Published online Sep 16, 2025. doi: 10.12998/wjcc.v13.i26.104876
Revised: April 18, 2025
Accepted: June 4, 2025
Published online: September 16, 2025
Processing time: 199 Days and 17.5 Hours
An ileal neobladder is a standardized form of urinary diversion that provides acceptable outcomes in terms of long-term quality of life. Urothelial carcinomas (UCs) arising in the ileal neobladder are extremely rare, and few reports on this have been published in the English language.
We report a case of UC that developed in the ileal neobladder of a 63-year-old man. The patient was diagnosed with UC in situ and underwent radical cystoprostatectomy and ileal neobladder creation. Ten years after the surgery and neoadjuvant chemotherapy, an UC developed in the ileal neobladder.
Ileal neobladder urothelial carcinoma can originate from the implanted urothe
Core Tip: The simultaneous or metachronous occurrence of multifocal tumors in the urinary tract is characteristic of urothelial carcinoma (UC), and many patients experience recurrence post-surgery. However, recurrence of UC in ileal neobladders is extremely rare. Most cases of UC in the ileal neobladder are initially diagnosed as muscle-invasive bladder cancers. In this case, the pathological stage of the resected bladder after neoadjuvant chemotherapy was UC in situ. Patients who have undergone ileal neobladder surgery should be carefully followed up for a long period because the exfoliated tumor cells could “seed and implant” in the bladder and non-urothelial epithelium.
- Citation: Murakami M, Noguchi H, Matsushita R, Tatarano S, Kirishima M, Tasaki T, Kitazono I, Higashi M, Enokida H, Tanimoto A. Urothelial carcinoma arising in orthotopic ileal neobladder reconstruction: A case report. World J Clin Cases 2025; 13(26): 104876
- URL: https://www.wjgnet.com/2307-8960/full/v13/i26/104876.htm
- DOI: https://dx.doi.org/10.12998/wjcc.v13.i26.104876
Bladder cancer was the 10th most common malignancy of the urinary tract worldwide in 2020, accounting for 3% of global cancer diagnoses. It is the 13th most common cause of cancer-related mortality worldwide, and its prevalence is particularly high in high-income nations. The risk factors for bladder cancer include advanced age, smoking, and occupational and environmental exposure to toxins[1]. The urothelial cells lining the bladder and urinary tract are exposed to several chemicals that can cause genetic mutations[1]. Transurethral resection of bladder tumors is the initial treatment for non-muscle-invasive bladder cancer (MIBC), and is essential for diagnosis and staging. The standard treatment for MIBC is radical cystectomy and ileal conduit or ileal neobladder construction preceded by cisplatin-based neoadjuvant chemotherapy (NAC) for invasive tumors in eligible patients[2]. Ileal neobladders have become the preferred form of urinary diversion in patients undergoing radical cystectomy because they are associated with a lower risk of secondary tumors than colonic neobladders[3]. Some cases of secondary adenocarcinoma developing in the ileal neobladder have been reported, as well as a few cases urothelial carcinoma (UC) replacing the bowel segment[4]. Although UCs are widely acknowledged to be multifocal, its etiology in urinary diversion remains unclear. Here, we report a unique case of UC that developed at the site of the ileal neobladder anastomosis after cystectomy for bladder cancer and NAC in a 63 year old man.
To the best of our knowledge, no case of UC in the ileal neobladder after radical cystectomy for UC in situ (UCIS) has ever been reported. This case suggests that any UC treated with an ileal neobladder should be carefully followed-up after radical cystectomy.
A 63-year-old man who had undergone radical cystoprostatectomy and ileal neobladder reconstruction 10 years previously presented with dysuria, hematuria, and nausea.
The patient’s symptoms started two months prior to admission. He had been treated for type 2 diabetes mellitus (T2DM) and diagnosed with chronic kidney disease.
Ten years prior, he was diagnosed with high-grade UC with muscle invasion of the urinary bladder. Laboratory data of creatinine (0.73 mg/dL, normal range: 0.6-1.1 mg/dL) and blood urea nitrogen (14.2 mg/dL, normal range: 8-22 mg/dL) were within normal limits. Computed tomography (CT) revealed no evidence of metastases. The patient received NAC consisting of two courses of cisplatin and gemcitabine. Radical cystoprostatectomy and ileal neobladder creation were performed. Histopathological findings revealed UCIS spreading from the apex to the trigone in the resected urinary bladder with no lymph node metastasis. No cancer was detected in the urethra and all surgical margins were negative. After the surgery, the patient was closely followed-up with urine cytology and imaging at regular intervals.
The patient was on medications for hypertension, T2DM, and dyslipidemia. The patient had smoked 30 cigarettes per day for 30 years until the age of 50 years. There was no relevant family history.
Upon admission, the systolic blood pressure was 113 mmHg, diastolic was 70 mmHg, and respiratory rate was 18/minute. His body weight, height, and surface area were 58.3 kg, 150 cm, and 1.38 m2 respectively.
Laboratory tests revealed elevated creatinine (1.63 mg/dL) and urine nitrogen levels (32.4 mg/dL). Urinalysis revealed increased red blood cell and leukocyte counts (19 cells/high-power field and 99 cells/high-power field, respectively).
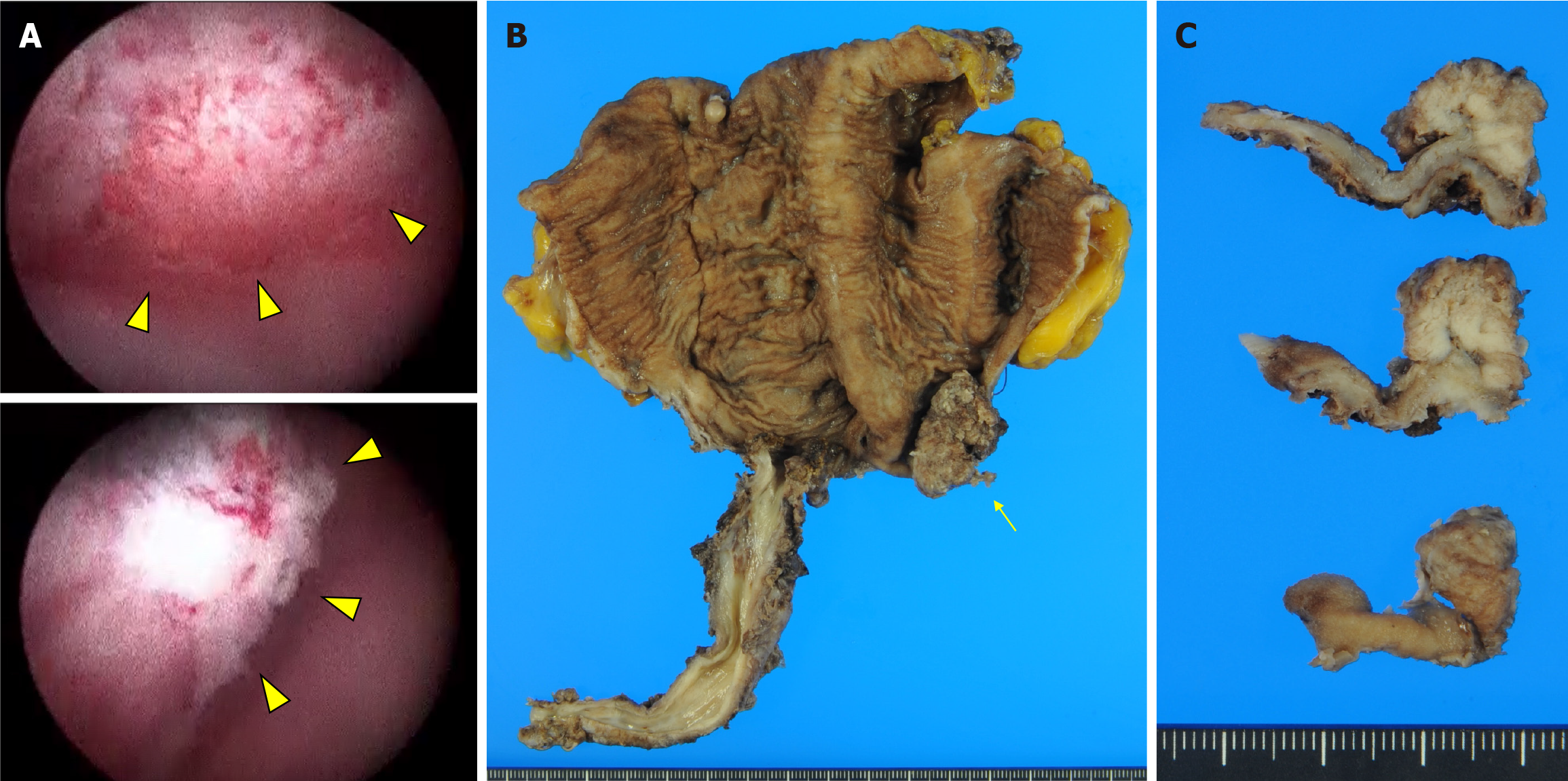
Cystoscopic examination revealed a papillary tumor measuring 20 mm × 15 mm, located in the left lateral region of the ileal neobladder (Figure 1A). The tumor was not connected to the anastomotic site. CT confirmed there was no metastasis to the pelvic lymph nodes nor any tumorous regions in the bilateral kidney pelvises and ureters. The biopsy specimen revealed UC with a papillary structure. Based on the diagnosis of UC of the ileal neobladder, the patient underwent neocystectomy.
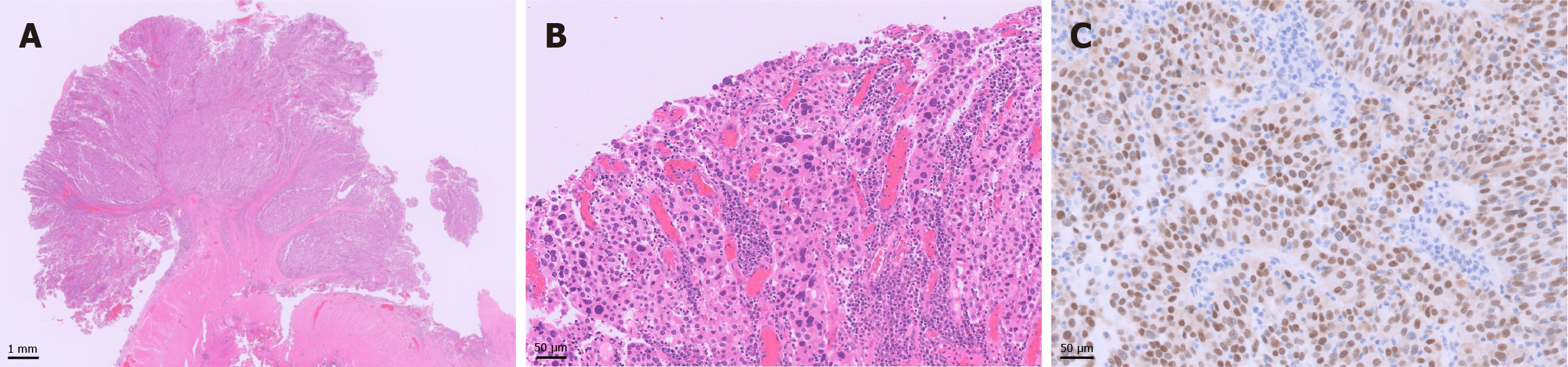
Gross examination revealed papillary tumor proliferation in the left lateral region of the ileal neobladder measuring 20 mm × 15 mm (Figure 1B). There were no tumors in the anastomosis or the remaining urethra. The cut surface of the tumor was gray to white in color (Figure 1C). Histological examination revealed a high-grade papillary UC without glandular differentiation (Figure 2A and B). The tumor cells were arranged in papillae with fibrous stroma. The boundary between the UC and the surrounding normal intestinal mucosa was clearly demarcated. Invasion of the submucosa of the ileal neobladder was also observed. Immunohistochemical examination revealed that the tumor cells were positive for GATA binding protein 3 (Figure 2C), but negative for cluster of differentiation 10 (not shown). No UC was observed in the urethra. Background ileal mucosa showed moderate atrophic changes; however, no urothelial metaplasia was observed.
The final diagnosis was UC of the ileal neobladder.
He underwent neocystectomy with lymphadenectomy and an ileal conduit reconstruction.
The patient was discharged 21 days postoperatively without any adverse events and received one-year postoperative chemotherapy with nivolumab. The patient was monitored at regular intervals by physical examination, urine cytology, and diagnostic imaging. The patient remained in stable condition without disease recurrence for 6 months after surgery.
Herein, we report a rare case of UC that developed in the ileal neobladder after radical cystoprostatectomy for UCIS. Primary malignancies after bladder substitution typically develop in the area of anastomoses with the remaining urinary tract or in the uretero-ileal or urethro-ileal anastomoses[5]. Although the most common histological subtype of malignancy in intestinal urinary diversion is adenocarcinoma[6], 20 cases of UC that developed in the ileal neobladder without urinary tract recurrence have been reported in the literature (Table 1)[4,7-15]. Most patients who develop neobladder carcinoma are initially diagnosed with high-grade UC with MIBC. Only one case of UCIS diagnosed before radical cystectomy has been reported; however, that case did not indicate where the recurrence occurred in the neobladder or urethra[7]. The recurrence period after the initial surgery ranges from 3 months to 161 months[4]. The UC recurrence rate in the urethra after radical cystectomy and ileal neobladder reconstruction ranges from 2.8% to 6%[16,17]. In our case, the patient was initially diagnosed with MIBC by transurethral resection of a bladder tumor and underwent NAC. The final diagnosis after radical cystoprostatectomy was UCIS without lymph node metastasis, and the ureteral margins were negative; however, UC developed in the ileal neobladder 10 years after surgery. This case shows that the initial diagnosis is important for predicting recurrence, even if the tumor volume is reduced after NAC.
| Ref. | Number of case | Age/sex | Pathological diagnosis | Initial stage | Localization of recurrence | Recurrence period |
| Oberneder et al[7] | 6 | NS | UC | pTis-pT4a | NS | NS |
| Hadzi-Djokić et al[8] | 1 | 65/M | UC, grade 2 | pT2 | Right wall | 12 years |
| Yamashita et al[9] | 1 | 74/M | UC, high grade | pT3a | Right and anterior wall | 3 years |
| Cakmak et al[10] | 1 | 40/F | UC, grade 2 | pT1 | Left wall (multifocal) | 11 years |
| Moeen et al[11] | 1 | 59/F | UC, high grade | pT2 | Bilateral wall (multifocal) | 18 months |
| Cherbanyk et al[12] | 1 | 66/M | UC, grade 2-3 | pT3a | Anterior and left wall | 9 years |
| Doshi et al[13] | 1 | 71/M | UC, high grade | pT3a | Left anterioinferior wall | 11 years |
| Pardalidis et al[14] | 1 | 56/M | UC, grade 3 | pT3a | NS | 10 years |
| Dadikhi et al[15] | 1 | 78/M | UC, grade 3 | pT2 | Entier (multifocal) | 4 years |
| Collins et al[4] | 6 | 57/M | UC, high grade | pT2a | NS | 30 months |
| 57/M | UC, sarcomatoid | pT2b | NS | 3 months | ||
| 58/M | UC, high grade | pT2a | NS | 41 months | ||
| 80/M | UC, glandular | ypT2b | NS | 44 months | ||
| 72/M | UC, plasmacytoid | ypT3a | NS | 12 months | ||
| 79/M | UC, micropapillary | pT2b | NS | 161 months | ||
| Present case | 1 | 63/M | UC, high grade | ypTis (pT2 befor neoadjuvant chemotherapy) | Left wall | 112 months |
Several studies have proposed two hypotheses to explain the mechanism of UC. The first is the “field carcinogenesis” theory, which posits that normal urothelial cells have malignant genetic background to develop UC[18]. The consequence is the multifocal occurrence of carcinoma in situ. Several molecular genetic studies support the theory that multifocal UCs result from clonal evolution of a single transformed cell[19,20]. The second hypothesis is that the exfoliated tumor cells seed and implant at another site in the urinary tract[21]. Papillary UC of the upper urinary tract frequently recurs in the bladder (15%-50%)[22]. The latter hypothesis explains the potential of UC implantation not only in the urothelium of the bladder, but also in the ileal mucosa of a neobladder[21].
The pathogenesis of the present case of UC that recurred in the small intestinal mucosa of ileal neobladder could be explained by the “seed and implantation” theory, because there was no recurrent UC lesion in the urinary tract or anastomosis site. Intraluminal tumor cell seeding as the source of multiple synchronous or metachronous urothelial tumors should be considered a potential factor contributing to the multifocal recurrence of UC in tissues other than the urothelium.
We have highlighted a rare case of UC of the ileal neobladder in this case report and highly recommend regular follow-up with standard management protocols for detecting early stage recurrence in the ileal neobladder.
| 1. | WHO Classification of Tumours Editorial Board. WHO Classification of Tumours, 5th Edition, Volume 8: Urinary and Male Genital Tumours. World Health Organization, 2022. |
| 2. | Nikolaidis CG, Gyriki D, Anitsakis C, Stavropoulou E. Tailoring treatment for elderly bladder cancer: a case report of personalized management of high-grade urothelial carcinoma with papillary features. Front Oncol. 2024;14:1434795. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 3. | Kälble T, Hofmann I, Riedmiller H, Vergho D. Tumor growth in urinary diversion: a multicenter analysis. Eur Urol. 2011;60:1081-1086. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 45] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 4. | Collins K, Yocum BP, Idrees MT, Saeed O. Carcinoma arising in ileal conduit or orthotopic ileal neobladder reconstruction: A 20-year single institute experience. Histopathology. 2024;85:182-189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 5. | Pickard R. Tumour formation within intestinal segments transposed to the urinary tract. World J Urol. 2004;22:227-234. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 27] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 6. | Cornell C, Khani F, Osunkoya AO, Matoso A, Miyamoto H, Gordetsky JB, Salaria SN, Giannico GA. Secondary malignancy after urologic reconstruction procedures: a multi-institutional case series. Hum Pathol. 2022;119:69-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Oberneder R, Staudte S, Waidelich R, Schmeller N, Hofstetter A. Local recurrence in patients after radical cystectomy and orthotopic ileal neobladder: impact on function. Int Urol Nephrol. 2003;35:175-179. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 8. | Hadzi-Djokić J, Pejcić T, Andrejević V, Djurasić L. Transitional cell carcinoma in orthotopic ileal neobladder 12 years after radical cystectomy. Vojnosanit Pregl. 2013;70:1062-1064. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 9. | Yamashita R, Matsuzaki M, Niwakawa M, Ito I. Bacillus Calmette-Guérin treatment of urothelial carcinoma arising in the ileal neobladder after radical cystectomy. Int J Urol. 2014;21:333-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 10. | Cakmak O, Tarhan H, Celik O, Kucuk U, Ilbey YO. Transitional cell carcinoma in orthotopic ileal neobladder. Case Rep Urol. 2014;2014:218615. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 11. | Moeen A. Recurrence of isolated transitional cell carcinoma in an orthotopic ileal neobladder: A case report. Afr J Urol. 2015;21:214-216. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 12. | Cherbanyk F, Prod'homme M, Pezzetta E, Chevaux B, Anaye A, Boillat JJ. Urothelial Carcinoma Recurrence in an Ileal Neobladder Nine Years after Primary Surgery with Muir-Torre Syndrome. Case Rep Urol. 2016;2016:4762514. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Doshi CP, Barkan GA, Quek ML. Urothelial Carcinoma Recurrence in an Orthotopic Neobladder without Urethral or Upper Urinary Tract Involvement. Case Rep Urol. 2019;2019:8458706. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 14. | Pardalidis PN, Andriopoulos NA, Kosmaoglou EV, Pardalidis VN, Pardalidis NP. Urothelial Carcinoma Recurrence in an Ileal Orthotopic Neobladder 10 Years After Primary Robotic Radical Cystoprostatectomy. J Endourol Case Rep. 2020;6:16-18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Dadikhi K, Mueller F, Montani M, Thalmann GN, Kiss B. Case of the Month from the University Hospital of Bern, Switzerland: Urothelial carcinoma in an orthotopic neobladder: reported cases and pathophysiological hypotheses. BJU Int. 2022;130:38-42. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Iselin CE, Robertson CN, Webster GD, Vieweg J, Paulson DF. Does prostate transitional cell carcinoma preclude orthotopic bladder reconstruction after radical cystoprostatectomy for bladder cancer? J Urol. 1997;158:2123-2126. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 51] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 17. | Studer UE, Danuser H, Hochreiter W, Springer JP, Turner WH, Zingg EJ. Summary of 10 years' experience with an ileal low-pressure bladder substitute combined with an afferent tubular isoperistaltic segment. World J Urol. 1996;14:29-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 78] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Palmeira C, Lameiras C, Amaro T, Lima L, Koch A, Lopes C, Oliveira PA, Santos L. CIS is a surrogate marker of genetic instability and field carcinogenesis in the urothelial mucosa. Urol Oncol. 2011;29:205-211. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Pycha A, Mian C, Hofbauer J, Brössner C, Haitel A, Wiener H, Marberger M. Multifocality of transitional cell carcinoma results from genetic instability of entire transitional epithelium. Urology. 1999;53:92-97. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Habuchi T. Origin of multifocal carcinomas of the bladder and upper urinary tract: molecular analysis and clinical implications. Int J Urol. 2005;12:709-716. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 63] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 21. | Herawi M, Leppert JT, Thomas GV, De Kernion JB, Epstein JI. Implants of noninvasive papillary urothelial carcinoma in peritoneum and ileocolonic neobladder: support for "seed and soil" hypothesis of bladder recurrence. Urology. 2006;67:746-750. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 28] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Witjes JA, Compérat E, Cowan NC, De Santis M, Gakis G, Lebret T, Ribal MJ, Van der Heijden AG, Sherif A; European Association of Urology. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778-792. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 697] [Cited by in RCA: 761] [Article Influence: 63.4] [Reference Citation Analysis (0)] |