Risk Factors, and Interventions of Postpartum Depression in Refugees and Asylum-Seeking Women: A Systematic Review and Meta-Analysis. Gynecol Obstet Invest. 2024;89:11-21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 43. | Skoog M, Hallström IK, Vilhelmsson A. Health care professionals' experiences of screening immigrant mothers for postpartum depression-a qualitative systematic review. PLoS One. 2022;17:e0271318. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 44. | Chow R, Huang E, Li A, Li S, Fu SY, Son JS, Foster WG. Appraisal of systematic reviews on interventions for postpartum depression: systematic review. BMC Pregnancy Childbirth. 2021;21:18. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 17] [Cited by in RCA: 34] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 45. | Davis JA, Ohan JL, Gibson LY, Prescott SL, Finlay-Jones AL. Understanding Engagement in Digital Mental Health and Well-being Programs for Women in the Perinatal Period: Systematic Review Without Meta-analysis. J Med Internet Res. 2022;24:e36620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 15] [Reference Citation Analysis (0)] |
| 46. | Iturralde E, Hsiao CA, Nkemere L, Kubo A, Sterling SA, Flanagan T, Avalos LA. Engagement in perinatal depression treatment: a qualitative study of barriers across and within racial/ethnic groups. BMC Pregnancy Childbirth. 2021;21:512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 43] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 47. | McGinty EE, Alegria M, Beidas RS, Braithwaite J, Kola L, Leslie DL, Moise N, Mueller B, Pincus HA, Shidhaye R, Simon K, Singer SJ, Stuart EA, Eisenberg MD. The Lancet Psychiatry Commission: transforming mental health implementation research. Lancet Psychiatry. 2024;11:368-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 37.0] [Reference Citation Analysis (0)] |
| 48. | Naslund JA, Aschbrenner KA, Araya R, Marsch LA, Unützer J, Patel V, Bartels SJ. Digital technology for treating and preventing mental disorders in low-income and middle-income countries: a narrative review of the literature. Lancet Psychiatry. 2017;4:486-500. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 305] [Article Influence: 38.1] [Reference Citation Analysis (0)] |
| 49. | Richardson S, Lawrence K, Schoenthaler AM, Mann D. A framework for digital health equity. NPJ Digit Med. 2022;5:119. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 209] [Reference Citation Analysis (0)] |
| 50. | Torous J, Lipschitz J, Ng M, Firth J. Dropout rates in clinical trials of smartphone apps for depressive symptoms: A systematic review and meta-analysis. J Affect Disord. 2020;263:413-419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 270] [Article Influence: 54.0] [Reference Citation Analysis (0)] |
| 51. | Anthes E. Mental health: There's an app for that. Nature. 2016;532:20-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 224] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 52. | Sun W, Kang X, Dong X, Zeng Z, Zou Q, Su M, Zhang K, Liu G, Yu G. Effect of transcranial direct current stimulation on postpartum depression: A study protocol for a randomized controlled trial. Front Psychol. 2023;14:990162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 53. | Treatment and Management of Mental Health Conditions During Pregnancy and Postpartum: ACOG Clinical Practice Guideline No. 5. Obstet Gynecol. 2023;141:1262-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 83] [Article Influence: 41.5] [Reference Citation Analysis (0)] |
| 54. | Meltzer-Brody S, Colquhoun H, Riesenberg R, Epperson CN, Deligiannidis KM, Rubinow DR, Li H, Sankoh AJ, Clemson C, Schacterle A, Jonas J, Kanes S. Brexanolone injection in post-partum depression: two multicentre, double-blind, randomised, placebo-controlled, phase 3 trials. Lancet. 2018;392:1058-1070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 290] [Cited by in RCA: 395] [Article Influence: 56.4] [Reference Citation Analysis (0)] |
| 55. | Li X, Laplante DP, Paquin V, Lafortune S, Elgbeili G, King S. Effectiveness of cognitive behavioral therapy for perinatal maternal depression, anxiety and stress: A systematic review and meta-analysis of randomized controlled trials. Clin Psychol Rev. 2022;92:102129. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 46] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 56. | Pan J, Luo W, Zhang H, Wang Y, Lu H, Wang C, Li C, Fu L, Hu Y, Li Y, Shen M. The Effects of Online Cognitive Behavioral Therapy on Postpartum Depression: A Systematic Review and Meta-Analysis. Healthcare (Basel). 2025;13:696. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 57. | Ngai FW, Gao LL. Effect of couple-based interpersonal psychotherapy on postpartum depressive symptoms: A randomised controlled trial. Asian J Psychiatr. 2022;78:103274. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 58. | Kang HK, Bisht B, Kaur M, Alexis O, Worsley A, John D. Effectiveness of interpersonal psychotherapy in comparison to other psychological and pharmacological interventions for reducing depressive symptoms in women diagnosed with postpartum depression in low- and middle-income countries: A systematic review. Campbell Syst Rev. 2024;20:e1399. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 59. | Kasujja R, Birungi P, Bhamidipati K, Assefa F, Kim HY, Peterson KM, Kohrt BA, Bershteyn A. Six-Week Problem Area-Concordant vs 8-Week Problem Area-Discordant Group Interpersonal Psychotherapy: A Randomized Clinical Trial. JAMA Netw Open. 2025;8:e255242. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 60. | Martín-Gómez C, Moreno-Peral P, Bellón JA, Conejo-Cerón S, Campos-Paino H, Gómez-Gómez I, Rigabert A, Benítez I, Motrico E. Effectiveness of psychological interventions in preventing postpartum depression in non-depressed women: a systematic review and meta-analysis of randomized controlled trials. Psychol Med. 2022;52:1001-1013. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 61. | Xu H, Liu R, Wang X, Yang J. Effectiveness of aerobic exercise in the prevention and treatment of postpartum depression: Meta-analysis and network meta-analysis. PLoS One. 2023;18:e0287650. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 62. | Davenport MH, McCurdy AP, Mottola MF, Skow RJ, Meah VL, Poitras VJ, Jaramillo Garcia A, Gray CE, Barrowman N, Riske L, Sobierajski F, James M, Nagpal T, Marchand AA, Nuspl M, Slater LG, Barakat R, Adamo KB, Davies GA, Ruchat SM. Impact of prenatal exercise on both prenatal and postnatal anxiety and depressive symptoms: a systematic review and meta-analysis. Br J Sports Med. 2018;52:1376-1385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 150] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 63. | Song J, Zhou B, Kan J, Liu G, Zhang S, Si L, Zhang X, Yang X, Ma J, Cheng J, Liu X, Yang Y. Corrigendum: Gut microbiota: Linking nutrition and perinatal depression. Front Cell Infect Microbiol. 2022;12:1053553. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 2] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 64. | Szajna A, Tekkalaki B, Nandagaon V, Udapi G, Sogalad M, Dandagi S, Kole U, Patil S, Raddi S, Short V, Kelly PJ. Feasibility and acceptability of a community health worker administered behavioral activation intervention for postpartum depression: a single arm pilot study from India. Front Psychiatry. 2024;15:1284674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 65. | Boran P, Dönmez M, Barış E, Us MC, Altaş ZM, Nisar A, Atif N, Sikander S, Hıdıroğlu S, Save D, Rahman A. Delivering the Thinking Healthy Programme as a universal group intervention integrated into routine antenatal care: a randomized-controlled pilot study. BMC Psychiatry. 2023;23:14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 66. | Kumpf U, Palm U, Eder J, Ezim H, Stadler M, Burkhardt G, Dechantsreiter E, Padberg F. TDCS at home for depressive disorders: an updated systematic review and lessons learned from a prematurely terminated randomized controlled pilot study. Eur Arch Psychiatry Clin Neurosci. 2023;273:1403-1420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 67. | Zheng Q, Shi L, Zhu L, Jiao N, Chong YS, Chan SW, Chan YH, Luo N, Wang W, He H. Cost-effectiveness of Web-Based and Home-Based Postnatal Psychoeducational Interventions for First-time Mothers: Economic Evaluation Alongside Randomized Controlled Trial. J Med Internet Res. 2022;24:e25821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 5] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 68. | World Health Organization. Improving early childhood development: WHO guideline. Geneva: WHO. (2022). Available from: https://www.who.int/publications/i/item/97892400020986. |
| 69. | ACOG Committee Opinion No. 757: Screening for Perinatal Depression. Obstet Gynecol. 2018;132:e208-e212. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 278] [Cited by in RCA: 497] [Article Influence: 82.8] [Reference Citation Analysis (0)] |
| 70. | National Institute for Health and Care Excellence (NICE). (2014, updated 2020). Antenatal and postnatal mental health: clinical management and service guidance (CG192). Available from: https://www.nice.org.uk/guidance/cg192. |
| 71. | US Preventive Services Task Force, Curry SJ, Krist AH, Owens DK, Barry MJ, Caughey AB, Davidson KW, Doubeni CA, Epling JW Jr, Grossman DC, Kemper AR, Kubik M, Landefeld CS, Mangione CM, Silverstein M, Simon MA, Tseng CW, Wong JB. Interventions to Prevent Perinatal Depression: US Preventive Services Task Force Recommendation Statement. JAMA. 2019;321:580-587. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 216] [Cited by in RCA: 334] [Article Influence: 55.7] [Reference Citation Analysis (0)] |
| 72. | Lang E, Colquhoun H, LeBlanc JC, Riva JJ, Moore A, Traversy G, Wilson B, Grad R; Canadian Task Force on Preventive Health Care. Recommendation on instrument-based screening for depression during pregnancy and the postpartum period. CMAJ. 2022;194:E981-E989. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 73. | Malhi GS, Bell E, Bassett D, Boyce P, Bryant R, Hazell P, Hopwood M, Lyndon B, Mulder R, Porter R, Singh AB, Murray G. The 2020 Royal Australian and New Zealand College of Psychiatrists clinical practice guidelines for mood disorders. Aust N Z J Psychiatry. 2021;55:7-117. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 328] [Article Influence: 82.0] [Reference Citation Analysis (0)] |
| 74. | La Verde M, Luciano M, Fordellone M, Sampogna G, Lettieri D, Palma M, Torella D, Marrapodi MM, Di Vincenzo M, Torella M. Postpartum Depression and Inflammatory Biomarkers of Neutrophil-Lymphocyte Ratio, Platelet-Lymphocyte Ratio, and Monocyte-Lymphocyte Ratio: A Prospective Observational Study. Gynecol Obstet Invest. 2024;89:140-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 18] [Article Influence: 18.0] [Reference Citation Analysis (0)] |
| 75. | Coecke S, Panzarella G, Firth J, Sarris J, Buckman JE, Toczyski P, van Rijn E, Quétel CR, Kephalopoulos S, Vignes M, Kourti N, Bobach C, Pladunova S, Solmi M, van Kamp I, Ronchi F, Pevere M, Gallo S, De Bernardi F, Sachana M, Laureys S, Torres F, Palacios Sanchez L, Cairney S, Kaihsiang Y, Charveriat C, Pang C, Vernetti C, Baggio M, Kovacic M, Di Gioia R, Munoz A, Puertas-Gallardo A, Takki M, Petrillo M, Gallo A, Alcaro S, Gawlik BM, Tavazzi S, Lombardi I, Hupont-Torres I, Romeo P, Schade S, Barreda Angeles M, Bakogianni I, Maragkoudakis P, Ceresa M, Querci M, Caldeira S, Wiesenthal T. Decoding depression: Exploring the environome across life course. Securing mental health systems: Preparedness, innovation, and policy for global resilience, Publications Office of the European Union. 2025. Available from: https://publications.jrc.ec.europa.eu/repository/handle/JRC139918. |
| 76. | Le SM, Quang HT. Digital Journey in Primary Health Care - Empowering Patients with Noncommunicable Diseases in Vietnam (English). Health; Nutrition; and Population (HNP) Discussion Paper Washington, DC: World Bank Group. Available from: http://documents.worldbank.org/curated/en/099017103132412996. |
| 77. | Dachew B, Ayano G, Duko B, Lawrence B, Betts K, Alati R. Paternal Depression and Risk of Depression Among Offspring: A Systematic Review and Meta-Analysis. JAMA Netw Open. 2023;6:e2329159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 78. | Bruno A, Celebre L, Mento C, Rizzo A, Silvestri MC, De Stefano R, Zoccali RA, Muscatello MRA. When Fathers Begin to Falter: A Comprehensive Review on Paternal Perinatal Depression. Int J Environ Res Public Health. 2020;17:1139. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 21] [Cited by in RCA: 54] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 79. | Wang D, Li YL, Qiu D, Xiao SY. Factors Influencing Paternal Postpartum Depression: A Systematic Review and Meta-Analysis. J Affect Disord. 2021;293:51-63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 77] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 80. | Konrad K, Gerloff C, Kohl SH, Mehler DMA, Mehlem L, Volbert EL, Komorek M, Henn AT, Boecker M, Weiss E, Reindl V. Interpersonal neural synchrony and mental disorders: unlocking potential pathways for clinical interventions. Front Neurosci. 2024;18:1286130. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 9] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 81. | Payne JL, Maguire J. Pathophysiological mechanisms implicated in postpartum depression. Front Neuroendocrinol. 2019;52:165-180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 239] [Article Influence: 39.8] [Reference Citation Analysis (0)] |
| 82. | Kargbo RB. Neurosteroids and Postpartum Depression: The Mechanism, Efficacy, and Approval of Brexanolone and Zurzuvae. ACS Med Chem Lett. 2023;14:1326-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 83. | Dagher RK, Bruckheim HE, Colpe LJ, Edwards E, White DB. Perinatal Depression: Challenges and Opportunities. J Womens Health (Larchmt). 2021;30:154-159. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 67] [Cited by in RCA: 130] [Article Influence: 32.5] [Reference Citation Analysis (0)] |
| 84. | Björvang RD, Vrettou M, Bujanda Cundin X, Del Prete E, Rüegg J, Lager S, di Bernardo D, Comasco E, Skalkidou A. Differentially expressed transcripts associated with depressive symptoms during pregnancy and postpartum. Mol Psychiatry. 2025;. [PubMed] [DOI] [Full Text] |